
The predictive value of minute ventilation versus carbon dioxide production in pulmonary hypertension associated with left heart disease
Introduction
Pulmonary hypertension (PH) is a common complication of left heart disease (LHD), with patients with LHD accounting for 65–80% of PH cases (1). PH associated with LHD (PH-LHD) is associated with poor prognosis and low exercise tolerance (2). PH-LHD is divided into combined post- and pre-capillary PH (Cpc-PH) and isolated post-capillary PH (Ipc-PH), Cpc-PH represent the presence of pre-capillary components in patients with PH-LHD. The best way to describe the presence of pre-capillary components in post-capillary pH is still controversial; all hemodynamic variables used to describe PH-LHD are not unlimited. Recent guidelines have revised the distinction between Cpc-PH and Ipc-PH. In the previous guidelines, Cpc-PH was referred to as reactive PH (3), and the transpulmonary gradient (TPG) was used as the distinguishing standard. At the 5th World Symposium on Pulmonary Hypotension (WSPH) in 2013, the combination (4,5) of pulmonary vascular resistance (PVR) and diastolic pulmonary pressure gradient (DPG) was presented as a diagnostic marker for distinguishing Cpc-PH and Ipc-PH. Recent guidelines have proposed PVR alone to be a suitable marker (6). Although hemodynamics is the gold standard currently, there are still some limitations in its clinical application. Therefore, further study of noninvasive methods for early diagnosis of Cpc-PH is worthy of attention. Furthermore, cardiopulmonary exercise testing (CPET) can be used assess the exercise capacity of patients with PH-LHD, but it is unclear whether it can distinguish between those with Ipc-PH and those with Cpc-PH.
Modern CPET systems allow analysis of gas exchange during rest, exercise, and recovery, and measurement of oxygen uptake (VO2), carbon dioxide output (VCO2), and ventilation (VE). CPET is the gold standard for evaluating cardiopulmonary function (7,8), and it may have important diagnostic utility for PH-LHD (9-11). Exercise-induced hyperventilation occurs in patients with Ipc-PH, while patients with Cpc-PH have even higher hyperventilation (10). Hyperventilation was characterized by an increase in VE versus VCO2 (VE/VCO2) in the few studies on CPET in patients with PH-LHD (9-11), VE/VCO2 relationship is most often expressed as VE/VCO2 slope, lowest VE/VCO2 and peak VE/VCO2. Except PH-LHD, pulmonary arterial hypertension (PAH) is a common type of PH. The end-tidal CO2 (PETCO2), as an index of hyperventilation, may be valuable in the differential diagnosis of PAH (12). Cpc-PH is associated with decreased exercise capacity and is similar to PAH phenotype (10). Therefore, we hypothesized that a pre-capillary component in PH-LHD might be identified by the gas exchange parameters derived from CPET. In the current study, we investigated the differences in CPET parameters between patients with Ipc-PH and patients with Cpc-PH, and evaluated their correlations with hemodynamic diagnostic indicators, so as to identify reliable predictors of CPET for the diagnosis of Cpc-PH.
We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/atm-21-366).
Methods
Study design and patient population
Patients with LHD who underwent in-hospital assessment for PH between May 2015 and May 2020 at Shanghai Pulmonary Hospital Affiliated to Tongji University School of Medicine were retrospectively included in the study. Clinical and laboratory tests were conducted to assess the World Health Organization functional class (WHO FC), N-terminal pro-B type natriuretic peptide (NT-pro-BNP) and 6-minute walk distance (6MWD) of all patients. Right heart catheterization (RHC) was performed on all patients. PH was defined as mean pulmonary artery pressure (PAP) >20 mmHg and pulmonary artery wedge pressure (PAWP) >15 mmHg at rest (6,13). Cpc-PH and Ipc-PH were further defined as PVR ≥3 Wood units (WU) and PVR <3 WU, respectively (6,13).
The inclusion criteria were: patients confirmed as PH-LHD by RHC, including those with PH due to heart failure with preserved left ventricular ejection fraction (LVEF) (HFpEF), those with PH due to heart failure with reduced LVEF (HFrEF), those with valvular heart disease, and those with congenital/acquired cardiovascular conditions leading to post-capillary PH. Patients also needed to have CPET data, with complete hemodynamic data by 2-dimensional echocardiography within 7 days of RHC. Patients were excluded if they could not perform a valid baseline exercise test or presented with any of the following clinical conditions: acute decompensated heart failure; severe cardiogenic shock requiring inotropic support or urgent mechanical circulatory support; signs of ischemia during CPET; or any other comorbidity with a life expectancy of <1 year. Patients with pulmonary arterial hypertension (PAH) or PH resulting from other diseases were excluded. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was reviewed and approved by the Ethics Committee of Shanghai Pulmonary Hospital (K16-317). Individual consent for this retrospective analysis was waived.
RHC
RHC was conducted using the Swan-Ganz catheter (7- or 7.5-Fr; Edwards Lifesciences LLC, Irvine, CA), and the PAP and PAWP tracings were recorded. Cardiac output (CO) was measured using the thermodilution method with cold saline. PAWP was measured by digitized mean. DPG = diastolic PAP-PAWP, TPG = mean PAP-PAWP, PVR in WU was calculated as: (mean PAP-PAWP)/CO, total pulmonary resistance (TPR) = mean PAP/CO.
Transthoracic echocardiography
Transthoracic echocardiography was performed according to the recommendations from the latest guidelines published by the American Society of Echocardiography (14). Tricuspid regurgitation was quantified by Doppler in accordance with current recommendations (15). Right ventricular (RV) end-diastolic transverse dimension (RVEDTD), right atrial transverse dimension (RATD), and end-systolic-stage eccentricity index (ENDSEI) were measured to evaluate the presence of right heart enlargement. Pulmonary artery systolic pressure (PASP) was measured by tricuspid regurgitation velocity (TRV) (14). RV function was assessed using tricuspid annular plane systolic excursion (TAPSE) (14). LVEF was measured using M-mode in the parasternal long-axis view.
CPET
CPET was performed on an electromagnetically braked cycle ergometer (Master Screen CPET, Jaeger Crop., Hoechberg, Germany), and gas exchange data were recorded over 10-second intervals via a breath-by-breath system. A test was terminated if any of the following conditions were observed: fatigued, dyspnea, chest tightness, or any other uncomfortable feeling reported by the patient. Measurements included heart rate (HR), O2 uptake (VO2), oxygen pulse (O2 pulse), PET CO2, VE/VCO2, VO2/ VE, oxygen uptake efficiency plateau (OUEP), and oxygen uptake efficiency slope (OUES).
The HR, VO2, PET CO2, VE/VCO2, VO2/VE, and O2 pulse values at peak exercise were measured according to the highest 30-second averaged value obtained during peak exercise. The lowest VE/VCO2 was calculated by averaging the 9 lowest consecutive 10-second-averaged data points of VE/VCO2. The VE/VCO2 slope was obtained from linear regression analysis of the relationship of VE with VCO2. The lowest VE/VCO2 and VE/VCO2 slope predicted values depends on age, sex, and size (16). percentage of predicted value (%pred) = Measured value/predicted value × 100%. The OUEP was the highest consecutive values for VO2 (mL/min)/VE (L/min) at 90 seconds (17). Using linear square regression, we computed the OUES according to the following equation: VO2 = a × lgVE + b (‘a’ is OUES) (17).
Statistical analysis
Data were analyzed using SPSS 19.0 (SPSS Inc.; Chicago, IL, USA). Characteristics of patients in the two groups (Ipc-PH and Cpc-PH) were compared using the independent-samples t-test and the Mann-Whitney U test for parametric and nonparametric data, respectively. Differences in categorical variables between groups were assessed using the χ2 test. Correlations between CPET parameters and PVR, DPG, and TPG were assessed using Pearson’s correlation coefficient. Univariate and multivariate logistic regression analyses were used to evaluate the diagnostic value of CPET for Cpc-PH. Further, the ability of CPET parameters to identify Cpc-PH was assessed through receiver operating characteristic (ROC) curve analysis. Patients were subdivided into two groups according to the cutoff value of the CPET parameter with the highest diagnostic value, and hemodynamic differences were compared between the groups. For all analyses, statistical significance was indicated by a 2-sided P value of P<0.05.
Results
Patient characteristics
A total of 90 patients with PH-LHD were included in this study. These patients were further classified into two groups according to their PVR measured by RHC: the Cpc-PH group (n=47) and the Ipc-PH group (n=43). Sixteen patients with Cpc-PH had DPG ≥7 mmHg, and all patients with Ipc-PH had DPG <7 mmHg. The patients had an average age of 64.0 (56.5, 72.3) years, and 36 patients (40%) were male. There were no significant differences in age, sex, body mass index (BMI), WHO FC, PH-LHD classification, or comorbidities between the two groups. The mean 6MWD was shorter in the Ipc-PH and Cpc-PH groups than in the non-PH group, and the NT-pro-BNP was higher. Key baseline characteristics are listed in Table 1.
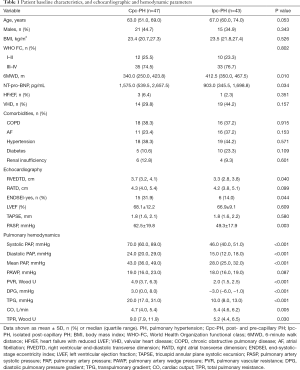
Full table
Comparison of echocardiographic and hemodynamic parameters between Cpc-PH and Ipc-PH
As Table 1 shows, the Cpc-PH group had more significant enlargement of the RV (P<0.05) (Figure 1), a significantly higher occurrence of ENDSEI (P<0.05), and significantly higher PASP (P<0.01) than the Ipc-PH group (Figure 1). Further, the right atrium was also more enlarged in the Cpc-PH group than in the Ipc-PH group, although the difference was not statistically significant (P>0.05). The TAPSE and LVEF showed no difference between the Cpc-PH and Ipc-PH groups.
Table 1 also shows the main hemodynamic parameters of all patients. Systolic PAP, diastolic PAP, mean PAP, PVR, DPG, and TPG in the Cpc-PH group were significantly higher than those in the Ipc-PH group (P<0.001). TPR was also higher (P<0.05), whereas CO was lower (P<0.01) in Cpc-PH group. There was no significant difference in PAWP between the two groups (P>0.05).
Comparison of CPET parameters between the Cpc-PH and Ipc-PH groups
Compared with the Ipc-PH patients, Cpc-PH patients showed greater abnormalities in CPET results (Table 2). The lowest VE/VCO2, lowest VE/VCO2%pred, VE/VCO2 slope % pred, and peak VE/VCO2 values of the Cpc-PH patients were significantly higher than those in the Ipc-PH group (P<0.001), and the VE/VCO2 slope value was also higher (P<0.01). However, in Cpc-PH group, the OUEP was significantly lower (P<0.001), and the peak PET CO2 and peak VO2/VE values were also lower than those in the Ipc-PH group (P<0.01). In Cpc-PH patients, peak VO2 was significantly decreased (P<0.05). The peak O2 pulse and work load values were lower, but the difference was not statistically significant (P>0.05). Meanwhile, the resting HR and peak HR values were higher, but this difference was also without statistical significance (P>0.05).
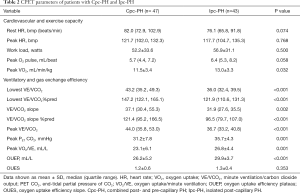
Full table
Correlations between CPET parameters and PVR, DPG, and TPG
Table 3 illustrates the correlation between CPET parameters and three hemodynamic parameters used to identify Cpc-PH in PH-LHD patients. Lowest VE/VCO2, lowest VE/VCO2% pred, VE/VCO2 slope, VE/VCO2 slope% pred, and peak VE/VCO2 showed a moderate positive correlation with PVR (r>0.5, P<0.001). VE/VCO2-related parameters had a stronger correlation with PVR than with DPG and TPG, and their correlations with PVR, DPG, and TPG were stronger than those with other CPET parameters. It could also be seen that the main echocardiographic indexes had weaker correlations with PVR, DPG, and TPG than with CPET parameters. Furthermore, RATD was more closely related to DPG, whereas PASP was more closely related to TPG.
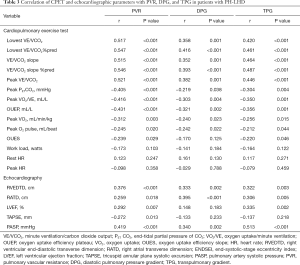
Full table
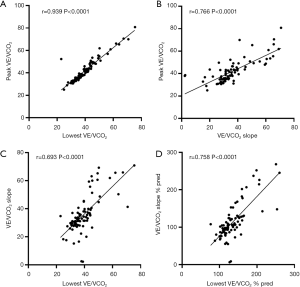
As shown in Figure 2, there was a strong positive correlation between lowest VE/VCO2 and peak VE/VCO2 (r=0.939, P<0.0001). Moreover, there were moderate positive correlations between peak VE/VCO2 and VE/VCO2 slope (r=0.766, P<0.0001), between lowest VE/VCO2 and VE/VCO2 slope (r=0.693, P<0.0001), and between lowest VE/VCO2%pred and VE/VCO2 slope %pred (r=0.758, P<0.0001).
Diagnostic value of CPET parameters for Cpc-PH
Logistic regression was used to investigate the value of the candidate CPET parameters in the diagnosis of Cpc-PH (Table 4). In the univariate analysis, peak VO2, peak VE/VCO2, lowest VE/VCO2, lowest VE/VCO2%pred, VE/VCO2 slope, VE/VCO2 slope %pred, peak PETCO2, peak VO2/VE, and OUEP were significant predictors of Cpc-PH. Subsequently, age, BMI, and all factors with a P value <0.05 were included in the multivariate logistic regression (forward) analysis, which revealed that only lowest VE/VCO2% was a significant independent predictor of Cpc-PH (odds ratio 0.957, 95% CI: 0.934–0.981; P<0.001).

Full table
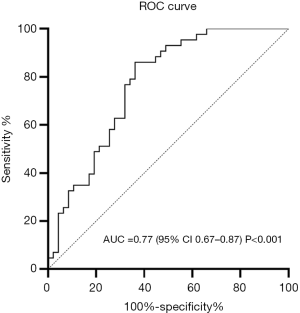
ROC curve analysis was then performed to evaluate the independent predictors of Cpc-PH (Figure 3). Lowest VE/VCO2%pred was the best predictor for Cpc-PH, as demonstrated by an area under the curve (AUC) of 0.77. Lowest VE/VCO2%pred ≥137.0% was considered to be the best cutoff value for predicting Cpc-PH, with a sensitivity of 63.8% and a specificity of 86.0%.
Effects of independent predictors on hemodynamics
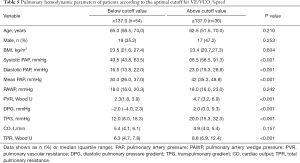
The patients were divided into two groups using the best cutoff value for lowest VE/VCO2% pred (≥137%) as the threshold (Table 5). Systolic PAP, diastolic PAP, mean PAP, PVR, DPG, TPG, and TPR in the above threshold group were significantly higher than those in the below threshold group (P<0.001). However, there was no significant difference in PAWP and CO between the two groups (P>0.05).
Full table
Discussion
In this retrospective study, we assessed exercise cardiopulmonary function, hemodynamics, and echocardiographic parameters in patients with Cpc-PH and Ipc-PH, and simultaneously investigated the pathophysiological mechanisms underlying abnormal CPET parameters in PH-LHD. We observed that patients with Cpc-PH had a poorer hemodynamic profile, lower cardiopulmonary exercise capacity, and worse RV dysfunction than patients with Ipc-PH. The DPG did not provide Cpc-PH patients with additional cardiopulmonary capacity information beyond that provided by PVR. VE/VCO2-related parameters were moderately correlated with PVR, and played a more significant role in distinguishing Cpc-PH from Ipc-PH. VE/VCO2-related parameters also showed significant correlation with each other. Lowest VE/VCO2%pred had the best predictive capacity for diagnosing Cpc-PH. On the basis of hemodynamics as the gold standard, CPET appears to be superior to echocardiography as a noninvasive method for identifying Cpc-PH. Hemodynamic differences were obvious between patients with PH-LHD grouped according to the lowest VE/VCO2%pred value.
Our previous research with a small sample size suggested that DPG does not provide additional cardiopulmonary capacity information for patients with Cpc-PH beyond that provided by PVR (18). After increasing the sample size in the current study, the same results were observed. All but no patients with Cpc-PH had DPG <7 mmHg, while none of the patients with Ipc-PH had DPG ≥7 mmHg; only 34% of patients with Cpc-PH had DPG ≥7 mmHg. CPET parameters were more strongly correlated with PVR than with DPG. The results suggested that it was reasonable to study the role of CPET between Cpc-PH and Ipc-PH according to the latest PVR standard. Noninvasive measures of pulmonary gas exchange, including VE/VCO2, PET CO2, have been shown in previous studies to help in the differentiation of Cpc-PH from Ipc-PH (19,20). Compared with those previous studies, our diagnosis was based only on PVR without DPG, but the results were similar.
CPET is still the gold-standard exercise assessment as well as a standard of care in patients with left-sided heart failure (8,21), and the role of routine CPET in cardiology has been extended to left-sided PH patient populations (22). Our results suggest that CPET seems to have more potential for distinguishing Cpc-PH from Ipc-PH compared with echocardiography, which is worthy of further study.
Ventilatory inefficiency (high VE/VCO2) is a characteristic abnormality in patients with pulmonary vascular disease (23). Current evidence indicates that ventilatory efficiency may be an important marker for assessing the severity of valvular heart disease, include aortic stenosis (24) and mitral valve disease/dysfunction (25). We found that patients with Cpc-PH displayed higher VE/VCO2 (peak, lowest, and slope) and lower peak PET CO2, peak VO2/VE, and OUEP values than Ipc-PH patients, indicating that gas exchange was worse in patients with Cpc-PH. A lowest VE/VCO2 of ≥155% predicted was considered to be the single best predictor of mortality in patients with heart failure (26). Compared with the VE/VCO2 slope, the lowest VE/VCO2 is considered to be a more stable and labor-saving method of measurement. In this study, peak VE/VCO2, lowest VE/VCO2, lowest VE/VCO2%pred, VE/VCO2 slope, and VE/VCO2 slope %pred were helpful in distinguishing Cpc-PH from Ipc-PH, and there was obvious correlation between them. However, logistic regression and ROC curve analyses showed that lowest VE/VCO2%pred was most the helpful parameter for guiding PH-LHD classification. The lowest VE/VCO2%pred actually had a better ability to identify Cpc-PH than other CPET parameters.
The typical CPET response in PH (27) and heart disease (28,29) is observed and characterized by a severe reduction in peak VO2 (30), work rate, and O2 pulse. In the present study, only peak VO2 differed significantly between the Cpc-PH and Ipc-PH groups, and it was negatively correlated with PVR; however, its guiding significance was not as good as that of ventilation efficiency-related indicators. We believe that ventilation inefficiency is the main characteristic of CPET in Cpc-PH patients. Many factors are believed to account for hyperventilation induced by excessive exercise, including ventilation/perfusion mismatch with increased dead-space ventilation, pulmonary vascular function, RV function, and nervous reflex. Chemoreflex was also shown by Vicenzi et al. to be effective in PH patients with different hyperventilation patterns, and the increase in chemosensitivity made higher VE/VCO2 and lower PET CO2 more likely (31). According to the results of the present study, we consider chemoreflex to be the key factor in the difference in ventilation efficiency between Cpc-PH and Ipc-PH patients.
RV afterload is considered to play an important role in the regulation of ventilation efficiency during exercise (32). In Methvin et al.’s study, the measurement of RV systolic function and dimensions by echocardiography showed that the RV condition in patients with left HF was associated with ventilatory inefficiency (33), which is consistent with the concept that RV extension and rigidity increase secondary chemosensitivity (34). Both diastolic stiffness and increased RV afterload in patients with PAH were also found to be related to exercise hyperventilation. RV dilation is the obvious reason for the increase in atrial and RV stretch and stiffness, which are important triggers of chemosensitivity and sympathetic nervous system tone in PAH patients (35). At present, the relationship between ventilatory inefficiency and RV dysfunction in severe LHD patients is consistent with that reported by studies on PAH patients (33). Our data displayed that RV dilation and ventilatory inefficiency were more obvious in Cpc-PH patients than in Ipc-PH patients, while systolic PAP, diastolic PAP, mean PAP, and TPR were obviously higher in patients above the lowest VE/VCO2%pred threshold. These observations suggest that RV dilation in patients with Cpc-PH may increase ventilation waste, and that increased RV afterload was related to poor ventilatory efficiency in PH-LHD, which may be related to the increased chemosensitivity.
The alterations in the ventilatory efficiency response to exercise in patients with heart failure also likely result from a reduced blood flow to the pulmonary blood vessels with a consequent increase in the inhomogeneity of ventilation-perfusion matching (36). Hypoperfusion may also affect the stimulation of the peripheral chemoreceptors, leading to an enhanced ventilatory response (37). In the Cpc-PH patients, cardiac output decreased, whereas PVR and TPR increased significantly, which could have had an aggravating effect on hypoperfusion.
A study by Butler et al. (38) showed that impairment of exercise capacity was obvious in patients with elevated PVR, and the degree of exercise abnormalities is related to the severity of PVR. In the present study, peak VO2 in patients with Cpc-PH was reduced, which may have further aggravated ventilation inefficiency. The decreases in the skeletal muscle blood supply and arterial hypoxemia can alter the concentration of CO2 and oxygen in the blood, and stimulate the response of peripheral chemoreceptors to exercise, thus resulting in an enhanced hyperventilation response. Our results showed that peak VO2 was lower in Cpc-PH patients than in Ipc-PH patients, while the peak VO2/VE and OUEP values were significantly lower in Cpc-PH patients, suggesting that VE showed a more significant increase in the Cpc-PH group. Taken together, the other mechanisms underlying exercise-induced hyperventilation might centrally interact with chemoreflex in such a way to increase ventilation inefficiency. Finally, the condition of the Cpc-PH patients was poorer than that of the Ipc-PH patients, with a worse pattern of ventilatory control derangement.
We found a significant correlation between PVR and VE/VCO2, which may indicate a possible association of the presence and range of a pre-capillary component with the degree of exercise-induced hyperventilation. PVR was obviously lower in patients below the lowest VE/VCO2%pred threshold. A decrease in PVR in patients with PH-LHD may reduce the tendency of inefficient ventilation, whereas an increase in PVR may provide obvious physiological stimulation for exercise hyperventilation. Based on our findings, we conclude that careful evaluation of VE/VCO2 may be helpful in identifying subjects with elevated PVR levels in RHC.
Our study is based on the established relationship between abnormal ventilation and poor pulmonary hemodynamics, suggesting that hyperventilation parameters may have diagnostic value in identifying the presence of pre-capillary components in patients with PH-LHD. As the potential treatment methods of PH-LHD patients continue to be explored, an effective noninvasive evaluation method may also guide the treatment intervention.
Our study has some limitations, Firstly, it is a single-center study with a limited sample size, and the study design is retrospective, which may have provided less relevant evidence than randomized controlled trials. A study of a larger population involving multiple clinical centers is required to further assess the validity of our results. Secondly, the study only focused on associations between hemodynamic parameters at rest and CPET. The correlation between hemodynamic indexes under exercise and CPET indexes calls for further study. Thirdly, there was no assessment of patients’ prognoses, which will be accounted for in the design of our future research.
Conclusions
CPET is useful in assisting the evaluation of patients with PH-LHD and can provide information to differentiate between Cpc-PH and Ipc-PH. Our observations suggest that the degree of exercise-induced hyperventilation can indicate the presence of pre-capillary components, and VE/VCO2 may be an acceptable noninvasive marker for the diagnosis of Cpc-PH in future.
Acknowledgments
Funding: This study was supported by the Program of National Natural Science Foundation of China (81700045), Program Supported by the Fundamental Research Funds for the Central University (22120180539), and Scientific Research Fund of Shanghai Municipal Health and Family Planning Commission (2018LP039). The funders had no role in the study design, data collection or analysis, decision to publish, or preparation of the manuscript.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/atm-21-366
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-21-366
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-21-366). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was reviewed and approved by the Ethics Committee of Shanghai Pulmonary Hospital (K16-317). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rosenkranz S, Gibbs JS, Wachter R, et al. Left ventricular heart failure and pulmonary hypertension. Eur Heart J 2016;37:942-54. [Crossref] [PubMed]
- Rosenkranz S, Lang IM, Blindt R, et al. Pulmonary hypertension associated with left heart disease: Updated Recommendations of the Cologne Consensus Conference 2018. Int J Cardiol 2018;272S:53-62. [Crossref] [PubMed]
- Guazzi M, Borlaug BA. Pulmonary hypertension due to left heart disease. Circulation 2012;126:975-90. [Crossref] [PubMed]
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015;46:903-75. [Crossref] [PubMed]
- Naeije R, Gerges M, Vachiery JL, et al. Hemodynamic Phenotyping of Pulmonary Hypertension in Left Heart Failure. Circ Heart Fail 2017;10:e004082. [Crossref] [PubMed]
- Vachiéry JL, Tedford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J 2019;53:1801897. [Crossref] [PubMed]
- Guazzi M, Adams V, Conraads V, et al. EACPR/AHA Scientific Statement. Clinical recommendations for cardio-pulmonary exercise testing data assessment in specific patient populations. Circulation 2012;126:2261-74. [Crossref] [PubMed]
- Arena R, Ozemek C. Intracardiac multimorbidity: assessing right ventricular function in left-sided heart failure through cardiopulmonary exercise testing. Expert Rev Cardiovasc Ther 2019;17:331-3. [Crossref] [PubMed]
- Guazzi M, Cahalin LP, Arena R. Cardiopulmonary Exercise Testing as a Diagnostic Tool for the Detection of Left-sided Pulmonary Hypertension in Heart Failure. J Card Fail 2013;19:461-7. [Crossref] [PubMed]
- Caravita S, Faini A, Deboeck G, et al. Pulmonary hypertension and ventilation during exercise: Role of the pre-capillary component. J Heart Lung Transplant 2017;36:754-62. [Crossref] [PubMed]
- Lim HS, Theodosiou M. Exercise ventilatory parameters for the diagnosis of reactive pulmonary hypertension in patients with heart failure. J Card Fail 2014;20:650-7. [Crossref] [PubMed]
- Welch CE, Brittain EL, Newman AL, et al. End-Tidal Carbon Dioxide as a Prognostic Feature in Pulmonary Arterial Hypertension. Ann Am Thorac Soc 2017;14:896-902. [Crossref] [PubMed]
- Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53:1801913. [Crossref] [PubMed]
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685-713, quiz 786-8. [Crossref] [PubMed]
- Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003;16:777-802. [Crossref] [PubMed]
- Sun XG, Hansen JE, Garatachea N, et al. Ventilatory efficiency during exercise in healthy subjects. Am J Respir Crit Care Med 2002;166:1443-8. [Crossref] [PubMed]
- Sun XG, Hansen JE, Stringer WW. Oxygen uptake efficiency plateau (OUEP): physiology and reference value. Eur J Appl Physiol 2012;112:919-28. [Crossref] [PubMed]
- Zhong XJ, Tang J, Zhao QH, et al. Can the diastolic pulmonary pressure gradient provide cardiopulmonary capacity information in patients with pulmonary hypertension and left heart disease? Int J Cardiol 2020;305:138. [Crossref] [PubMed]
- Correale M, Tricarico L, Ferraretti A, et al. Cardiopulmonary exercise test predicts right heart catheterization. Eur J Clin Invest 2017;47. [Crossref] [PubMed]
- Taylor BJ, Smetana MR, Frantz RP, et al. Submaximal Exercise Pulmonary Gas Exchange in Left Heart Disease Patients with Different Forms of Pulmonary Hypertension. J Card Fail 2015;21:647-55. [Crossref] [PubMed]
- Guazzi M, Arena R, Halle M, Piepoli MF, et al. 2016 focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur Heart J 2018;39:1144-61. [Crossref] [PubMed]
- Guazzi M, Bandera F, Ozemek C, et al. Cardiopulmonary Exercise Testing: What Is its Value? J Am Coll Cardiol 2017;70:1618-36. [Crossref] [PubMed]
- Weatherald J, Farina S, Bruno N, et al. Cardiopulmonary Exercise Testing in Pulmonary Hypertension. Ann Am Thorac Soc 2017;14:S84-S92. [Crossref] [PubMed]
- Bandera F, Generati G, Pellegrino M, et al. Paradoxical low flow/low gradient aortic stenosis: Can cardiopulmonary exercise test help in identifying it? Int J Cardiol 2016;203:37-9. [Crossref] [PubMed]
- Izumo M, Suzuki K, Moonen M, et al. Changes in mitral regurgitation and left ventricular geometry during exercise affect exercise capacity in patients with systolic heart failure. Eur J Echocardiogr 2011;12:54-60. [Crossref] [PubMed]
- Sun XG, Hansen JE, Beshai JF, et al. Oscillatory breathing and exercise gas exchange abnormalities prognosticate early mortality and morbidity in heart failure. J Am Coll Cardiol 2010;55:1814-23. [Crossref] [PubMed]
- Paolillo S, Farina S, Bussotti M, et al. Exercise testing in the clinical management of patients affected by pulmonary arterial hypertension. Eur J Prev Cardiol 2012;19:960-71. [Crossref] [PubMed]
- Malhotra R, Bakken K, D'Elia E, et al. Cardiopulmonary Exercise Testing in Heart Failure. JACC Heart Fail 2016;4:607-16. [Crossref] [PubMed]
- Balady GJ, Arena R, Sietsema K, et al. Clinician’s Guide to cardiopulmonary exercise testing in adults: A scientific statement from the American Heart Association. Circulation 2010;122:191-225. [Crossref] [PubMed]
- Mancini D, LeJemtel T, Aaronson K. Peak VO(2): a simple yet enduring standard. Circulation 2000;101:1080-2. [Crossref] [PubMed]
- Vicenzi M, Deboeck G, Faoro V, et al. Exercise oscillatory ventilation in heart failure and in pulmonary arterial hypertension. Int J Cardiol 2016;202:736-40. [Crossref] [PubMed]
- Lewis GD, Shah RV, Pappagianopolas PP, et al. Determinants of ventilatory efficiency in heart failure: the role of right ventricular performance and pulmonary vascular tone. Circ Heart Fail 2008;1:227-33. [Crossref] [PubMed]
- Methvin AB, Owens AT, Emmi AG, et al. Ventilatory inefficiency reflects right ventricular dysfunction in systolic heart failure. Chest 2011;139:617-25. [Crossref] [PubMed]
- Farina S, Correale M, Bruno N, et al. The role of cardiopulmonary exercise tests in pulmonary arterial hypertension. Eur Respir Rev 2018;27:170134. [Crossref] [PubMed]
- Tello K, Dalmer A, Vanderpool R, et al. Impaired right ventricular lusitropy is associated with ventilatory inefficiency in pulmonary arterial hypertension. Eur Respir J 2019;54:1900342. [Crossref] [PubMed]
- Taylor BJ, Olson TP, Kim CH, et al. Use of Noninvasive Gas Exchange to Track Pulmonary Vascular Responses to Exercise in Heart Failure. Clin Med Insights Circ Respir Pulm Med 2013;7:53-60. [Crossref] [PubMed]
- Johnson RL Jr. Gas exchange efficiency in congestive heart failure. Circulation 2000;101:2774-6. [Crossref] [PubMed]
- Butler J, Chomsky DB, Wilson JR. Pulmonary hypertension and exercise intolerance in patients with heart failure. J Am Coll Cardiol 1999;34:1802-6. [Crossref] [PubMed]
(English Language Editor: J. Reynolds)


