
Effect of norepinephrine challenge on cardiovascular determinants assessed using a mathematical model in septic shock: a physiological study
Introduction
Norepinephrine (NE) is recommended as the first choice to maintain mean arterial pressure (MAP) during the resuscitation from septic shock (1). The primary aim of an NE infusion is to increase and maintain MAP. However, other effects of NE on hemodynamics, such as effects on the mean systemic filling pressure (Pmsf), venous return, systemic circulation, and cardiac function, must be considered in clinical practice (2). Recent attention focused on the venous return [equal to cardiac output (CO)] response to incremental NE infusions in critically ill patients. Notably, the CO response to NE is variable, as determined by the interaction of the venous return curve and cardiac function curve. Researchers demonstrated that the effect of NE on venous return varies in critically ill patients (3,4). Maas et al. found the NE-induced change in CO was determined by the balance of volume recruitment (increase in mean systemic filling pressure as assessed using the inspiratory-hold method), change in resistance for venous return, and baseline heart function (3). Persichini et al. found that the decrease of NE was responsible for a decrease in venous return due to the decrease in mean systemic pressure as measured using the inspiratory/expiratory-hold method (4). However, the inspiratory/expiratory-hold method requires deep sedation, and it is a complex procedure. It is inconvenient for a physician to use the inspiratory/expiratory-hold method to measure Pmsf and related parameters at the bedside. Therefore, a simple method to measure Pmsf would help the interpretation of the hemodynamic effect of NE in septic shock patients. According to an electrical analog simplification of Guyton circulatory physiology, Parkin proposed a mathematical model analog of mean systemic pressure (Pmsa) that may be calculated from directly measured right atrial pressure (RAP), MAP and CO (5). Werner-Moller et al. recently compared the agreement of Pmsf between various indirect measured methods (inspiratory-hold method, instantaneous beat-to-beat venous return method and mathematical model analog method) and the direct method (measured at zero flow using right atrial balloon occlusion) at various volume statuses in an animal study (6). The authors found that the mathematical model analog method had better agreement with the direct measured method of Pmsf than the other indirect methods (6). The same team further found that an increase in NE increased extracorporeal membrane oxygenation (ECMO) flow via the combined effect of recruit volume, increased Pmsf, as measured using a stop flow maneuver, and increased venous return and pump afterload in an animal model of ventricular fibrillation with venoarterial ECMO (VA-ECMO) therapy (7). Therefore, the use of this model would include the Pmsa and Pmsa-derived variables in the interpretation of the effects of NE on CO at bedside. To the beset of our knowledge, no published study quantified the CO response to increased NE using Pmsa and Pmsa-derived variables in clinical practice.
The present study investigated the CO response to an NE challenge (titrated to achieve an increase of 10 mmHg MAP) in septic shock patients. The quantitative relationship of hemodynamic variables, including a mathematical model to calculate Pmsa and related parameters, to the response of CO to the NE challenge was investigated.
Some of the patients in the present study were included in a previously reported study on the effects of NE on the peripheral perfusion index (8). We present the following article in accordance with the STARD reporting checklist (available at http://dx.doi.org/10.21037/atm-20-6686).
Methods
Patients
The investigation was performed according to the principles outlined in the Declaration of Helsinki (as revised in 2013). The Institutional Research and Ethics Committee of the Peking Union Medical College Hospital approved this study for human subjects (No. ZS-910, approved at 2015). Written informed consent was obtained from all patients or next of kin before the data were included in the study.
All adult patients with septic shock who were prospectively admitted to the Department of Critical Care Medicine of Peking Union Medical College Hospital and who required pulse index continuous cardiac output (PiCCO) plus CO hemodynamic monitoring for resuscitation were eligible for the study when the research team was available. The attending intensivists based decisions on PiCCO catheter placement on the severity of the patient’s condition after early hemodynamic support. The PiCCO catheter was placed in the femoral artery using a standard operating procedure. Septic shock was defined as severe sepsis with sepsis-induced hypotension that persisted despite adequate fluid resuscitation and required the administration of vasopressors (9). All of the included patient received NE to maintain the individualized MAP levels, which were set based on the patient’s usual levels and organ perfusion via clinical decision.
Measurements
Information collected at enrollment included demographic characteristics, such as age, sex, Acute Physiology and Chronic Health Evaluation II score (APCHE II) (10), and primary site and type of infection. The flow chart of NE titration and hemodynamic data collection was presented in the previous study (8).
The NE challenge was defined as an elevation of 10 mmHg MAP that occurred from the NE increase. NE initially induced a 10-mmHg reduction in baseline MAP (MAPbase), which we called the MAP−10mmHg level. Second, NE was increased to restore the MAPbase level. Last, NE was increased further to obtain a 10-mmHg increase from MAPbase, which was the MAP+10mmHg level. The two NE challenge tests were captured during the NE titration: the 1st test was from MAP−10mmHg to MAPbase levels, and the 2nd test was from MAPbase to MAP+10mmHg levels. We allowed 10 minutes for hemodynamic adaptation at the three MAP levels. The heart rate (HR), central venous pressure (CVP), systolic/diastolic blood pressure (SBP/DBP), MAP, continuous cardiac output (CCO) and related parameters were continuously and simultaneously recorded. When a significant increase of CVP (>3 mmHg) and/or a decrease of CO (>2 L/min) was observed, the balancing time of hemodynamics to the NE increase was reduced to decrease hemodynamic disturbances in response to the increased NE.
Determination of Pmsa and related variables
- Pmsa = (a × CVP) + (b × MAP) + (c × CO), where a and b are dimensionless constants (a + b =1; typically a =0.96 and b =0.04), and c is calculated by the patient’s the height and weight. The mathematical model of the systemic circulation was composed of compliant arterial and venous compartments and resistances to blood flow (5). MPA and CO were measured by the PiCCO machine.
- Resistance of systemic vascular beds (RSYS) = (MAP – CVP)/CO. Total vascular systemic resistance was calculated as the ratio of the pressure difference between MAP and CVP to the CO.
- Resistance of venous return (Rvr) = (Pmsa – CVP)/CO. Pmsa was the average pressure in the systemic circulation, and Rvr was the resistance encountered to the heart (11). This formula is used to describe venous return during transient states of imbalances (12).
- Pressure gradient for venous return (PGVR) = Pmsa − CVP. The PGvr was defined as the pressure difference between Pmsa and CVP.
- Efficiency of the heart (Eh) = (Pmsa – CVP)/Pmsa (5). This equation was proposed by Parkin WG for the measurement of heart performance. During the cardiac stop ejection, CVP is equal to the Pmsa, and Eh approaches zero.
Statistical analysis
Descriptive analyses were performed. All data are expressed as medians (25–75% percentile) or means ± standard deviation. Comparisons of related parameters based on the different MAP levels were performed using a general linear model repeated measures (GLMRM) model (13,14). This model is an extension of the classic ANOVA and allows assessments of fixed effects (MAP levels) and random effects (patient). The GLMRM model takes into account the correlation between multiple measurements of one patient. Therefore, the estimated marginal means were adjusted for the covariates, and the trends of related hemodynamic parameters corresponding to the different MAP levels are shown. Repeated measurements were analyzed using analysis of variance or analysis of variance on ranks. Paired data were compared using the t-test or the Wilcoxon signed-rank test. Comparisons of two continuous variables were performed using a linear regression. Discrimination of values for the prediction of CO/Eh directional changes (increase or decrease) were assessed using receiver operating characteristic (ROC) analysis. All comparisons were two-tailed, and P<0.05 was required to exclude the null hypothesis. The statistical analyses were performed using the software package SPSS 21.0 (SPSS Inc., Chicago, IL, USA).
Results
Twenty-seven septic shock patients were enrolled, and the age was 59 [41–73] years. The demographic and clinical characteristics of the study group are shown in Table 1. There were no adverse effects associated with the NE challenges.
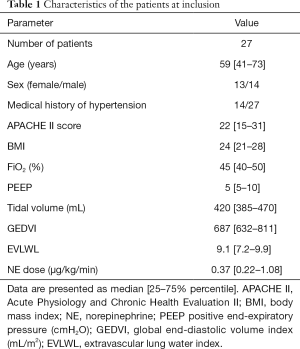
Full table
Effects of NE on the hemodynamic variables
The doses of the NE infusion were significantly increased from 0.32 (0.09–0.89) to 0.37 (0.22–1.08) and 0.49 (0.26–1.15) µg/kg/min for the different assigned MAP levels. The NE-induced variations in hemodynamic variables are listed in Table 2. Increasing the dose of NE was associated with consistent increases in SBP, DBP, MAP, CVP and dP/dtmax (dPmax). A significant decrease in stroke volume variation (SVV) was observed during MAP titration. However, there were no significant or consistent changes in the trends for CO or HR. Figure 1 shows the variations in the estimated marginal means for CO, MAP CVP and HR in the 27 patients at the three MAP levels.
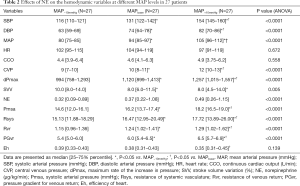
Full table
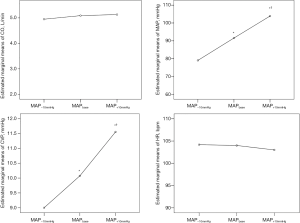
There was individual variability in the CO changes at different MAP levels produced by the increased NE. With the same directional change, a consistent decrease in CO in 8 patients and an increase in CO in 14 patients was observed during the stepwise incremental NE process. The CO change in direction was not inconsistent in 5 patients.
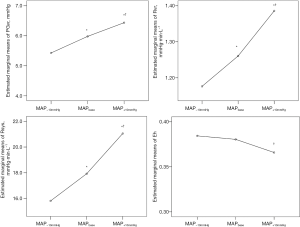
The increased NE infusion produced a consistent and significant increase in Pmsa, Rsys, Rvr and PGvr with a linear trend (P<0.0001). However, the change in Eh showed no significant linear change trend (Table 1). There was no significant difference in Eh between MAP−10mmHg and MAPbase levels. Figure 2 shows the variation in estimated marginal means for PGvr, Rsys, Rvr, and Eh at the three MAP levels.
Correlation between changes in CO and changes in related parameters in the 54 NE challenge episodes
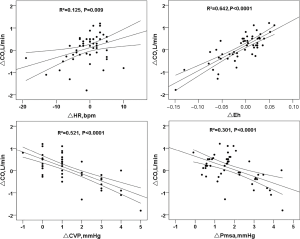
The ΔCO positively and significantly correlated with ΔPGvr (r=0.917, P<0.0001), ΔEh (r=0.802, P<0.0001) (Figure 3) and ΔHR (r=0.354, P=0.009) (Figure 3). ΔCO negatively and significantly correlated with ΔCVP (r=−0.722, P<0.0001) (Figure 3), ΔPmsa (r=−0.549, P<0.0001) (Figure 3), ΔRvr (r=−0.597, P<0.0001), and ΔRsys (r=−0.597, P<0.0001). However, there was no significant relationship between ΔCO and ΔSVV (r=−0.284, P=0.841) or ΔMAP (r=−0.099, P=0.478).
Prediction of CO and Eh responses to the 54 NE challenges
For the CO responses, the 54 NE challenges were divided into CO-decreasing (ΔCO <0, n=20) and CO-increasing (ΔCO >0, n=32, including 30 ΔCO >0 and 2 ΔCO =0) episodes. For the Eh responses, the 54 NE challenges were divided into Eh-amplifying and Eh-reducing episodes. The CO-decreasing group had a significantly higher ΔCVP than the CO-increasing group [ΔCVP (mmHg): CO-increasing vs. CO-decreasing, 0.6+0.9 vs. 2.4+1.3, respectively; P<0.0001]. The NE challenges in the Eh-reducing episodes (n=26, ΔEh <0) had significantly higher ΔCVP than the Eh-amplifying episodes (n=28, ΔEh >0) [ΔCVP (mmHg): Eh-reducing vs. Eh-amplifying, 2.2+1.2 vs. 0.3+0.6, respectively; P<0.0001].
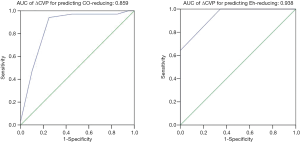
To assess the ability of ΔCVP to predict the NE-induced decrease in CO, the area under the curve (AUC) analysis was used, and the result was 0.859 with 95% CI: 0.743–0.975 (Figure 4). A cutoff of ΔCVP >1.5 mmHg for detecting decreased CO resulted in a sensitivity of 75% and a specificity of 94.1%. Using ΔCVP to predict an NE-induced amplification of Eh, the AUC was 0.938 with 95% CI: 0.881–0.995 (Figure 4). A cutoff of ΔCVP >1.5 mmHg for detecting decreased Eh resulted in a sensitivity of 64.3% and a specificity of 100%.
Discussion
The present study showed that (I) a stepwise increase in NE infusion dose produced a significant and continuously increasing trend in the driving pressure of venous return, Rvr and systemic circulation. (II) There were highly divergent responses in Eh and CO to NE challenge episodes following an NE-induced 10 mmHg increase in MAP. (III). An increase in CVP may be an early alarm to identify the reduction in CO/Eh during the NE-induced increase in MAP.
Effect of increasing NE on venous return
Pmsf is the precise physiologically variable that is used to assess the systemic volume state, and the venous return is determined as the pressure gradient between Pmsf and RAP (15,16), which is defined as the pressure in the vascular system during circulatory arrest (17). The Pmsf has attracted increasing interest in critically ill patients, and it was used to interpret the effect of NE on venous return (3,4).
The present study used a mathematical model to calculate the mean systemic pressure (Pmsa) and derived variables to assess the effect of increasing NE on venous return. Increases in venous and systemic resistance (ΔRvr and ΔRsys) were related to a decrease in CO, and the increase in driving pressure venous return was related to an NE-induced increase in CO in the present study. An increase in the NE infusion rate induced a continuous increase in the driving pressure of venous return and the resistance of venous and system. Werner-Moller et al. found that Pmsa was a good surrogate for standard Pmsf measured at zero blood flow (6). Our study also supported the feasibility of Pmsa to assess the effect of NE on venous return in clinical practice. A formal experimental demonstrated that CVP, as backpressure to venous return, inversely correlated to CO (18). A consistent result was found in the present study.
Changes in CO and Eh in the NE challenges
The present study used the venous return curve and cardiac function curve to interpret cardiovascular responses based on Guyton’s theory. A schematic diagram of the effects of NE on CO is shown in Figure 5. The CO responses to NE are divided into three conditions. (I) CO-increasing and Eh-amplifying responses to NE (Figure 5A): NE shifted the venous return curve to the right (and increased Pmsa and Rvr) without affecting the cardiac function curve at a steep stage. As a result, NE caused an increase in CO with a smaller change in CVP. A slight change in CVP and a greater increase in CO indicated Eh amplification. Two studies reported that the early administration of an NE infusion or an increased infusion rate recruited unstressed volume to stressed volume and increased the cardiac preload (defined as left ventricular end-diastolic area) and CO in septic shock patients (19,20). (II) CO-little changed an Eh-reducing response to NE (Figure 5B): NE shifted the venous return curve to the right (and increased Pmsa and Rvr) without affecting the cardiac function curve, which was at a relatively flat stage. As a result, NE caused an increase in CO-unchanged with an increase in CVP. an increase in CVP and a slight change in CO demonstrated that NE reduced Eh. Several studies found that NE infusion was associated with an unchanged CO in cardiogenic shock (21,22), head trauma, and septic patients (23). (III) CO-decreasing and Eh-reducing responses to NE (Figure 5C): NE shifted the venous return curve to the right (and increased Pmsa with a greater increase in Rvr) and shifted the cardiac function curve to the left (i.e., the ability of heart ejection may be impaired with an NE-induced increase of afterload, and the increase of afterload was always inversely correlated to CO). As a result, NE caused a decrease in CO with an increase in CVP. Desjars et al. observed that NE-induced increased MAP produced a drop in CO in septic patients (24). Conversely, López et al. found that the use of nitric oxide synthase inhibition to increase MAP induced a decrease in CO in hypotensive septic shock patients (25). A recent animal study found that an increase of NE and volume expansion increased ECMO (7). NE increased vascular resistance and pump afterload, but volume expansion caused a decrease of vascular resistance and pump afterload (7). The CVP-increasing response combined with the CO-decreasing response demonstrated that NE impaired Eh.

In summary, all three of the above-mentioned CO responses to NE were reported in previous clinical studies. The role of HR should also be considered in the interpretation of the directional change of CO. An increase in NE may directly stimulate β-adrenergic receptors and further amplify CO. the increase in HR correlated with an increase in the NE challenge-induced CO response in the present study. A previous study also found that HR decreased in patients with a CO-decreasing response (3).
Clinical relevance of the NE challenge
Guarracino et al. reported that the assessment of cardiovascular response using the mathematical model used in this study was highly heterogeneous during guideline-based resuscitation in septic shock patients (26). We found that an increase in CVP may be an early alarm for the reduction in CO and Eh following the administration of NE. Therefore, the dynamic monitoring of changes in CVP may provide useful information for the identification of impaired cardiac function caused by increasing NE. The cutoff value of ΔCVP related to NE-induced impaired cardiac function was, to our knowledge, determined for the first time in septic shock patients in the present study. The present study showed that an elevation of CVP >1.5 mmHg was related to the reductions in CO and Eh during the increased NE.
The CO was not equal to the Eh. Eh reflects the efficiency of the heart as a global measure and was calculated by changes in CVP, CO and MAP. The present study showed that ΔCO was significantly related to ΔEh. However, some patients had a small increase in CO with a reduction in Eh in response to NE. We emphasize that the change in Eh may provide further useful information to optimize cardiac function during the use of NE to maintain MAP in septic shock patients.
Limitations
First, the present study should be regarded as a physiological study because all of the patients were in a stable condition without hypotension. The initial MAPbase was optimized based on local hemodynamic therapy principle. Our main finding is that an increase of ΔCVP following NE titration indicates the presence of an NE-induced impaired cardiac function. Therefore, ΔCVP following NE may be an alarm indicator for the identification of NE-induced heart impairment. Second, we arbitrarily choose an NE-induced increase of 10 mmHg MAP as an NE challenge to the heart. For medical safety, the adjustment of MAP was required to be mild. Notably, an NE-induced 10-mmHg change in MAP caused a variety of changes in CO and Eh. Maas et al. found that NE-induced 20-mmHg increases in arterial pressure were associated with an increase or decrease in CO in stable postoperative cardiac surgery patients (3). Third, mathematical coupling may exist between the related parameters (CVP, MAP, CO and Pmsa) because Pmsa was derived from a calculated formula in our study. Recent clinical studies showed that volume-induced changes in Pmsf were reliably tracked by changes in Pmsa, and the Pmsa and Pmsa-derived variables provided an assessment of the efficiency of volume expansion in postsurgical cardiac patients (27-30). An animal study showed that Pmsa had a good ability to assess the standard Pmsf (6). Pmsa may be a promising indicator in bedside hemodynamic monitoring. Fourth, because two NE challenges were included for each patient, the individual patient information at baseline is not appropriate for analysis in the NE challenge test. Fifth, Eh is an indirect measure of the ability of the heart to maintain the stressed volume. It may be more interesting and relevant to relate Eh to the stress volume in the present study. Some clinical studies showed that the stressed volume could be calculated during fluid challenge. Mass et al. calculated the stress volume using Pmsf (using the inspiratory hold method) and systemic compliance at the difference volume status (3). Pmsf was measured before and after fluid administration. Stressed volume was determined by extrapolating the Pmsf-volume curve to the zero-pressure intercept. Moreover, the systemic compliance was assumed to be unchanged (31). However, the related condition was different in our study. A known value of volume change was lacking in the present study, and the systemic compliance always was variable during the NE change. Further study is required to investigate the effect of NE on the stress volume.
Conclusions
Divergent responses in CO and Eh to NE challenge were interpreted via the application of a mathematical model for the calculation of mean systemic pressure. An increase in CVP may be an early alarm to identify the reduction in CO /Eh during the NE-induced increase of MAP.
Acknowledgments
Funding: This work was supported by the Beijing Natural Science Foundation (No. 7202157), Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (CIFMS) from Chinese Academy of Medical Sciences (grant number 2020-I2M-2-005), and the National Key Research and Development Program of China (grant number 2020YFC0841300). 2018 Beijing Dongcheng District Excellent Talents Training Funding Project.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-6686
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-6686
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-6686). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The investigation was performed according to the principles outlined in the Declaration of Helsinki (as revised in 2013). The institutional review board of Peking Union Medical College Hospital approved the study protocol (No. ZS-910, approved at 2015). Written informed consent was obtained from all patients or next of kin before the data were included in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- He H, Long Y, Zhou X, et al. Oxygen-Flow-Pressure Targets for Resuscitation in Critical Hemodynamic Therapy. Shock 2018;49:15-23. [Crossref] [PubMed]
- Foulon P, De Backer D. The hemodynamic effects of norepinephrine: far more than an increase in blood pressure! Ann Transl Med 2018;6:S25. [Crossref] [PubMed]
- Maas JJ, Pinsky MR, de Wilde RB, et al. Cardiac output response to norepinephrine in postoperative cardiac surgery patients: interpretation with venous return and cardiac function curves. Crit Care Med 2013;41:143-50. [Crossref] [PubMed]
- Persichini R, Silva S, Teboul JL, et al. Effects of norepinephrine on mean systemic pressure and venous return in human septic shock. Crit Care Med 2012;40:3146-53. [Crossref] [PubMed]
- Parkin WG, Leaning MS. Therapeutic control of the circulation. J Clin Monit Comput 2008;22:391-400. [Crossref] [PubMed]
- Werner-Moller P, Sondergaard S, Jakob SM, et al. Effect of volume status on the estimation of mean systemic filling pressure. J Appl Physiol (1985) 2019;126:1503-13. [Crossref] [PubMed]
- Moller PW, Hana A, Heinisch PP, et al. The Effects of Vasoconstriction And Volume Expansion on Veno-Arterial ECMO Flow. Shock 2019;51:650-8. [Crossref] [PubMed]
- He HW, Liu W L, Zhou X, et al. Effect of mean arterial pressure change by norepinephrine on peripheral perfusion index in septic shock patients after early resuscitation. Chin Med J (Engl) 2020;133:2146-52. [Crossref] [PubMed]
- Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003;31:1250-6. [Crossref] [PubMed]
- Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818-29. [Crossref] [PubMed]
- Berger D, Moller PW, Takala J. Reply to "Letter to the editor: Why persist in the fallacy that mean systemic pressure drives venous return?". Am J Physiol Heart Circ Physiol 2016;311:H1336-7.
- Berger D, Moller PW, Weber A, et al. Effect of PEEP, blood volume, and inspiratory hold maneuvers on venous return. Am J Physiol Heart Circ Physiol 2016;311:H794-806. [Crossref] [PubMed]
- Roy A. Estimating correlation coefficient between two variables with repeated observations using mixed effects model. Biom J 2006;48:286-301. [Crossref] [PubMed]
- McCulloch CE, and Searle SR. Generalized, Linear, and Mixed Models. John Wiley and Sons, 2000.
- Magder S. Point: the classical Guyton view that mean systemic pressure, right atrial pressure, and venous resistance govern venous return is/is not correct. J Appl Physiol (1985) 2006;101:1523-5. [Crossref] [PubMed]
- Guyton AC. Determination of cardiac output by equating venous return curves with cardiac response curves. Physiol Rev 1955;35:123-9. [Crossref] [PubMed]
- Jansen JR, Maas JJ, Pinsky MR. Bedside assessment of mean systemic filling pressure. Curr Opin Crit Care 2010;16:231-6. [Crossref] [PubMed]
- Moller PW, Winkler B, Hurni S, et al. Right atrial pressure and venous return during cardiopulmonary bypass. Am J Physiol Heart Circ Physiol 2017;313:H408-20. [Crossref] [PubMed]
- Hamzaoui O, Georger JF, Monnet X, et al. Early administration of norepinephrine increases cardiac preload and cardiac output in septic patients with life-threatening hypotension. Crit Care 2010;14:R142. [Crossref] [PubMed]
- Monnet X, Jabot J, Maizel J, et al. Norepinephrine increases cardiac preload and reduces preload dependency assessed by passive leg raising in septic shock patients. Crit Care Med 2011;39:689-94. [Crossref] [PubMed]
- Rokyta R Jr, Tesarová J, Pechman V, et al. The effects of short-term norepinephrine up-titration on hemodynamics in cardiogenic shock. Physiol Res 2010;59:373-8. [Crossref] [PubMed]
- Albanèse J, Leone M, Garnier F, et al. Renal effects of norepinephrine in septic and nonseptic patients. Chest 2004;126:534-9. [Crossref] [PubMed]
- Martin C, Papazian L, Perrin G, et al. Norepinephrine or dopamine for the treatment of hyperdynamic septic shock? Chest 1993;103:1826-31. [Crossref] [PubMed]
- Desjars P, Pinaud M, Potel G, et al. A reappraisal of norepinephrine therapy in human septic shock. Crit Care Med 1987;15:134-7. [Crossref] [PubMed]
- López A, Lorente JA, Steingrub J, et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: Effect on survival in patients with septic shock. Crit Care Med 2004;32:21-30. [Crossref] [PubMed]
- Guarracino F, Bertini P, Pinsky MR. Cardiovascular determinants of resuscitation from sepsis and septic shock. Crit Care 2019;23:118. [Crossref] [PubMed]
- Maas JJ, Pinsky MR, Geerts BF, et al. Estimation of mean systemic filling pressure in postoperative cardiac surgery patients with three methods. Intensive Care Med 2012;38:1452-60. [Crossref] [PubMed]
- Cecconi M, Aya HD, Geisen M, et al. Changes in the mean systemic filling pressure during a fluid challenge in postsurgical intensive care patients. Intensive Care Med 2013;39:1299-305. [Crossref] [PubMed]
- Gupta K, Sondergaard S, Parkin G, et al. Applying mean systemic filling pressure to assess the response to fluid boluses in cardiac post-surgical patients. Intensive Care Med 2015;41:265-72. [Crossref] [PubMed]
- Maas JJ, Geerts BF, Jansen JR. Evaluation of mean systemic filling pressure from pulse contour cardiac output and central venous pressure. J Clin Monit Comput 2011;25:193-201. [Crossref] [PubMed]
- Maas JJ, Pinsky MR, Aarts LP, et al. Bedside assessment of total systemic vascular compliance, stressed volume, and cardiac function curves in intensive care unit patients. Anesth Analg 2012;115:880-7. [Crossref] [PubMed]


