Docetaxel maintenance therapy versus best supportive care after first-line chemotherapy with different dose docetaxel plus cisplatin for advanced non-small cell lung cancer (TFINE study, CTONG-0904): an open-label, randomized, phase III trial
Introduction
Non-small cell lung cancer (NSCLC) represents 80% of lung cancer cases and accounts for 1.6 million new cases worldwide each year (1,2). Systemic chemotherapy with platinum-based doublets remains the backbone treatment for patients with advanced NSCLC, although novel targeted agents have also been used in some populations with specific genotypes (3-6). Recent studies have shown that maintenance therapy after first-line chemotherapy could improve patient survival with manageable toxicity (7-10). However, most of these studies were limited to newer targeted drugs or pemetrexed, which are believed to have lower toxicity. Given the limited number of NSCLC patients who can benefit from targeted therapies, further investigations of traditional cytotoxic agents in the maintenance setting or dose optimization may bring additional benefits for the overall population.
Docetaxel, an anti-microtubule taxane widely used in several types of cancer, was approved for both the first- and second-line treatment of advanced NSCLC (11). Recently, Fidias et al. reported that the use of docetaxel in switch maintenance therapy demonstrated significantly improved patient survival in a Caucasian population, with manageable toxicities (9). However, no studies have investigated the effect of continuation maintenance with docetaxel in advanced NSCLC. Maximizing the effectiveness of specific agents could delay the use of subsequent chemotherapeutics and drug resistance, thus prolonging progression-free survival (PFS) or even overall survival (OS).
Moreover, differences in the therapeutic dose of docetaxel between Caucasians and Asians have been reported (12-14). While the standard dose recommended for the systemic treatment of NSCLC in Caucasians is 75 mg/m2, pilot studies performed in East Asian countries demonstrated similar efficacy and lower toxicity with the 60 mg/m2 dose compared to 75 mg/m2 (12,15). Additional studies are warranted to identify the optimal docetaxel dose in East Asian populations, although some studies presenting the potential mechanisms behind these differences between ethnic populations, including genetic variation involved in the metabolism and transport of docetaxel, have been presented (14,16).
We conducted this phase III study to evaluate the efficacy and safety of docetaxel as maintenance therapy in East Asian patients with advanced NSCLC, and to determine the preferred dose of docetaxel (75 or 60 mg/m2) when combined with cisplatin as first-line treatment. The primary objective of the present trial was to compare the PFS during maintenance therapy with best supportive care (BSC) and docetaxel plus BSC. The secondary objectives included an assessment of the best response rate of first-line therapy, OS, time to progression (TTP) during maintenance therapy, and safety during the study. We present the following article in accordance with the CONSORT reporting checklist (available at http://dx.doi.org/10.21037/atm-20-8078).
Methods
Study design
This was a multicenter, open-label, dynamic randomized study conducted in 15 hospitals across China. It had 2 randomizations and 2 phases: the first-line therapy phase and the maintenance phase. The trial was registered in Clincaltrials.gov with the identifier NCT01038661.
Patients
Eligible patients had cytologically or histologically confirmed stage IIIB or IV NSCLC. Other inclusion criteria were: age 18 to 75 years, Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1, no previous chemotherapy for advanced NSCLC, life expectancy ≥12 weeks, at least 1 evaluable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.0) (17), and adequate hematological, liver, and kidney functions. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The protocol was submitted to the independent ethics committees and institutional review boards of each participating institution for review and written approval (No. YP2009126) and informed consent was taken from all the patients.
Randomization and treatment
In the first phase, eligible patients were randomized (R1, 1:1) to receive either docetaxel 75 mg/m2 (DC75 group) or 60 mg/m2 (DC60 group) in combination with cisplatin 75 mg/m2 for up to 4 cycles. R1 was stratified according to ECOG PS (0 vs. 1), gender, tumor stage (IIIB vs. IV), and histological type (squamous vs. non-squamous). Patients in both groups, who achieved disease control after 4 cycles of first-line chemotherapy, entered the maintenance phase and were randomly assigned (R2, 1:2) to receive BSC or docetaxel continuation maintenance (DCM) therapy of 60 mg/m2 plus BSC for up to 6 cycles. R2 was stratified according to the tumor response [objective response vs. stable disease (SD)] after first-line treatment and docetaxel dose groups (DC75 vs. DC60). A centralized interactive web response system was used for both randomizations.
Dose adjustments for both docetaxel and cisplatin were performed according to pre-defined criteria for each type of toxicity (mainly grade 4 neutropenia and grade 3–4 neuropathy). Two dose reductions were allowed for DC75 (first to 60 mg/m2, second to 50 mg/m2), but only 1 reduction was allowed for DC60 and DCM therapy (to 50 mg/m2). Dose re-escalation was not allowed in patients with reductions due to toxicity.
Procedures
Baseline evaluation included complete disease history, physical examination, ECOG PS, hematology and biochemistry examinations, magnetic resonance imaging (MRI) or computed tomography (CT) scans of the brain, and bone scans or positron emission tomography. Initial tumor lesions were assessed using MRI or CT within 21 days prior to the first randomization. Tumor responses were assessed every 2 cycles based on the RECIST 1.0 guidelines (17) during study treatment, and confirmed at least 4 weeks apart from the initial assessment. For those who did not progress after completion of study treatments, tumor responses were assessed every 6 weeks until progressive disease (PD), administration of other anti-tumor treatment, or death. Data on patient survival and subsequent anti-tumor therapies were collected every 8 weeks after study discontinuation until patient death or loss to follow-up. Hematology and biochemistry were collected 1 day before dosing in each cycle and weekly thereafter. Vital signs were evaluated at each study visit and special examinations were performed based on clinical judgment.
Outcomes
The primary endpoint of the study was to compare the PFS of patients treated with either BSC or continuation maintenance therapy with docetaxel plus BSC, defined as the duration from the second randomization to tumor progression or death due to any cause.
The secondary endpoints were best response rate during first-line treatment, including disease control rate (DCR), objective response rate (ORR), OS, TTP, and toxicities of investigated drugs.
Adverse events (AEs) and serious adverse events (SAEs) were monitored and recorded throughout the study. AE severity and toxicity profiles were assessed according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC) 3.0 (18).
For the evaluation of both primary and secondary endpoints, tumor responses were assessed at the center level and images were interpreted by the same investigator/radiologist throughout the study.
Statistical analysis
The evaluation of the primary endpoint PFS was based on the superiority test. Assuming that a 50% improvement in PFS with docetaxel maintenance therapy would indicate superiority over the BSC group (4.5 vs. 3.0 months), at the level of two-sided α=0.05, β=0.2, with a 2:1 randomization, an enrollment period of 18 months, and a follow-up period of 24 months, the estimated sample size was 270 patients (180 in the maintenance group and 90 in the BSC group). Considering a 20% chance of PD in the first-line treatment and a drop-out rate of 10%, a total sample size of 380 patients was calculated.
No interim analyses comparing treatment groups were planned. Median PFS and its 95% confidence interval (CI) in treatment groups was assessed using the Kaplan-Meier method, and a log-rank test in the intent-to-treat (ITT) population (enrolled to R2). The Cox proportional hazards model was applied to estimate the treatment hazard ratio (HR) and its 95% CI. The model was fitted using the stratification factors in the second randomization and Kaplan-Meier estimates were calculated.
Comparisons of DCR and ORR were performed by between-groups Chi-square or Fisher’s exact tests in the evaluable population. The DCR in the first-line treatment was assessed by a non-inferiority test in the evaluable population, defined as the patients who had at least 1 post-chemotherapy assessment of tumor response. Based on this non-inferiority design, assuming a 70% DCR in the control group (75 mg/m2) and a non-inferiority margin of 15%, at the level of α=0.05, β=0.1, we calculated a sample size of 160 in each group.
A two-sided 0.05 significance level was applied to all tests. Safety analyses included all patients receiving at least 1 dose of the investigated drugs. Descriptive statistics were presented for an overview of AEs, the incidence of AEs, treatment-related AEs, severity of AEs, and SAEs. Analyses were performed using the SAS 9.2 (SAS Institute Inc., Cary, NC, USA) software.
Results
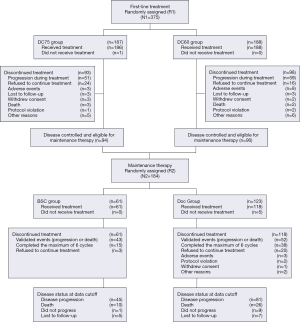
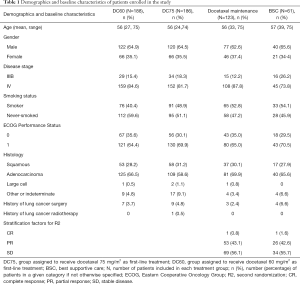
A total of 375 patients were enrolled in the first-line treatment phase (187 assigned to DC75 and 188 assigned to DC60; ITT population for this period; Figure 1) between December 2009 and August 2011. Of these patients, 374 (99.7%) were treated and included in the safety population for this phase and 314 (83.7%) had at least 1 post-treatment assessment of tumor response (the evaluable population). A total of 184 patients without PD entered the second randomization and 179 were treated (safety population for the maintenance phase; Figure 1, Table S1). Treatment groups after both randomizations were balanced in terms of patient demographics and clinical characteristics (Table 1). Overall, the main reason for discontinuation was death (276 patients), with other specific reasons presented in Figure 1. Treatment delivery was similar between groups during first-line chemotherapy, with a median of 4 cycles in both groups (Table 2).
Full table

Full table
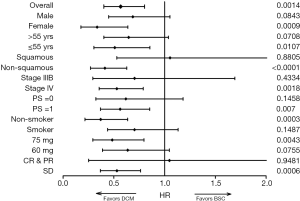
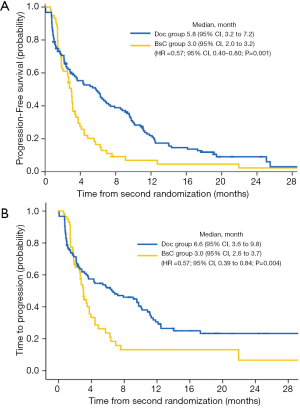
After a median follow-up of 22.8 months, there were 162 validated events for the primary analysis, including 126 PD and 36 deaths. During the second study phase, PD was observed in 45 of 61 patients (73.8%) from the BSC group and in 81 of 123 patients (65.9%) from the DCM group. The median TTP and PFS since R2 were significantly longer for patients receiving DCM therapy compared to BSC (PFS: 5.8 vs. 3.0 months, P=0.0022; TTP: 6.6 vs. 3.0 months; P=0.0088; Figure 2). A Cox analysis using the stratification factors in R2 confirmed the difference (HR =0.57; 95% CI: 0.40−0.80; P=0.001). The 1-year PFS Kaplan-Meier estimate was 21.9% (95% CI: 14.9−29.9) in the DCM group, and 6.9% in the BSC group (95% CI: 2.0−16.0). The subgroup analysis demonstrated the PFS superiority of DCM over BSC across main subgroups as seen in the forest plot (Figure 3).
The median PFS since R1 was 4.9 months in the DC75 group compared to 4.7 months in the DC60 group (P=0.99). For those who received R2 after DC75 treatment, the PFS since R1 was 8.3 months in the DCM group and 5.3 months in the BSC group. For patients from the DC60 group, the PFS since R1 was 9.2 months in the DCM group and 6.1 months in the BSC group.
At the data cutoff for OS, there were 139 and 137 deaths in the DC75 and DC60 group, respectively. The median OS was 11.8 months in the DC75 group compared to 13.0 months in the DC60 group (P=0.26). For those who received R2, patients had a median OS of 12.3 months in the DCM group and 13.7 months in the BSC group (P=0.77).
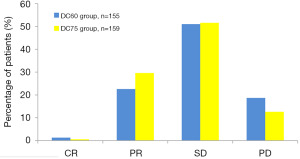
The comparison of best response between groups was performed in the evaluable population for first-line treatment, which included 159 and 155 patients in the DC75 and DC60 group, respectively. In the DC60 group, there were 37 patients (23.9%) achieving ORR and 116 (74.8%) had DCR, compared to 30.2% ORR and 81.8% DCR in the DC75 group (Figure 4). The difference in ORR between groups was not statistically significant (P=0.17). In regard to the DCR difference, it had a 95% CI of −17.40% to 1.77% which covered the non-inferiority margin (−15%), suggesting that this trial failed to demonstrated non-inferiority of DC60 over DC75 in the first-line treatment for advanced NSCLC.
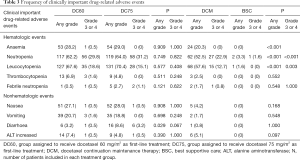
During the first-line treatment, 1,398 AEs were reported by 175 patients [86 (47.8%) patients in the DC75 group and 89 (45.7%) in the DC60 group; Table S2]. The most common treatment-related AEs included leukopenia, neutropenia, anemia, nausea, vomiting, diarrhea, and alanine aminotransferase (ALT) elevation, with diarrhea being significantly more frequent in the DC75 group compared with the DC60 group (8.6% vs. 3.2%, P=0.029) (Table 3). Grade ≥3 AEs were comparable between groups, although grade ≥3 diarrhea seemed to be more frequent in the DC75 group (3.2% vs. 0.5%, P=0.067). During the maintenance treatment, at least 1 AE was reported for 87 patients (73.7%) receiving DCM and 7 patients (11.5%) with BSC. Grade ≥3 AEs were limited to hematologic events in both groups, but they were significantly more frequent in the DCM group than in the BSC group (Table 3). No death-leading AE related to study treatments was reported during the first-line therapy or maintenance phase.
Full table
Numerically, more patients in the BSC group (n=35, 57.4%) received second-line treatments, including docetaxel, EGFR tyrosine kinase inhibitors, or pemetrexed, than those in maintenance group (n=56, 45.5%), although the difference was not statistically significant (P=0.13).
Discussion
In the present study, we have shown that continuation maintenance therapy with a lower dose of docetaxel following 4 cycles of platinum-based chemotherapy containing docetaxel significantly improved PFS in patients with advanced NSCLC. DCM therapy resulted in a 43% reduction in the risk of PD or death, without any new safety signals. This study also suggests, in view of fewer toxicities and similar efficacy, that the 60 mg/m2 docetaxel dose may be the preferred dose when combined with cisplatin in East Asian populations.
To date, numerous clinical trials have evaluated maintenance therapy for advanced NSCLC (7-9,19,20), including continuation maintenance or switch maintenance therapy. However, trials which introduced cytotoxic maintenance therapy were compromised due to dose-accumulating toxicities (21). Nevertheless, clinical trials introducing pemetrexed and gemcitabine as maintenance therapy following platinum-based first-line therapy have shown benefits in selected groups of patients (either with SD or with non-squamous histology) (7,8,22). Recently, Fidias et al. reported a significant PFS improvement (5.7 vs. 2.7 months) with docetaxel switch maintenance treatment without an increase in toxicity (9). However, docetaxel as continuation maintenance therapy has not been previously investigated in NSCLC patients. To evaluate the clinical benefit from already available chemotherapy drugs, we conducted this study and found that the difference in PFS between the study groups exceeded the pre-set superiority criteria of 50% longer PFS in the maintenance group compared with the BSC group (5.8 vs. 3.0 months; P=0.002). This difference was maintained when the results were analyzed for the entire study duration, and for all patients initially enrolled and across main subgroups, thus providing the first evidence of a prolonged PFS in a Chinese population when using a non-cross-resistant agent. Although these primary results are positive, they should be interpreted with caution due to the lower number of patients enrolled in the maintenance phase compared to the estimated needed sample size (184 vs. 270). This low number of patients was due to differences between the estimated and actual values for disease progression (20% vs. 29.3%) and drop-out rate (10% vs. 21.6%).
OS was also an important indicator when studying maintenance therapy in advanced NSCLC. In the present TFINE study, the secondary endpoint OS was not significantly different between the DCM and BSC groups. The failure in translating the PFS improvement into OS was partially due to post-progression therapy, as more patients in the BSC group (57.4%) received second-line treatments than those in the maintenance group (45.5%), although the difference was not statistically significant (P=0.13). Furthermore, subgroup analysis revealed that the PFS superiority of DCM was mainly attributed to female, young, non-squamous, and never-smoker patients. These patients were more likely to receive targeted therapies in subsequent treatment, thus attenuated the survival benefit from the DCM therapy. However, we did not have the genotype test when this trial was initiated in early 2009, which limited the use of more precise biomarkers in maintenance therapy.
In our study, both first-line therapy and docetaxel maintenance therapy were relatively well tolerated, with myelosuppression being the major cause of toxicities. Only 2 patients from the DC75 group and 4 patients from the maintenance group discontinued treatment due to AEs. Of note is the high number of patients that were either lost to follow-up or withdrew consent, and this may have also been due to AEs. Moderate and severe AEs were reported for 25.4% patients in the maintenance group. The most frequently reported AEs were consistent with previous clinical trials showing a high frequency of hematological toxicity in patients treated with docetaxel (23-26). Although AEs were more frequent in patients receiving maintenance docetaxel than in the BSC group, no unexpected AEs were reported and the observed safety experience of docetaxel 60 mg/m2 for patients receiving maintenance treatment was consistent with the reported safety characteristics of docetaxel in other populations.
In clinical studies, the traditional 75 mg/m2 dose of docetaxel has been associated with common side effects, including severe myelotoxicity (11,23-26). Racial differences in drug efficacy and toxicity with the same taxane dose have been reported, and these differences were explained by genotype diversity associated with taxane metabolism (27). Thus, finding the optimal dose of docetaxel in East Asian populations remains a challenge. Due to concerns regarding toxicities, clinical studies evaluating the efficacy and safety of 60 mg/m2 docetaxel have been performed in Japan and Korea (12,15), and all studies showed either comparable efficacy or non-inferiority of this dose, but with an improved safety profile compared to 75 mg/m2. In our study, the difference between DCR in the 2 treatment groups was −7.8% (−17.40% to 1.77%), with the lower limit of the 95% CI for the intergroup DCR difference lower than the pre-set non-inferiority margin of −15%. Although the non-inferiority of DC60 in terms of DCR could not be demonstrated, ORR was similar in both the DC60 and DC75 groups, consistent with previously published data in East Asian populations (12).
In conclusion, we have shown that docetaxel maintenance therapy following first-line platinum-based chemotherapy containing docetaxel significantly improved PFS in East Asian patients with advanced NSCLC. The dose of 60 mg/m2 docetaxel as first-line treatment may be a promising alternative to the higher docetaxel dose in this population. However, further studies which are adequately powered to demonstrate the non-inferiority of this dose are warranted.
Acknowledgments
The authors would like to thank Adriana Rusu (XPE Pharma & Science on behalf of Sanofi China) for writing assistance and editorial support.
Funding: This work was supported by Sanofi China. Sanofi was the sponsor of this study and took responsibility for all costs associated with this study and for the development and publishing of the present manuscript.
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-8078
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-8078
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-8078). LZ has received research support from Boehringer Ingelheim, AstraZeneca, Eli Lilly, and Sanofi; SL has received research support from Boehringer Ingelheim, AstraZeneca, Roche and Sanofi; YLW has received speaker fees from AstraZeneca, Roche, Eli Lilly, and Sanofi Aventis; CZ is advisor of Eli Lily, Boehringer Ingelheim, Roche and Clovis and has received honoraria from Eli Lilly, Roche, Sanofi, Pfizer and AstraZeneca. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The protocol was submitted to the independent ethics committees and institutional review boards of each participating institution for review and written approval (No. YP2009126). Informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [Crossref] [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2013. CA Cancer J Clin 2013;63:11-30. [Crossref] [PubMed]
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic Lymphoma Kinase Inhibition in Non-Small-Cell Lung Cancer. N Engl J Med 2010;363:1693-703. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or Carboplatin-Paclitaxel in Pulmonary Adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III Study of Afatinib or Cisplatin Plus Pemetrexed in Patients with Metastatic Lung Adenocarcinoma with EGFR Mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Sun S, Schiller JH, Spinola M, et al. New Molecularly Targeted Therapies for Lung Cancer. J Clin Invest 2007;117:2740-50. [Crossref] [PubMed]
- Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 2009;374:1432-40. [Crossref] [PubMed]
- Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010;11:521-9. [Crossref] [PubMed]
- Fidias PM, Dakhil SR, Lyss AP, et al. Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J Clin Oncol 2009;27:591-8. [Crossref] [PubMed]
- Qi WX, Tang LN, He AN, et al. Erlotinib and pemetrexed as maintenance therapy for advanced non-small-cell lung cancer: a systematic review and indirect comparison. Curr Med Res Opin 2012;28:643-50. [Crossref] [PubMed]
- Saloustros E, Georgoulias V. Docetaxel in the treatment of advanced non-small-cell lung cancer. Expert Rev Anticancer Ther 2008;8:1207-22. [Crossref] [PubMed]
- Kim K. Comparison of docetaxel/cisplatin dosages of 75/60 and 60/60 mg/m(2)for the treatment of non-small cell lung cancer. Exp Ther Med 2012;4:317-22. [Crossref] [PubMed]
- Sekine I, Yamamoto N, Nishio K, et al. Emerging ethnic differences in lung cancer therapy. Br J Cancer 2008;99:1757-62. [Crossref] [PubMed]
- Yamamoto N, Tamura T, Murakami H, et al. Randomized pharmacokinetic and pharmacodynamic study of docetaxel: dosing based on body-surface area compared with individualized dosing based on cytochrome P450 activity estimated using a urinary metabolite of exogenous cortisol. J Clin Oncol 2005;23:1061-9. [Crossref] [PubMed]
- Mukohara T, Takeda K, Miyazaki M, et al. Japanese experience with second-line chemotherapy with low-dose (60 mg/M2) docetaxel in patients with advanced non-small-cell lung cancer. Cancer Chemother Pharmacol 2001;48:356-60. [Crossref] [PubMed]
- Bosch TM, Huitema AD, Doodeman VD, et al. Pharmacogenetic screening of CYP3A and ABCB1 in relation to population pharmacokinetics of docetaxel. Clin Cancer Res 2006;12:5786-93. [Crossref] [PubMed]
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 2000;92:205-16. [Crossref] [PubMed]
- National Cancer Institute. NCI guidelines for investigators: adverse event reporting requirements for DCTD (CTEP and CIP) and DCP INDs and IDEs. 2012. Available online: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/aeguidelines.pdf. Accessed August 27, 2014.
- Paz-Ares L, de Marinis F, Dediu M, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol 2012;13:247-55. [Crossref] [PubMed]
- Zhang L, Ma S, Song X, et al. Gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non-small-cell lung cancer (INFORM; C-TONG 0804): a multicentre, double-blind randomised phase 3 trial. Lancet Oncol 2012;13:466-75. [Crossref] [PubMed]
- Stinchcombe TE, Socinski MA. Maintenance Therapy in Advanced Non-Small Cell Lung Cancer: Current Status and Future Implications. J Thorac Oncol 2011;6:174-82. [Crossref] [PubMed]
- Perol M, Chouaid C, Milleron B, et al. Maintenance with either gemcitabine or erlotinib versus observation with predefined second-line treatment after cisplatin-gemcitabine induction chemotherapy in advanced NSCLC: IFCT-GFPC 0502 phase III study. J Clin Oncol 2010;28:abstr 7507.
- Okano Y, Ando M, Asami K, et al. Randomized phase III trial of erlotinib (E) versus docetaxel (D) as second- or third-line therapy in patients with advanced non-small cell lung cancer who have wild-type or mutant epidermal growth factor receptor (EGFR): Docetaxel and Erlotinib Lung Cancer Trial (DELTA). J Clin Oncol 2013;31:abstr 8006.
- Garassino MC, Martelli O, Bettini A, et al. TAILOR: A phase III trial comparing erlotinib with docetaxel as the second-line treatment of NSCLC patients with wild-type(wt) EGFR. J Clin Oncol 2012;30:abstr LBA7501.
- Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small cell lung cancer previously treated with platium-based chemotherapy. J Clin Oncol 2000;18:2095-103. [Crossref] [PubMed]
- Schiller JH, Harrington D, Belani C, et al. Comparison of four chemotherapy regimens for advanced non-small cell lung cancer. N Engl J Med 2002;346:92-8. [Crossref] [PubMed]
- Gandara DR, Kawaguchi T, Crowley J, et al. Japanese-US common-arm analysis of paclitaxel plus carboplatin in advanced non–small-cell lung cancer: a model for assessing population-related pharmacogenomics. J Clin Oncol 2009;27:3540-6. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)