High fat diet-induced obesity increases the formation of colon polyps induced by azoxymethane in mice
Introduction
Obesity is increasing worldwide, and represents a high proportion of the population in developed countries. It not only causes diabetes, heart disease and hyper-cholesterolemia, but also is linked to cancer (1). Epidemiological studies have shown that obesity increases the incidence of several cancers including endometrial, esophageal, breast, liver, renal and colon cancer (1). Obesity also results in poorer prognosis for colon cancer (2,3). However, the mechanisms of obesity-associated colon cancer are not fully understood. Further research could elucidate these mechanisms as well as provide effective prevention and treatment approaches. However, to do this we require good animal models.
At present, there are limited animal models available. The most commonly used animal models for obesity include ob/ob mice, db/db mice and Zucker rats; all of them have gene mutations (4,5). These models have been used to study the effect of obesity on colon cancer induced by azoxymethane (AOM) showing that obesity does increase the incidence of colon cancer (6,7). The db/db mouse model has also been used in preventative studies using branch amino acids (5), green tea (8) and dietary flavonoids (9). A study using kk-A(y) obese mice showed that the number of polyps induced by AOM had a 22-fold increase when compared to the controls (4). However, these animals either lack leptin or have a mutation that inactivates the leptin receptor Ob-Rbl, leading to the disruption of leptin-induced intracellular signalling (4). Leptin can promote colorectal cancer development by activating multiple signalling pathways such as MAPK and PI3K/Akt (10). Therefore, it is desirable to use animals with intact leptin and the leptin receptor Ob-Rbl to investigate obesity-associated colorectal cancer.
Obese C57Bl/6 mice have been used to study obesity and colon cancer. One particular study showed that a high-fat diet can increase polyp formation induced by AOM in mice (11). However, another study using the same model reported that a high-fat diet for 15 weeks did not affect the growth of the xenograft tumour although body weight increased (12). This discrepancy may be caused by not distinguishing diet-induced obese (DIO) and diet-resistant (DR) mice. Previous studies have shown that mice or rats fed a high-fat diet may either develop obesity or resist to obesity development as they have about the same body weight as mice fed lab chow diet (13-16). The DR mice or rats have quite different blood profiles of cancer risk factors that mediate obesity-increased cancer such as blood levels of leptin and insulin (17,18). The gene expression in DIO and DR is also different (19,20). Using non-obese DR mice in the experiments could dilute the effect of obesity on carcinogenesis. In the present study, we aimed to investigate the effects of obesity on AOM-induced colon polyp formation using DIO mice in comparison with DR mice. We showed that DIO mice had a greater susceptibility to AOM-induced colon polyp formation than DR mice, although they were fed an identical high-fat diet. This demonstrated that the DIO mice could be a good model for studying obesity-associated colon cancer.
Methods
Animals
The 6-week-old C57Bl/6 male mice were purchased from Animal Centre (Perth, Australia), and allowed 1 week acclimatisation to laboratory conditions. Mice were housed three per plastic cage with wood chips as bedding in a barrier-sustained animal room at 24±2 °C and 55% humidity on a 12-hour light/dark cycle and fed either a high-fat diet (3.80 kcal/g, 40% of energy from fat) or a low-fat diet (2.59 kcal/g, 10% of energy from fat). Food and water were available ad libitum. The animals were observed daily for clinical signs including general activity, hair and mortality. Body weight was measured weekly. All experiments were performed according to the requirement of Animal Ethics Committee for University of Wollongong (AE09/02), Australia.
AOM treatment
Following 6 weeks of either a high or low-fat diet, the mice were divided into three groups including DIO, DR and LF. All mice were injected with AOM intraperitoneally at dose of 10 mg/kg once a week for 6 weeks. The mice were then fed a high- or a low-fat diet for another 14 weeks before being sacrificed for the examination of colon polyp formation.
Examination of polyp formation
The entire colons of these mice were isolated and fixed in 10% formalin. The formation of polyps in the colon was examined. The colons were stained with 0.2% methylene blue and polyps were counted under a light microscope.
Statistical analysis
Statistics were performed using one-way ANOVA (SPSS, Chicago, USA), where P<0.05 was considered as statistically significant.
Results
To study the effect of obesity on colon cancer, 6-week-old mice were fed a high-fat diet for a period of 6 weeks. Mice were then separated into three groups as per our previous studies (21). Those with the highest weight gains were designated as DIO mice and those with the lowest weight gains were grouped as DR mice. The mice with medium weight gains were not used for the following experiments. Statistical analyses showed that the DIO mice were significantly heavier (15%) than the DR and control mice. The DR mice had a similar body weight gain as the LF mice with no statistical difference.
The number of colon polyp formed was counted in the three groups of mice (DIO, DR and LF). It was found that the DIO mice developed 2.5 times of polyps compared to the DR mice and 3.4 times of polyps compared to the low-fat fed mice (Figure 1A). Large polyps were also observed in DIO mice with AOM treatment (Figure 1B). Histological examination showed the disarrangement of the colon epithelial cells (Figure 1C).
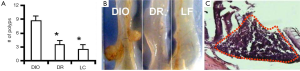
Discussion
In the present study, we showed that the DIO mice have increased polyp formation after the injection of AOM when compared to the DR mice and control mice fed a low-fat diet. This result suggests the DIO mice could be a suitable model for studying obesity-associated colon cancer, and that the DR may need to be excluded in such experiments. Distinguishing DIO and DR phenotypes can provide improved animal models for studying obesity-associated colon cancer. Although a high-fat diet has been shown to increase body weight and increase polyp formation (22), the DIO mice could be a more accurate model. Our previous study showed that not every mouse responded identically to high-fat diet (13,14). It takes three stages to develop DIO with an increased intolerance to leptin (14). After being fed a high-fat diet, some mice became obese while others remained lean. DIO and DR mice have totally different profiles in many aspects including the expression levels of agouti-related peptide and neuropeptide Y in the brain (23). It has been shown that DIO mice have different gene expression profiles by using microarray and quantitative PCR such as genes involved in Wnt and BMP signalling pathways as well as the gene for mesoderm specific transcript (19,20). As all mice are inbred, these differences are considered to be caused by different epigenetic mechanisms. The differences in the activation of the signalling pathways caused by gene expression may also account for the increased polyps in DIO mice. Activation of survival signalling pathways can increase cell proliferation and decrease apoptosis to promote carcinogenesis (24-26). Taken together, we think that it could be necessary to use DIO not DR mice in order to investigate the link between obesity and colon cancer.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569-78. [PubMed]
- Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr 2007;86:556-65. [PubMed]
- Chen J, Huang XF, Qiao L, et al. Insulin caused drug resistance to oxaliplatin in colon cancer cell line HT29. J Gastrointest Oncol 2011;2:27-33. [PubMed]
- Teraoka N, Mutoh M, Takasu S, et al. High susceptibility to azoxymethane-induced colorectal carcinogenesis in obese KK-Ay mice. Int J Cancer 2011;129:528-35. [PubMed]
- Shimizu M, Shirakami Y, Iwasa J, et al. Supplementation with branched-chain amino acids inhibits azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db mice. Clin Cancer Res 2009;15:3068-75. [PubMed]
- Ealey KN, Lu S, Archer MC. Development of aberrant crypt foci in the colons of ob/ob and db/db mice: evidence that leptin is not a promoter. Mol Carcinog 2008;47:667-77. [PubMed]
- Lee WM, Lu S, Medline A, et al. Susceptibility of lean and obese Zucker rats to tumorigenesis induced by N-methyl-N-nitrosourea. Cancer Lett 2001;162:155-60. [PubMed]
- Shimizu M, Shirakami Y, Sakai H, et al. (-)-Epigallocatechin gallate suppresses azoxymethane-induced colonic premalignant lesions in male C57BL/KsJ-db/db mice. Cancer Prev Res (Phila) 2008;1:298-304. [PubMed]
- Miyamoto S, Yasui Y, Ohigashi H, et al. Dietary flavonoids suppress azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db mice. Chem Biol Interact 2010;183:276-83. [PubMed]
- Jaffe T, Schwartz B. Leptin promotes motility and invasiveness in human colon cancer cells by activating multiple signal-transduction pathways. Int J Cancer 2008;123:2543-56. [PubMed]
- Endo H, Hosono K, Fujisawa T, et al. Involvement of JNK pathway in the promotion of the early stage of colorectal carcinogenesis under high-fat dietary conditions. Gut 2009;58:1637-43. [PubMed]
- Wheatley KE, Williams EA, Smith NC, et al. Low-carbohydrate diet versus caloric restriction: effects on weight loss, hormones, and colon tumor growth in obese mice. Nutr Cancer 2008;60:61-8. [PubMed]
- Xin X, Storlien LH, Huang XF. Hypothalamic c-fos-like immunoreactivity in high-fat diet-induced obese and resistant mice. Brain Res Bull 2000;52:235-42. [PubMed]
- Lin S, Thomas TC, Storlien LH, et al. Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int J Obes Relat Metab Disord 2000;24:639-46. [PubMed]
- Lauterio TJ, Bond JP, Ulman EA. Development and characterization of a purified diet to identify obesity-susceptible and resistant rat populations. J Nutr 1994;124:2172-8. [PubMed]
- Gorski JN, Dunn-Meynell AA, Levin BE. Maternal obesity increases hypothalamic leptin receptor expression and sensitivity in juvenile obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 2007;292:R1782-91. [PubMed]
- Levin BE, Dunn-Meynell AA, Balkan B, et al. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol 1997;273:R725-30. [PubMed]
- Levin BE, Dunn-Meynell AA, Banks WA. Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling before obesity onset. Am J Physiol Regul Integr Comp Physiol 2004;286:R143-50. [PubMed]
- Koza RA, Nikonova L, Hogan J, et al. Changes in gene expression foreshadow diet-induced obesity in genetically identical mice. PLoS Genet 2006;2:e81. [PubMed]
- Koza RA, Rogers P, Kozak LP. Inter-individual variation of dietary fat-induced mesoderm specific transcript in adipose tissue within inbred mice is not caused by altered promoter methylation. Epigenetics 2009;4:512-8. [PubMed]
- Huang XF, Yu Y, Li Y, et al. Ventromedial hypothalamic NPY Y2 receptor in the maintenance of body weight in diet-induced obesity in mice. Neurochem Res 2008;33:1881-8. [PubMed]
- Fujisawa T, Endo H, Tomimoto A, et al. Adiponectin suppresses colorectal carcinogenesis under the high-fat diet condition. Gut 2008;57:1531-8. [PubMed]
- Yu Y, Deng C, Huang XF. Obese reversal by a chronic energy restricted diet leaves an increased Arc NPY/AgRP, but no alteration in POMC/CART, mRNA expression in diet-induced obese mice. Behav Brain Res 2009;205:50-6. [PubMed]
- Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer 2008;8:387-98. [PubMed]
- Krausova M, Korinek V. Wnt signaling in adult intestinal stem cells and cancer. Cell Signal 2014;26:570-9. [PubMed]
- Chen J. Signaling pathways in HPV-associated cancers and therapeutic implications. Rev Med Virol 2015;25 Suppl 1:24-53. [PubMed]


