
Y-chromosome loss is frequent in male renal tumors
Introduction
Aneuploidy is a hallmark of many cancer types. It promotes tumorigenesis through copy number gain of oncogenes or loss of tumor suppressor genes. Chromosomal loss is a common event in aneuploid tumors and may occur at any stage of tumorigenesis (1,2). As the likelihood of chromosomal loss relates inversely to chromosome size and gene density (as a determinant of risk for losing essential genes for cellular homeostasis), it is logical that Y-loss is among the more common structural genomic variations in tumors (1,2).
Earlier studies on the frequency of Y-loss in different cancer types yielded variable results, partly due to different analysis methods (3-5). For example, loss of the Y-chromosome has been reported in 62–68% of squamous cell carcinomas (6,7), 59–69% of gastric cancers (4,8), 33–36% of pancreatic neoplasms (9,10), 23–34% of bladder cancers (11-13), 0.6–3% of prostate cancers (14,15), as well as 3–10% of hematologic diseases (16,17). The tumorigenic potential of Y-loss has traditionally been questioned, and many authors suggest that Y-loss might be a phenotypically silent bystander event occurring during tumor development (18,19). Y-chromosome loss was even found in non-neoplastic tubular epithelium in end-stage kidney disease (20). More recently, however, a prospective study in elderly males has demonstrated Y-loss in peripheral blood cells to confer risk for non-hematologic cancer (21). In kidney tumors, Y-chromosome loss has been reported in clear cell (48%, n=75), papillary (92%, n=25), and chromophobe carcinoma (46%, n=13) as well as in oncocytomas (45%, n=9) (22-25), but the size of the cohorts were not large enough to thoroughly investigate the clinical role of this chromosomal loss in different kidney tumor subtypes.
In this study, 1,252 male kidney tumors of all subtypes were analysed to evaluate a possible association between Y-chromosome loss and clinical outcome. We present the following article in accordance with the REMARK reporting checklist (available at http://dx.doi.org/10.21037/atm-20-3061).
Methods
A tissue microarray (TMA) containing one 0.6 mm tissue core each from a total of 1,805 kidney tumors was constructed in 2016 and used for this study. Only the 1,252 male samples were used for further analyses. The TMA was made from consecutive tissue samples of patients who underwent surgery between 1994 and 2016, and their tumors were histopathologically evaluated according to the WHO classification criteria of 2015 by two pathologists with a special focus on urogenital pathology (FB, CF) at the Institute of Pathology of the University Medical Center Hamburg-Eppendorf. WHO/ISUP, Fuhrman and Thoenes grading was performed for each tumor (26-28). Only tissue samples with sufficient amounts of cancer making it suitable for TMA construction were included. The TMA consists of four blocks, one of which had been constructed earlier (29). TMA manufacturing has been described in detail elsewhere (30). Hematoxylin- and eosin-stained TMA slides were inspected for presence or absence of renal neoplasm. Clinical and pathological parameters of the arrayed tumors are summarized in Table S1.
The manufacturing of tissue microarrays from left-over routine diagnostic material and its usage for research purposes is in accordance with local laws (HmbKHG, §12a) and was approved by the local ethic committee (Ärztekammer Hamburg no. WF-049/09). Written informed consent was not obtained from the patients. All work was carried out in accordance with the Helsinki Declaration (as revised in 2013).
Fluorescence in-situ hybridization (FISH)
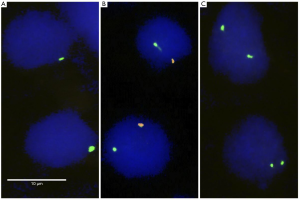
Freshly cut 4 µm sections of the TMA were prepared for FISH. A commercially available kit (paraffin pretreatment reagent set; Abbott, Chicago, USA) was used for proteolytic pretreatment of the slides. TMA sections were deparaffinized, air dried and dehydrated in an ascending series of ethanol (70%, 85% and 100%), followed by a 5-minute denaturation step in 70% formamid 2x SSC solution at 74 °C. The commercial AneuVYSION® FISH probe (Abbott, #05J38-010) was used for Y chromosome copy number analysis. The kit includes probes against the Y-chromosome (spectrum orange) and the X chromosome (spectrum green). The slides were hybridized overnight in a humidified chamber at 37 °C, washed, and counterstained with 0.2 µmol/L 4'-6-diamidino-2-phenylindole in anti-fade solution. Each tissue spot was evaluated by visual inspection of the red and green fluorescence signals under an epifluorescence microscope. Deletion of the Y-chromosome was assumed when the orange Y-chromosome signal was absent in ≥90% of tumor cells while the X-chromosome signal was retained. Adjancent non-neoplastic tissue served as the internal control for the hybridization quality. Tumors lacking the orange Y signal in less than 90% of the tumor cells were considered as normal because it was assumed that such incomplete signal loss could be attributed to technical factors. Many cell nuclei are not fully represented in 4µm thick tissue sections, resulting in a predictable loss of FISH signals in a fraction of these cells. Representative FISH images are present in Figure 1.
Statistical analysis
JMP 12.0 software (SAS Institute Inc., NC, USA) was used. Contingency tables and chi-square (likelihood) tests were employed to study the relationship between Y-loss, histological tumor type and tumor grade. Log-rank testing and Kaplan-Meier plots were performed to study the impact of histological and molecular parameters on patient outcome using recurrence-free survival as an endpoint.
Results
A total of 1,045 (83.5%) of 1,252 cases were evaluable for both centromere probes (X and Y). 207 tumors were not informative because of missing tissue spots, absence of tumor cells in the tissue spot, or insufficient hybridization quality.
Y-loss in renal tumor subtypes
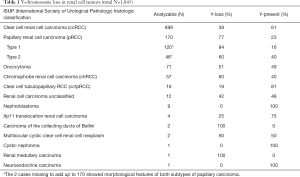
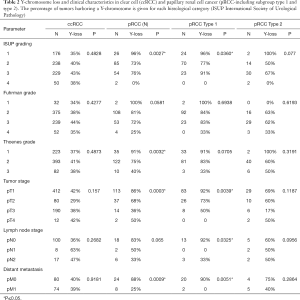
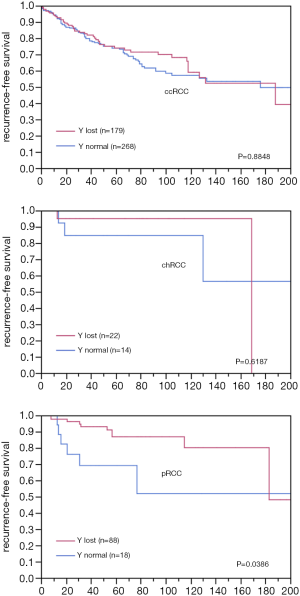
Chromosome Y-loss was always unequivocal and typically observed in virtually all tumor cells of a TMA spot. A Y-chromosome loss was seen in 496 of the 1,045 analyzable male tumors (47%). The frequency of Y-chromosome losses depended on the histologic tumor type (Table 1). Among the major tumor types, Y-loss was most frequent in papillary carcinoma (77%) and least common in clear cell carcinoma (39%). It was higher in type 1 papillary carcinomas (84%) as compared to type 2 carcinomas (60%). Comparison with tumor phenotype did not reveal significant associations for clear cell (Table 2) and chromophobe carcinoma (data not shown), but one was seen for papillary carcinoma (Table 2). It is of note that in papillary RCC, only lower tumor stages showed an increased Y-loss rate. Accordingly, Y-loss was unrelated to recurrence-free survival in clear cell and chromophobe carcinomas (Figure 2). There was, however, a Y-loss related difference in patient outcome in papillary carcinomas (P=0.0386). Here, 88 patients with Y-loss cancer had significantly less disease recurrences than 18 patients whose tumors had retained the Y-chromosome (Figure S1). However, subgroup analyses showed no significant difference between type 1 and type 2 papillary carcinoma. In addition, multivariate analysis including tumor grade (ISUP/WHO), tumor stage (pT), and Y-chromosome status shows that Y-loss has no independent prognostic significance for clinical outcome (Table S2).
Full table
Full table
Association of Y-loss with tumor phenotype
Y-chromosome loss was related to higher patient age at diagnosis (without Y-loss: mean age 59 (95% CI: 58–61) years, with Y-loss: mean age 65 (64–66 years, P<0.0001). This association held true in the subgroup of 699 clear cell carcinomas but not in 170 papillary carcinomas or 57 chromophobe carcinomas (Table S3). To better understand the impact of patient age on the associations between Y-loss and tumor agressiveness in papillary RCC, we performed additional subset analyses in patients aged <50 years, 51–70 years, and >70 years. It showed that the impact of Y-loss was more pronounced in younger patients. Significant associations with ISUP, Fuhrman grade, Thoenes grade, pT, hematologic metastases and patient prognosis were most prevalent in the subset of patients aged less than 50 years but became less evident in elderly patients. All data are summarized in Table S4 and Figure S2. Tumor size was inversely related to Y-loss. Y-loss was associated with smaller tumor size in all cancers (n=1,028; P=0.0052) and in clear cell RCC (n=688; P=0.0075), but not in papillary (n=166; P=0.0809) or chromophobe tumors (Table S5).
Discussion
The data from our study identify kidney cancer as a tumor type with a high frequency of Y-loss (47%). Using similar TMAs containing one tissue sample per patient, we had earlier identified markedly lower frequencies of Y-loss in carcinomas of the urinary bladder (22%) (12) and the prostate (0.6%) (14). In these tumor types, presence or absence of Y-loss was largely unrelated to tumor phenotype and patient outcome. The frequency of Y-loss appears to be related to the renal tumor subtype rather than to the aggressiveness of the tumor. This thesis is supported by the striking frequency differences between the different types of renal tumors and the high incidence of Y-losses in oncocytomas (36 out of 71 oncocytoma with Y-loss, 51%), which are benign neoplasms.
It was not unexpected that Y-loss was most frequently observed in papillary cell carcinoma, as Y-loss was identified early as a hallmark of this tumor type along with trisomy 7 and 17 (3). However, chromophobe carcinomas (60% Y-loss), oncocytomas (51%) and clear cell carcinomas (39%) also had higher Y-loss rates than any other tumor type previously analysed with identical methods. This shows that renal epithelial tissue is unusually susceptible to the loss of its Y-chromosome and demonstrates that Y-chromosome analysis cannot be a suitable tool for subtyping renal tumors. Indeed, Y-loss was already described earlier in non-tumorous renal tubule epithelium (20,31).
Y-chromosome loss was associated with a more favorable disease outcome in papillary carcinomas, which is of potential interest due to the strong association of Y-loss with this particular kidney cancer subtype. To date, no tumor type has been described in a study cohort of comparable size that had a higher Y-loss rate than papillary renal cell carcinoma. In addition, there are only a few tumor types with recurrent molecular alteration that occur with such a frequency (>70%). Therefore, it is possible that Y-loss plays a pathogenetic role in papillary renal cancer, and that these few non-Y-loss papillary carcinomas are a distinct disease entity with increased biological aggressiveness. In contrast, Klatte et al. reported an improved progression-free survival in metastatic clear cell renal cell cancer with Y-loss in univariate analysis (32). It is unclear what caused these differences, but there is evidence that the effects of chromosome Y-loss on tumor biology may differ substantially in different cancer types. For example, Y-loss was linked to better prognosis in chronic myelomonocytic leukemia (33) and to worse prognosis in head & neck cancer (34) and multiple myeloma (35), but had no effect on the prognosis of prostate (14) and bladder cancer (12). Furthermore, Y-loss in the germline of males has been associated with lung cancer risk (36), Alzheimer disease (37) and a generally increased carcinogenesis (38).
The significant association of Y-loss with older patient age fits well with previous data suggesting that Y-chromosome loss is an age-related phenomenon. The link between age and Y-chromosome loss has already been reported in some hematological disorders (16), bladder cancer (11) and clear cell renal cell cancer (32). Y-loss has been found in normal tissues including hematological, renal or urothelial cells (11,16,18,20,31). In a study examining the Y chromosome in neoplastic and healthy bone marrow, an increasing incidence of Y-loss was observed with increasing age, suggesting that the loss of the Y-chromosome is age-related and not diagnostic (20). That the prognostic impact of Y-loss was lost in older patients in our study further argues for a decreasing role of Y-loss in tumor biology with increasing age. The mechanism of action of Y-chromosome loss is not known. However, it has been speculated that removal of the Y-chromosome, which may not be required for some adult tissues, may be beneficial for healthy cells as it reduces the amount of DNA that needs to be doubled during cell division. A subsequent neoplastic development from healthy older cells that have lost their Y-chromosome would also be compatible with the observation of Y-chromosome losses were found in high- and low-grade dysplasia and even in intestinal metaplasia next to esophageal cancer (5).
A limitation of this study is that only one 0.6 mm TMA spot per individual was analyzed. It cannot be excluded that the fraction of tumors harboring Y-loss has been underestimated due to intratumoral heterogeneity, which is a frequent feature of renal cell tumors (39). However, the aim of this study was to find associations between a molecular feature (Y-loss) and renal tumor phenotype. A multitude of studies comparing single-spot TMA data with clinical or molecular features have successfully reproduced all previously established associations between molecular parameters and other features in renal cell tumors (29,40,41) and other tumor types (42-46).
In summary, our data demonstrate, that Y-loss is a highly common phenomenon in kidney neoplasm and that it may be linked to a more favorable patient outcome in papillary carcinoma. Although differences in Y-loss frequency exist between histologic subtypes, in multivariate analysis Y-loss status is not prognostically relevant for tumor classification.
Acknowledgments
We are grateful to Janett Lütgens, Sünje Seekamp, Inge Brandt, Navid Shadanpour, Clemens Bannenberg, Luca Simmendinger and Nina Peters for excellent technical assistance.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-3061
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-3061
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-3061). The authors have no conflicts of interest to declare.
Ethical Statement: The manufacturing of tissue microarrays from left-over routine diagnostic material and its usage for research purposes is in accordance with local laws (HmbKHG, §12a) and was approved by the local ethic committee (Ärztekammer Hamburg no. WF-049/09). Written informed consent was not obtained from the patients. All work was carried out in accordance with the Helsinki Declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Duijf PH, Benezra R. The cancer biology of whole-chromosome instability. Oncogene 2013;32:4727-36. [Crossref] [PubMed]
- Mertens F, Johansson B, Hoglund M, et al. Chromosomal imbalance maps of malignant solid tumors: a cytogenetic survey of 3185 neoplasms. Cancer Res 1997;57:2765-80. [PubMed]
- Brunelli M, Eble JN, Zhang S, et al. Metanephric adenoma lacks the gains of chromosomes 7 and 17 and loss of Y that are typical of papillary renal cell carcinoma and papillary adenoma. Mod Pathol 2003;16:1060-3. [Crossref] [PubMed]
- van Dekken H, Alers JC, Riegman PH, et al. Molecular cytogenetic evaluation of gastric cardia adenocarcinoma and precursor lesions. Am J Pathol 2001;158:1961-7. [Crossref] [PubMed]
- Walch AK, Zitzelsberger HF, Bruch J, et al. Chromosomal imbalances in Barrett's adenocarcinoma and the metaplasia-dysplasia-carcinoma sequence. Am J Pathol 2000;156:555-66. [Crossref] [PubMed]
- Hunter S, Gramlich T, Abbott K, et al. Y chromosome loss in esophageal carcinoma: an in situ hybridization study. Genes Chromosomes Cancer 1993;8:172-7. [Crossref] [PubMed]
- Worsham MJ, Benninger MJ, Zarbo RJ, et al. Deletion 9p22-pter and loss of Y as primary chromosome abnormalities in a squamous cell carcinoma of the vocal cord. Genes Chromosomes Cancer 1993;6:58-60. [Crossref] [PubMed]
- Beuzen F, Dubois S, Flejou JF. Chromosomal numerical aberrations are frequent in oesophageal and gastric adenocarcinomas: a study using in-situ hybridization. Histopathology 2000;37:241-9. [Crossref] [PubMed]
- Kowalski J, Morsberger LA, Blackford A, et al. Chromosomal abnormalities of adenocarcinoma of the pancreas: identifying early and late changes. Cancer Genet Cytogenet 2007;178:26-35. [Crossref] [PubMed]
- Missiaglia E, Moore PS, Williamson J, et al. Sex chromosome anomalies in pancreatic endocrine tumors. Int J Cancer 2002;98:532-8. [Crossref] [PubMed]
- Sauter G, Moch H, Wagner U, et al. Y chromosome loss detected by FISH in bladder cancer. Cancer Genet Cytogenet 1995;82:163-9. [Crossref] [PubMed]
- Minner S, Kilgue A, Stahl P, et al. Y chromosome loss is a frequent early event in urothelial bladder cancer. Pathology 2010;42:356-9. [Crossref] [PubMed]
- Panani AD, Roussos C. Sex chromosome abnormalities in bladder cancer: Y polysomies are linked to PT1-grade III transitional cell carcinoma. Anticancer Res 2006;26:319-23. [PubMed]
- Stahl PR, Kilgue A, Tennstedt P, et al. Y chromosome losses are exceedingly rare in prostate cancer and unrelated to patient age. Prostate 2012;72:898-903. [Crossref] [PubMed]
- Baretton GB, Valina C, Vogt T, et al. Interphase cytogenetic analysis of prostatic carcinomas by use of nonisotopic in situ hybridization. Cancer Res 1994;54:4472-80. [PubMed]
- Herens C, Brasseur E, Jamar M, et al. Loss of the Y chromosome in bone marrow cells: results on 1907 consecutive cases of leukaemia and preleukaemia. Clin Lab Haematol 1999;21:17-20. [Crossref] [PubMed]
- Zhang LJ, Shin ES, Yu ZX, et al. Molecular genetic evidence of Y chromosome loss in male patients with hematological disorders. Chin Med J (Engl) 2007;120:2002-5. [Crossref] [PubMed]
- Guttenbach M, Koschorz B, Bernthaler U, et al. Sex chromosome loss and aging: in situ hybridization studies on human interphase nuclei. Am J Hum Genet 1995;57:1143-50. [PubMed]
- Wong AK, Fang B, Zhang L, et al. Loss of the Y chromosome: an age-related or clonal phenomenon in acute myelogenous leukemia/myelodysplastic syndrome? Arch Pathol Lab Med 2008;132:1329-32. [PubMed]
- Hes O, Sima R, Nemcova J, et al. End-stage kidney disease: gains of chromosomes 7 and 17 and loss of Y chromosome in non-neoplastic tissue. Virchows Arch 2008;453:313-9. [Crossref] [PubMed]
- Forsberg LA, Rasi C, Malmqvist N, et al. Mosaic loss of chromosome Y in peripheral blood is associated with shorter survival and higher risk of cancer. Nat Genet 2014;46:624-8. [Crossref] [PubMed]
- Kardas I, Mrozek K, Babinska M, et al. Cytogenetic and molecular findings in 75 clear cell renal cell carcinomas. Oncol Rep 2005;13:949-56. [Crossref] [PubMed]
- Kovacs G, Fuzesi L, Emanual A, et al. Cytogenetics of papillary renal cell tumors. Genes Chromosomes Cancer 1991;3:249-55. [Crossref] [PubMed]
- Lindgren V, Paner GP, Omeroglu A, et al. Cytogenetic analysis of a series of 13 renal oncocytomas. J Urol 2004;171:602-4. [Crossref] [PubMed]
- Speicher MR, Schoell B, du Manoir S, et al. Specific loss of chromosomes 1, 2, 6, 10, 13, 17, and 21 in chromophobe renal cell carcinomas revealed by comparative genomic hybridization. Am J Pathol 1994;145:356-64. [PubMed]
- Srigley JR, Delahunt B, Eble JN, et al. The International Society of Urological Pathology (ISUP) Vancouver Classification of Renal Neoplasia. Am J Surg Pathol 2013;37:1469-89. [Crossref] [PubMed]
- Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol 1982;6:655-63. [Crossref] [PubMed]
- Thoenes W, Storkel S, Rumpelt HJ. Histopathology and classification of renal cell tumors (adenomas, oncocytomas and carcinomas). The basic cytological and histopathological elements and their use for diagnostics. Pathol Res Pract 1986;181:125-43. [Crossref] [PubMed]
- Quaas A, Rahvar AH, Burdelski C, et al. betaIII-tubulin overexpression is linked to aggressive tumor features and shortened survival in clear cell renal cell carcinoma. World J Urol 2015;33:1561-9. [Crossref] [PubMed]
- Simon R, Mirlacher M, Sauter G. Immunohistochemical analysis of tissue microarrays. Methods Mol Biol 2010;664:113-26. [Crossref] [PubMed]
- van den Berg E, Dijkhuizen T, Storkel S, et al. Chromosomal abnormalities in non-neoplastic renal tissue. Cancer Genet Cytogenet 1995;85:152-4. [Crossref] [PubMed]
- Klatte T, Rao PN, de Martino M, et al. Cytogenetic profile predicts prognosis of patients with clear cell renal cell carcinoma. J Clin Oncol 2009;27:746-53. [Crossref] [PubMed]
- Nomdedeu M, Pereira A, Calvo X, et al. Clinical and biological significance of isolated Y chromosome loss in myelodysplastic syndromes and chronic myelomonocytic leukemia. A report from the Spanish MDS Group. Leuk Res 2017;63:85-9. [Crossref] [PubMed]
- Hollows R, Wei W, Cazier JB, et al. Association between loss of Y chromosome and poor prognosis in male head and neck squamous cell carcinoma. Head Neck 2019;41:993-1006. [Crossref] [PubMed]
- Shin SY, Eom HS, Sohn JY, et al. Prognostic Implications of Monosomies in Patients With Multiple Myeloma. Clin Lymphoma Myeloma Leuk 2017;17:159-64.e2. [Crossref] [PubMed]
- Qin N, Li N, Wang C, et al. Association of Mosaic Loss of Chromosome Y with Lung Cancer Risk and Prognosis in a Chinese Population. J Thorac Oncol 2019;14:37-44. [Crossref] [PubMed]
- Dumanski JP, Lambert JC, Rasi C, et al. Mosaic Loss of Chromosome Y in Blood Is Associated with Alzheimer Disease. Am J Hum Genet 2016;98:1208-19. [Crossref] [PubMed]
- Noveski P, Madjunkova S, Sukarova Stefanovska E, et al. Loss of Y Chromosome in Peripheral Blood of Colorectal and Prostate Cancer Patients. PLoS One 2016;11:e0146264. [Crossref] [PubMed]
- Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012;366:883-92. [Crossref] [PubMed]
- Eichelberg C, Chun FK, Bedke J, et al. Epithelial cell adhesion molecule is an independent prognostic marker in clear cell renal carcinoma. Int J Cancer 2013;132:2948-55. [Crossref] [PubMed]
- Moch H, Schraml P, Bubendorf L, et al. High-throughput tissue microarray analysis to evaluate genes uncovered by cDNA microarray screening in renal cell carcinoma. Am J Pathol 1999;154:981-6. [Crossref] [PubMed]
- Torhorst J, Bucher C, Kononen J, et al. Tissue microarrays for rapid linking of molecular changes to clinical endpoints. Am J Pathol 2001;159:2249-56. [Crossref] [PubMed]
- Al-Kuraya K, Schraml P, Torhorst J, et al. Prognostic relevance of gene amplifications and coamplifications in breast cancer. Cancer Res 2004;64:8534-40. [Crossref] [PubMed]
- Ruiz C, Seibt S, Al Kuraya K, et al. Tissue microarrays for comparing molecular features with proliferation activity in breast cancer. Int J Cancer 2006;118:2190-4. [Crossref] [PubMed]
- Schlomm T, Iwers L, Kirstein P, et al. Clinical significance of p53 alterations in surgically treated prostate cancers. Mod Pathol 2008;21:1371-8. [Crossref] [PubMed]
- Hinsch A, Chaker A, Burdelski C, et al. betaIII-tubulin overexpression is linked to aggressive tumor features and genetic instability in urinary bladder cancer. Hum Pathol 2017;61:210-20. [Crossref] [PubMed]


