
Systemic inflammation is associated with inferior disease control and survival in stage III non-small cell lung cancer
Introduction
Worldwide, lung cancer is the leading cause of cancer-related deaths, with non-small cell lung cancer (NSCLC) accounting for 87% of all lung cancers (1). Up to 25% of patients have regional metastasis (stage III) and up to 55% have distant metastatic disease at the time of diagnosis (2). Treatment of locally advanced NSCLC remains controversial but generally consists of a combination of radiation, surgery, and chemotherapy depending on patients’ stage and performance status. Recently, the addition of consolidative immunotherapy has been associated with improved outcomes in patients undergoing definitive chemoradiation (3).
Inflammation is an essential constituent of the tumor milieu and plays a substantial role in the development and progression of malignancies. The recent addition of immunotherapy in NSCLC treatment has evoked further inquiry into the dynamic relationship between the immune system and lung cancer (4,5). The systemic immune-inflammation index (SII)—calculated as platelet count × neutrophil/lymphocyte count—is a quantifiable factor hypothesized to describe the interplay of the immune system and tumor (6).
At the time of diagnosis, SII appears to have a prognostic value in multiple solid malignancies (7). Elevated SII values at diagnosis are associated with worse overall survival (OS) and progression-free survival (PFS) in patients with either localized or locally advanced NSCLC. Values of SII that confer prognostic significance for NSCLC have ranged from 521 to 1,270 in single institution retrospective studies (5,7-13). Limited data do not clarify which patient factors contribute to an elevated SII; however, hypothesized factors include NSAID use, corticosteroid use, and comorbid disease burden (14).
Given the lack of consensus on a prognostic cut-off of SII for NSCLC and limited evidence of factors that contribute to an elevated SII, we present our analysis to validate previously identified SII cut offs that have prognostic implications in survival and outcomes and identify factors potentially influencing SII values through a retrospective analysis of locally advanced NSCLC patients treated with definitive chemoradiation therapy (CRT) at our institution. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-6710).
Methods
Patient selection
We reviewed the medical records of 134 consecutive patients managed at our institution with stage III NSCLC who underwent first-line definitive CRT with curative intent between January 1st, 2010 and December 31st, 2019. The study population included adults (age ≥18 years) with a histological or cytological diagnosis of NSCLC. Patients were included if they were prescribed ≥5,400 cGy in conventional fractions (180–200 cGy). Patients were included if they underwent concurrent chemotherapy with or without consolidative immunotherapy. EGFR status was not determined due to lack of data. Patients who received surgical resection as a component of multimodality therapy were excluded from the study. Patients were excluded if they were found to have distant metastatic disease or Stage I-II disease regardless of treatment modality. SII was calculated as platelet count x neutrophil/lymphocyte count on the complete blood count (CBC) with differential available closest to the date of tissue diagnosis (6). Nine patients were excluded due to missing laboratory data to assess SII within 30 days of diagnosis and prior to treatment initiation leaving 125 evaluable patients.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The institutional Review Board at our institution approved this study (IRB #398-17-EP), and individual consent for this retrospective analysis was waived. Data were collected from electronic medical records; patients’ personal data have been secured.
Work-up and management
CT and PET-CT imaging were reviewed to assess extent of disease, size of disease involvement, and clinical stage. Based on diagnostic imaging, all staging was updated to AJCC 8th edition. The extent of mediastinal assessment was at the discretion of the managing physicians. All patients underwent a PET-CT as part of their work-up. Radiotherapy and chemotherapy were administered at the discretion of the managing physicians. Follow-up was performed at the discretion of the managing physicians but commonly included CT imaging every 3–6 months with brain MRIs at the time of symptoms development or identification of new metastatic disease.
Statistical analysis
The date of relapse was defined as the first observation documented on either clinical exam or imaging. OS was defined as the time from the start of CRT to death. Disease progression was defined according to the RECIST 1.0 criteria (15). PFS was defined as the time from the start of CRT to progression or death. Freedom from recurrence (FFR), freedom from locoregional recurrence (FFLRR), and freedom from distant recurrence (FFDR) were defined as the time from the start of CRT to any relapse or progression, locoregional progression, or metastatic relapse, respectively. Patients without tumor progression or death at data collection time were censored at their last date of evaluation. The associations of SII and OS, disease-specific survival (DSS), and PFS were examined with the log-rank test.
Clinic-pathological factors potentially correlated with patients’ prognoses were estimated. The list of evaluated factors included gender (male vs. female), age (<70 vs. ≥70 years), Eastern Cooperative Oncology Group Performance Status (ECOG PS) (0–1 vs. 2–3), smoking status (never smoker vs. history of tobacco use), Charlson comorbidity index (<6 vs. ≥6), race (Caucasian vs. non-Caucasian), SII (low vs. high), BMI (<30 vs. ≥30), use of supplemental oxygen (yes vs. no), weight loss ≥10% in preceding 6 months (yes vs. no), prescription for statin use (yes vs. no), prescription for NSAID use (yes vs. no), COPD exacerbation in preceding 6 months (yes vs. no), AJCC 8th edition group stage (IIIA vs. IIIB-C), tumor histology (adenocarcinoma vs. non-adenocarcinoma), concurrent chemotherapy type (Cisplatin and Etoposide vs. other).
Univariate and multivariate Cox regression analyses of potential factors affecting patients’ outcomes were performed. Multicollinearity was assessed by the variance inflation factor method. No multi-collinearity was appreciated between included factors. Significance level at the univariate model for inclusion in the multivariate model in the Cox regression analysis was set at 0.2 (16,17). The parallel-hazard assumption was satisfied for each included factor as assessed on Kaplan-Meier plots. All other significance levels were set at 0.05 and all P values were two-sided. Statistical analysis was performed using SPSS software, version 26 (IBM Corp., Armonk, NY, USA).
Results
From 2010 to 2019, 134 patients at our institution underwent definitive CRT. Of this population, 125 had records sufficient and were included in analysis. The median age was 67 years (range: 45–86 years), 20.8% had ECOG performance status ≥2, 55.2% had Charlson comorbidity index ≥6, 29.6% had FEV1 <60%, 11.2% with a DLCO <40%, and only 4.8% did not have a history of tobacco use while 28% of patients were active smokers throughout their treatment (Table 1). The median SII measured at diagnosis was 1,105.0 (range: 234.1–14,153.2). A large proportion of patients presented with locally advanced disease with 28.8% presenting with T4 disease, 59.2% presenting with ≥2 involved mediastinal nodal stations, and 33.6% presented with N3 nodal disease. Definitive radiotherapy was given to a median dose of 6,000 cGy (range: 5,400–7,040 cGy) with concurrent chemotherapy. The median interval from diagnosis to radiotherapy completion was 80 days (range: 41–210 days). A lower ECOG performance status (P=0.009) and no significant weight loss (P=0.002) were associated with the lower SII group. This clinicodemographic data existed for all patients included.
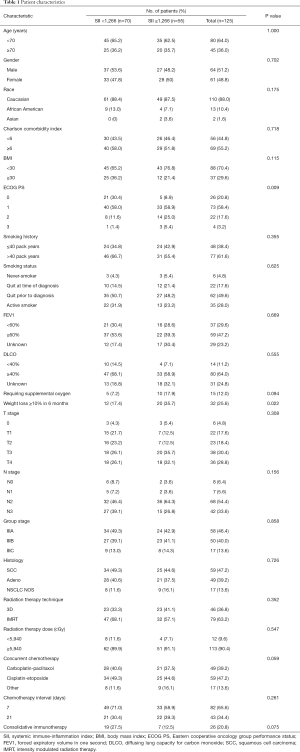
Full table
At a median follow-up of 12.2 months for all patients and 19.7 months for patients alive at last follow-up, we identified a total of 65 patients with recurrent disease (52%) and 83 deaths (66%). The median OS was 20.7 months, with OS rates at 3 and 5 years being 33.3% and 22.6%, respectively. The median DSS was 22.6 months, with DSS rates at 3 and 5 years being 42.3% and 33.3%, respectively. The median PFS was 11.9 months, with PFS rates at 3 and 5 years being 21.8% and 13.4% respectively. Of patients identified to have recurrent disease, the first site of recurrence was locoregional only in 23 patients (35.4%), locoregional and distant in 23 patients (35.4%) and distant only in 19 patients (29.2%). The median FFR, FFLRR, and FFDR was 15.4, 20.3, and 30.5 months, respectively.
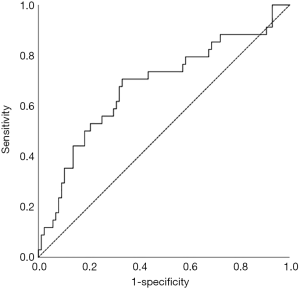
We examined SII as a prognostic indicator of OS in this patient population. The optimal cut-off point of 1,266 was determined using a receiver operating characteristic (ROC) curve with five-year OS as the endpoint (Figure 1). The area under the curve (AUC) for OS was 0.653 (95% CI: 0.540–0.765, P=0.008). We divided all patients into high-level group and low-level group based on the SII cut-off value of 1,266. As shown in Table 1, 70 patients (56.0%) had SII <1,266 and, and 55 patients (44.0%) had SII ≥1,266.
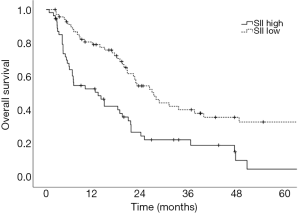
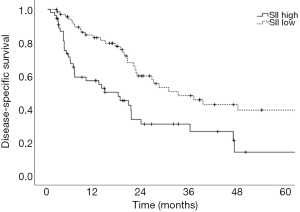
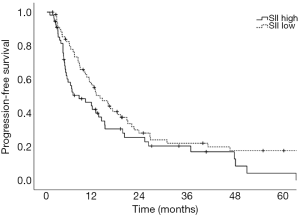
Prognostic factors for OS, DSS, and PFS were identified (Table 2). SII was identified as one such prognostic factor in both univariate (Table 3) and multivariate analyses (Table 4). Patients who presented with a low SII (<1,266) had an improved OS when compared with patients with a high SII (≥1,266), with median OS of 27.2 months versus 13.1 months, respectively (unadjusted HR: 0.414, 95% CI: 0.267–0.643, P<0.001) (see Figure 2). The five-year OS rate was 33.8% in patients with a low SII and 5.8% in patients with a high SII (P<0.001). A low SII was associated with an improved DSS rate with a median DSS of 33.4 months compared to 18.1 months for patients with a high SII (HR: 0.383, 95% CI: 0.228–0.645, P<0.001) (see Figure 3). The five-year DSS rates of patients with a low versus high SII was 40.7% versus 16.8% (P=0.004). A low SII was also associated with an improved PFS rate with a median PFS of 13.4 months compared to 8.1 months for patients with a high SII (unadjusted HR: 0.669, 95% CI: 0.446–1.004, P=0.052) which was not statistically significant (see Figure 4). The 5-year PFS rates of patients with a low versus high SII was 18.4% versus 4.7% (P=0.029). There was no significant associated between a low SII and FFR, (unadjusted HR: 0.987, 95% CI: 0.591–1.648, P=0.959) FFLRR (unadjusted HR: 0.895, 95% CI: 0.509–1.575, P=0.702), and FFDR (unadjusted HR: 0.875, 95% CI: 0.47–1.628, P=0.673).
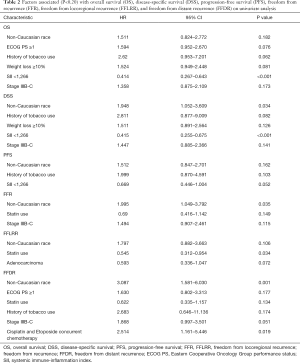
Full table
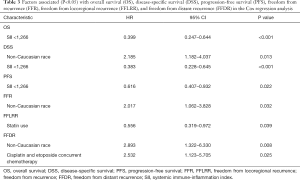
Full table
Full table
On multivariate analysis, a low SII was associated with an improved OS (HR: 0.399, 95%, CI: 0.247–0.644, P<0.001), DSS (HR: 0.383, 95%, CI: 0.228–0.645, P<0.001), and PFS (HR: 0.616, 95%, CI: 0.407–0.932, P=0.022). Statin usage was independently associated with an improved FFLRR (HR: 0.556, 95%, CI: 0.319–0.972, P=0.039). Non-Caucasian race was independently associated with a worse DSS (HR: 2.185, 95%, CI: 1.182–4.037, P=0.013), FFR (HR: 2.017, 95%, CI: 1.062–3.828, P=0.032), and FFDR (HR: 2.893, 95%, CI: 1.322–6.330, P=0.008). Cisplatin and Etoposide concurrent chemotherapy was associated with a worse FFDR (HR: 2.532, 95% CI: 1.123–5.705, P=0.025).
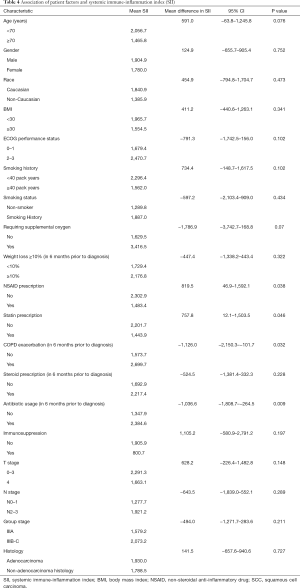
We examined the association of patient factors and SII (see Table 4). A lower SII was associated with a prescription for NSAIDs (2,302.9 vs. 1,483.4, 95% CI of mean difference: 46.9–1,592.1, P=0.038) and statins (2,201.7 vs. 1,443.9, 95% CI of mean difference: 12.1–1,503.5, P=0.046). A higher SII was associated with a COPD exacerbation within six months prior to diagnosis (1,573.7 vs. 2,699.7, 95% CI of mean difference: −2,150.25–101.655, P=0.032) and antibiotic usage within six months prior to diagnosis (1,347.9 vs. 2,384.6, 95% CI of mean difference: −1,808.695–264.53, P=0.009). We identified a non-statistically significant trend toward higher SII in patients age <70 (2,056.7 vs. 1,465.8, 95% CI of mean difference: −63.843–1,245.782, P=0.076), patients with an ECOG performance status 2–3 (1,679.4 vs. 2,470.7, 95% CI of mean difference: −1,742.5–159.9, P=0.102), ≥40 pack year smoking history (2,296.4 vs. 1,562.0, 95% CI of mean difference: −148.7–1,617.5, P=0.102), and supplemental oxygen requirement (1,629.5 vs. 3,416.5, 95% CI of mean difference: −3,742.7–168.8, P=0.07).
The prognostic implication of treatment factors following initiation of CRT including completed RT dose (<5,940 vs. ≥5,940 cGy) or use of consolidative immunotherapy were assessed on a subset of 114 patients (91.2%) with an OS of at least three months. Undergoing radiation therapy to a dose ≥5,940 cGy was associated with a non-significantly improved median OS of 22.0 vs. 8.6 months (P=0.190), DSS of 26.7 vs. 8.6 months (P=0.062), and PFS of 13.4 vs. 7.0 months (P=0.041). Consolidative immunotherapy was associated with an improved median OS of 27.3 vs. 19.2 months (P=0.013), DSS not yet reached vs. 21.4 months (P=0.013), PFS of 19.2 vs. 11.5 months (P=0.091) which was not statistically significant. Receiving an RT dose ≥5,940 (1,643.3 vs. 1,797.1, P=0.830) or consolidative immunotherapy (1,402.9 vs. 1,996.3, P=0.488) was not associated with a lower SII.
Discussion
The present study demonstrates the potential importance of SII as a pre-treatment prognostic indicator for OS and PFS in patients undergoing definitive CRT for stage III NSCLC. We identified that an SII cut-off of 1,266 was the most prognostic implication for OS in our patient population. An SII <1,266 was associated with an improved OS, DSS, and PFS compared to an elevated SII ≥1,266. However, we did not identify a significant association between SII and recurrent disease. This could be a result of the present study lacking power to reliably detect differences in FFLRR and FFDR. While prior studies have shown the predictive possibilities of SII as a marker for OS and PFS in this setting and midway through treatment, these results are novel in that they demonstrate the potential utility of SII as a prognostic indicator in PFS, DSS, and OS (8,18).
We evaluated the association between individual patient factors and SII to better explain both the variance of SII among this population and the association between SII and survival. We identified a positive association between COPD exacerbation and antibiotic prescription in the six-month period prior to diagnosis to be associated with an elevated SII. Concordantly, statin and NSAID prescriptions were associated with a lower SII. Worse ECOG performance status, ≥40 pack year smoking history, younger age (<70), and supplemental oxygen use were also associated with higher SII values but were not statistically significant. Within our patient population, we were unable to demonstrate that an elevated SII contributed to more extensive disease spread as NSCLC factors including histology, tumor stage, nodal stage, or group stage were not associated with elevated SII values.
In 2011, Hanahan et al. presented inflammation as a hallmark of cancer, emphasizing the interplay between cancer and the immune system (4). More recently, studies have associated patient inflammatory statuses with worse cancer mortality (19). An elevated SII indicates the patient is experiencing relative thrombocytosis, neutrophilia, and/or lymphopenia—all of which are factors with known contributions to cancer development and metastasis. Thrombocytosis may be involved in tumor angiogenesis and immune evasion (20). Neutrophilia contributes to the spread of inflammatory factors that are beneficial to tumor cells including VEGF which stimulates angiogenesis, NFκB which protects tumor cells from apoptosis, and CXCL8 which is a chemokine with growth stimulating effects (21,22). Lymphopenia results in a dampened ability of the body to detect and destroy malignant cells (23). Thus, patients with elevated SII may have diminished abilities to identify and inhibit tumor progression.
The reported prognostic cut-off of SII for OS in patients with stage III NSCLC has varied in the literature from 521 to 1,270 (7-9). Heterogeneous populations, treatment paradigm, and patient selection factors might explain variance. Our findings provide external validation for the SII cut-off of 1,270 which Berardi et al. proposed to be independently associated with OS and PFS in a retrospective series of patients in Italy who underwent definitive treatment of stage III-IV NSCLC (8). Discordantly, retrospective series of patients managed in China with stage III NSCLC identified SII cut-offs of 521–660 to be of prognostic significance for OS (8,9). Cultural or racial differences might influence the cut-off for which SII employs prognostic value.
The three aforementioned groups identified SII to be prognostic of OS and PFS (7-9). However, there is limited evidence to suggest SII is predictive of treatment response, locoregional control, or distant control. It is notable that Tong et al. identified SII to be predictive of response to CRT (9). However, further description of this finding is lacking. Similarly, we did not find SII to be prognostic for extent of disease at diagnosis, locoregional control, or distant control. These findings may be consistent with the association of SII and patient mortality rather than disease control. Further investigation in a larger sample size is warranted.
Previous studies have hypothesized interaction between patient medications and infectious processes contributing to SII (14). We present the first evidence that NSAIDs and statins are associated with a lower SII. Many studies have found an associated decreased risk of cancer development with use of NSAIDs or statins (24,25). NSAIDs reduce inflammation through the inhibition of the enzyme cyclooxygenase (COX) which thereby prevents prostaglandin production (26). Additionally, they have been found to augment both cellular and humoral immunity through the inhibition of NFκB (27,28). Statins have been found to decrease levels of C-reactive protein, interleukin 6, serum amyloid A, and soluble intercellular adhesion molecule-1 (29,30). Further investigation is necessary to determine the therapeutic implications of NSAIDs and statins in improving outcomes through lowering SII.
Limitations of this study include its relatively small sample size and retrospective nature. As a retrospective study, it may lack sufficient power to detect meaningful associations. We were unable to control for selection bias in physician choice of treatment regiments. SII is a limited surrogate for patient inflammation as it was captured at a single time point. Lastly, the duration of treatment with NSAIDs and statins could not be determined and standardized. However, this adds to the increasing body of evidence correlating the role of systemic inflammation with lung cancer outcomes. Hence, notwithstanding the aforementioned limitations, these results warrant further investigation.
Conclusions
Here, we demonstrate that a low SII was associated with improved OS, DSS, and PFS rates in patients with stage III NSCLC undergoing definitive CRT. These findings serve as an external validation of the previously described findings of Berardi et al. with a similarly derived SII cut-off, however, we additionally found that this was not significant for recurrent disease suggesting that SII may be prognostic for patient mortality rather than disease progression or response to treatment (8). We identified that NSAIDs and statin prescriptions may be associated with lower SII at diagnosis of NSCLC. Further investigation of the therapeutic potential of these agents in patients with an elevated SII in this setting may be warranted.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-6710
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-6710
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-6710). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The institutional Review Board at our institution approved this study (IRB #398-17-EP), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Grapatsas K, Leivaditis V, Tsilogianni Z, et al. Epidemiology, risk factors, symptomatology, TNM classification of non small cell lung cancer. An overview while waiting the 8th TNM classification. Oncomedicine 2017;2:12-23. [Crossref]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Dolan RD, McSorley ST, Horgan PG, et al. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: Systematic review and meta-analysis. Crit Rev Oncol Hematol 2017;116:134-46. [Crossref] [PubMed]
- Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res 2014;20:6212-22. [Crossref] [PubMed]
- Yang R, Chang Q, Meng X, et al. Prognostic value of Systemic immune-inflammation index in cancer: A meta-analysis. J Cancer 2018;9:3295-302. [Crossref] [PubMed]
- Berardi R, Santoni M, Rinaldi S, et al. Pre-treatment systemic immune-inflammation represents a prognostic factor in patients with advanced non-small cell lung cancer. Ann Transl Med 2019;7:572. [Crossref] [PubMed]
- Tong YS, Tan J, Zhou XL, et al. Systemic immune-inflammation index predicting chemoradiation resistance and poor outcome in patients with stage III non-small cell lung cancer. J Transl Med 2017;15:221-017-1326-1.
- Guo D, Zhang J, Jing W, et al. Prognostic value of systemic immune-inflammation index in patients with advanced non-small-cell lung cancer. Future Oncol 2018;14:2643-50. [Crossref] [PubMed]
- Kang J, Ning MS, Feng H, et al. Predicting 5-Year Progression and Survival Outcomes for Early Stage Non-small Cell Lung Cancer Treated with Stereotactic Ablative Radiation Therapy: Development and Validation of Robust Prognostic Nomograms. Int J Radiat Oncol Biol Phys 2020;106:90-9. [Crossref] [PubMed]
- Liu J, Li S, Zhang S, et al. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J Clin Lab Anal 2019;33:e22964. [Crossref] [PubMed]
- Guo W, Cai S, Zhang F, et al. Systemic immune-inflammation index (SII) is useful to predict survival outcomes in patients with surgically resected non-small cell lung cancer. Thorac Cancer 2019;10:761-8. [Crossref] [PubMed]
- Tomita M, Ayabe T, Maeda R, et al. Systemic Immune-inflammation Index Predicts Survival of Patients After Curative Resection for Non-small Cell Lung Cancer. In Vivo 2018;32:663-7. [PubMed]
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. [Crossref] [PubMed]
- Fagerland MW, Hosmer DW, Bofin AM. Multinomial goodness-of-fit tests for logistic regression models. Stat Med 2008;27:4238-53. [Crossref] [PubMed]
- Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol 1989;129:125-37. [Crossref] [PubMed]
- Biswas T, Kang KH, Gawdi R, et al. Using the Systemic Immune-Inflammation Index (SII) as a Mid-Treatment Marker for Survival among Patients with Stage-III Locally Advanced Non-Small Cell Lung Cancer (NSCLC). Int J Environ Res Public Health 2020;17:E7995. [Crossref] [PubMed]
- England BR, Sokolove J, Robinson WH, et al. Associations of Circulating Cytokines and Chemokines With Cancer Mortality in Men With Rheumatoid Arthritis. Arthritis Rheumatol 2016;68:2394-402. [Crossref] [PubMed]
- Metelli A, Wu BX, Riesenberg B, et al. Thrombin contributes to cancer immune evasion via proteolysis of platelet-bound GARP to activate LTGF-β. Sci Transl Med 2020;12:eaay4860. [Crossref] [PubMed]
- Atretkhany KN, Drutskaya MS, Nedospasov SA, et al. Chemokines, cytokines and exosomes help tumors to shape inflammatory microenvironment. Pharmacol Ther 2016;168:98-112. [Crossref] [PubMed]
- Zhu YM, Webster SJ, Flower D, et al. Interleukin-8/CXCL8 is a growth factor for human lung cancer cells. Br J Cancer 2004;91:1970-6. [Crossref] [PubMed]
- Shankaran V, Ikeda H, Bruce AT, et al. Pillars Article: IFNγ and Lymphocytes Prevent Primary Tumour Development and Shape Tumour Immunogenicity. Nature. 2001. 410: 1107-1111. J Immunol 2018;201:827-31. [PubMed]
- Wong RSY. Role of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) in Cancer Prevention and Cancer Promotion. Adv Pharmacol Sci 2019;2019:3418975. [Crossref] [PubMed]
- Xiao H, Yang CS. Combination regimen with statins and NSAIDs: a promising strategy for cancer chemoprevention. Int J Cancer 2008;123:983-90. [Crossref] [PubMed]
- Gilroy DW, Colville-Nash PR, Willis D, et al. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med 1999;5:698-701. [Crossref] [PubMed]
- Tanaka K, Tanaka H, Kanemoto Y, et al. The effects of nonsteroidal anti-inflammatory drugs on immune functions of human peripheral blood mononuclear cells. Immunopharmacology 1998;40:209-17. [Crossref] [PubMed]
- Cavallini L, Francesconi MA, Zoccarato F, et al. Involvement of nuclear factor-kappa B (NF-kappaB) activation in mitogen-induced lymphocyte proliferation: inhibitory effects of lymphoproliferation by salicylates acting as NF-kappaB inhibitors. Biochem Pharmacol 2001;62:141-7. [Crossref] [PubMed]
- Albert MA, Danielson E, Rifai N, et al. PRINCE Investigators. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA 2001;286:64-70. [Crossref] [PubMed]
- Weber C, Erl W, Weber KS, et al. HMG-CoA reductase inhibitors decrease CD11b expression and CD11b-dependent adhesion of monocytes to endothelium and reduce increased adhesiveness of monocytes isolated from patients with hypercholesterolemia. J Am Coll Cardiol 1997;30:1212-7. [Crossref] [PubMed]


