The evolution of image-guided lumbosacral spine surgery
History of lumbosacral spine surgery and pedicle screw placement
Vertebral fusion for surgical correction of spinal disorders has been employed in the lumbar spine since 1911 when it was initially described, first by Hibbs, then independently by Albee later that year (1-3). Hibbs reported a case of surgical fusion for treatment of a patient with chronic tuberculin osteomyelitis (Pott’s disease) in which the lamina and facets were decorticated and fused with autologous morselized bone graft. He would later modify his technique for use in spinal deformity due to scoliosis. Albee described a separate method of spinal fusion, using tibial bone grafts to fuse the spinous processes and laminae, which he also described initially as a treatment for spinal deformity related to Pott’s disease (1). Unfortunately, in order for these procedures to be effective, patients often required months of bed rest or immobilization with braces or body casts for vertebral fusion to occur (4).
Efforts to reduce prolonged post-operative immobilization and associated morbidity prompted the development of internal fixation devices. The first such method using surgical hardware was reported by King in 1944 in which metallic screws were placed across the facet in parallel orientation to the lamina, often augmented by autologous bone graft (4,5). This surgical method provided early evidence that internal fixation could promote post-procedural biomechanical stability without the need for extended bed rest and external stabilization devices (4). However, this and other similar techniques presented considerable operative morbidity including reports of symptomatic nerve root irritation (4) and unacceptable rates of pseudoarthrosis, which exceeded 50% in some studies (4,6).
Facet arthrodesis with short surgical screws remained the mainstay for rigid spine stabilization until 1959 when Boucher reported a method more closely resembling modern pedicle screw placement. This technique involved placement of a longer screw across the fact in an oblique fashion, in an orientation that allowed it to gain purchase in the face and posterior vertebral body (4,7). Boucher required up to 4 months of restricted activity post-operatively, but reported high rates of clinical success and lessened operative morbidity (7). Pedicle screw placement began to gain more widespread traction in 1969, when Harrington described placement of pedicle screws through the long axis of the pedicle for reduction of high-grade spondylolisthesis in a two patient series (8,9). Variations of transpedicular screw placement were refined in Europe over the ensuing decade and gained widespread acceptance in the United States, stimulated in part by a presentation by Roy-Camille at the American Academy of Orthopedics meeting in 1979 (8,10).
By the mid 1980’s convincing evidence had emerged supporting the use of pedicle screws fixed to an interlocking plate (8), decreasing rates of hardware failure by introducing multiple level load-sharing (11). Two variations of the adjustable interlocking plate were popularized and separately described by Roy-Camille and Steffee (8,12-14). Roy-Camille is credited as the first operator to use this technique, which he implemented in 1963 (4). These devices became the foundation for modern pedicle screw and rod constructs, the design of which continues to evolve as a result of multidisciplinary research by surgeons, engineers, radiologists, and physicists (15).
Over the past three decades, pedicle screw placement has become a common procedure for surgical correction of spinal trauma, deformity, and instability (4,8). The insertion of pedicle screws is a common but demanding technique and carries the risk of vascular, neurologic, and mechanical complication (16,17). The latter two of these complications are often attributable to inaccurate screw placement. In fact, the first transpedicular screw fixations reported by Boucher relied upon visual and palpable intraoperative landmarks, requiring a relatively large operative footprint and providing no mechanism to determine pedicle screw placement accuracy intraoperatively (7). It is not surprising that symptomatic nerve root irritation from malpositioned pedicle screws was noted in 2 of the 160 patients cohort (7).
Several in vitro studies have also demonstrated the biomechanical significance of malpositioned pedicle screws (16,18). Breaches of the medial and lateral pedicle cortex have been shown to reduce pullout strength by 8% and 21% respectively (18). It is generally assumed that a majority of a pedicle screw’s fixation strength derives from the segment contained within the pedicle (16,19). Purchase in the cancellous bone of the vertebral body and the anterior vertebral body cortex each add approximately 20% additional strength (16,19,20). These factors underscore the importance of accurate lumbosacral fusion hardware placement.
A number of methods have been devised to improve pedicle screw placement accuracy and reduce morbidity associated with their malposition. Irrigation of the drill path prior to pedicle screw insertion (21) and intraoperative visualization of the pedicle with a small flexible endoscope (22) have been used as adjuncts to visual and palpable aids. Intraoperative stimulus evoked electromyography (EMG) provides real-time physiologic feedback from nerve roots in the operative bed, giving immediate feedback if nerve impingement occurs intraoperatively (23,24). While these methods may be useful, the integration of imaging and image guided surgery (IGS) systems into lumbosacral fusion has played a critical role in promoting accurate pedicle screw placement and improving procedural success rates (3,16,25-30).
Early methods of image guidance
Early pedicle screw placement utilized plain radiographs for both operative planning and post-operative evaluation (14,31-33). In order to evaluate hardware placement and need for early revision, an intraoperative lateral radiograph was adapted by many surgeons (31). Odgers examined this technique in a prospective study of 72 patients who underwent transpedicular screw placement using only an intraoperative lateral radiograph for image guidance and found a 10.0% per screw pedicle breach rate. Odgers’ breach rates were similar to those later reported using fluoroscopic-guidance (34), and only two patients in Odgers’ cohort were found to have symptomatic neurologic complications. However, the validity of this technique was called into question by several studies showing poor accuracy of radiographs in diagnosing pedicle screw malposition (32,35).
The incorporation of fluoroscopy into spine surgery represented an important advancement in image guidance. Fluoroscopy offers several benefits over radiographic guidance including prompt, multiplanar imaging when a C-arm is used. These factors, coupled with the common availability of fluoroscopy imaging units, have prompted its widespread adaptation. According to a worldwide survey published in 2013, fluoroscopic navigation was used routinely as the primary method of image guidance by 78% of spine surgeons (36).
The factors contributing to hardware malposition when using fluoroscopic guidance are complex and include operator experience, vertebral level of the operation, and alterations to conventional spinal anatomy to name a few (9). In the best case scenario in which the operators are experienced and a majority of patients receive surgery for correction of degenerative disease, breach rates below 3% have been reported (37,38). A single center, multiple operator study by Parker et al showed an overall pedicle breach rate of 1.7% using fluoroscopic guidance to place 6,816 pedicle screws in both the lumbar and thoracic spine (38). However, other studies have reported breach rates exceeding 30% for fluoroscopically-guided pedicle screw placement (27,39). These relatively high rates are attributable to the limitations of two-dimensional (2D) image guidance in the evaluation of an anatomically complex, three-dimensional (3D) structure such as the vertebral body, relying on operator spatial awareness for accurate hardware placement. Although these examples represent two opposing extremes, pedicle screw malposition rates are generally assumed to be 10-15% using fluoroscopy, as four separate meta-analyses have shown breach rates of 13.1% (30), 15% (9), 14.5% (27), and 9.7% (34).
Preoperative CT-based stereotactic navigation
The term “stereotaxis” in the surgical literature initially referred to methods of localizing targets for surgery using a fixed external reference frame attached to or adjacent to the patient (40). The evolution of computer technology in the early 1990’s allowed for development of frameless systems, which gained first use in operative localization of brain tumors (41). Early frameless stereotaxis employed preoperative CT datasets loaded into an image post-processing workstation. These datasets were later registered with the patient’s spinal anatomy using a sonic probe (42). As the probe was maneuvered intraoperatively, the corresponding 3D imaging anatomy could be manipulated in multiple imaging planes at a variety of depths, and the probe was used to guide pedicle screw placement (42). This method showed early promise, with initial studies demonstrating a pedicle screw malposition rate of approximately 1% after placement of 150 total screws (42).
A number of variations on the frameless stereotactic system were developed over the ensuing decade, which consisted of an image workstation to compute either 2D or 3D images (Figure 1), a system to track surgical instruments, and a reference device (15,43). The sonic probe used by Kalfas in 1995 was largely supplanted by external systems that track surgical instruments using infrared light (Optotrac®) or light emitting diodes (LED) (Polaris®) (43). LED-based systems have either LEDs (active arrays) or reflective spheres (passive arrays) attached to the surgical instruments and the reference array so that they can be tracked by an external camera (44). The referencing device, or dynamic reference array (DRA) is secured to a bony landmark outside of the immediate operative field (Figure 2). A camera tracks the position of surgical hardware and instruments in the operative field with respect to the DRA and relays this information to the workstation (15,43,44). The workstation then displays the relative position of the surgical equipment. Additional non-optical systems using electromagnetic tracking systems have also been developed, which operate on the same basic principle (45).
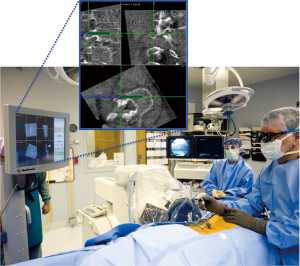

Systems based on preoperative imaging required a CT scan of the operative field, typically at least 150 1-mm slices for a single level fusion (43). This CT dataset would then be transferred into the navigation system and the window settings manually adjusted to the desired levels. There are several aspects of this process that potentially hinder technical success and accurate pedicle screw placement. Failure to adjust the imaging parameters to properly exclude non-osseous structures is one such example that can lead to hardware malposition (43,45).
Another important source of potential error is the process of registration, whereby the spinal anatomy in the operative field is linked to the preoperative CT. Several methods have been used in registration, including CT-fluoroscopy and paired point matching (15,43). In paired-point matching, important landmarks are marked on the imaging system and their corresponding location in the spinal anatomy is denoted and registered in the system. CT-fluoroscopy matching uses multiple 2D fluoroscopy images at varying planes to match with the pre-operative CT dataset (15). The third method referred to as surface mapping matches a number of surface points presented to the system with a computer-generated 3D reconstruction of the spine surface (15). While the combination of surface mapping and paired point matching has been shown to increase registration accuracy, the effect is likely not clinically significant (46). For these reasons, a number of operators using this navigation method prefer the paired-point matching technique (46).
Registration accuracy is affected by a number of additional factors, including the intrinsic capabilities of each system. Intraprocedural movement of the reference frame is a potential cause of misregistration and associated pedicle screw malposition. For paired point matching, the reference points must be selected on un-injured vertebrae, so as to reduce the possibility of intraoperative shifting (43,45). Another significant factor affecting the accuracy of navigation systems, particularly those based on pre-operative CT imaging, derives from differences in patient positioning (15,43,44). Patients typically undergo preoperative imaging in the supine position, whereas lumbosacral fusion is conventionally performed in the prone position. These positional differences can alter intersegmental relationships by several millimeters, but are unaccounted for by the navigation system (15). Efforts to mitigate the effects of misregistration and intersegmental shift include registration of bony landmarks at multiple levels in the lumbosacral spine and on multiple serial occasions (47). This method results in considerably increased operative times, potentially without clinical benefit, as a study by Papadopoulos concluded that single-time multisegmental registration provided sufficient accuracy to avoid symptomatic pedicle breach (47).
IGS based on preoperative CT imaging have improved the accuracy of pedicle screw placement, with breach rates of approximately 2% in the lumbosacral spine (48-50). Improved accuracy over conventional methods were also corroborated in the cervical spine (25,29,51,52) and thoracic spine (29,49,50,53). However, pre-operative CT-based systems also have limitations and potential drawbacks. First, each patient must undergo pre-operative CT imaging which adds a level of cost, inconvenience, and radiation exposure that the patient would not otherwise experience (44,45). Further, the registration process is cumbersome and time consuming. Increased operative times caused by registration are undesirable and potentially increase rates of infection (38). The accuracy of registration is also operator-dependent and susceptible to error, largely due to differences in patient positioning (45).
Fluoroscopy-based navigation
As of 2013, a majority of surgeons routinely use fluoroscopic guidance for pedicle screw placement (36) (Figure 3). Therefore computer-aided image guidance based on conventional C-arm imaging is attractive to many surgeons. 2D fluoroscopy-based navigation, often referred to as “virtual fluoroscopy”, uses intraoperative fluoroscopy to guide hardware placement. Like pre-operative CT-based systems, virtual fluoroscopy includes an image workstation, a stationary DRA, and customized surgical instruments capable of being tracked in the surgical field (44). Instead of a pre-operative CT scan, multiple fluoroscopic projections are acquired intra-operatively to construct a computer-generated model of the operative level (43). This imaging step is performed while the operating room staff is at a sufficient distance from the image intensifier (II) to mitigate significant radiation exposure (44). Modeling is an automated process, eliminating the often lengthy registrations required by pre-operative CT-based systems (15). An electro-optic camera then tracks the position of surgical instruments and relays this information to the computer system, which incorporates C-arm angle into the model images to triangulate hardware position and trajectory (43,44).
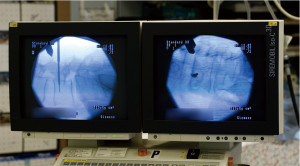
Fluoroscopic navigation improves pedicle screw placement accuracy when compared to conventional fluoroscopy (54). In a meta-analysis published in 2014, Mason noted overall breach rates of 31.9% and 15.7% using 2D fluoroscopy and virtual fluoroscopy respectively (54). However, virtual fluoroscopy is associated with a number of important drawbacks compared with 3-D navigation techniques. Primarily, fluoroscopic navigation does not impart the three-dimensional spatial feedback provided by 3D guidance systems and thus results in higher rates of pedicle screw malposition (54). Image quality is also highly dependent on the imaging system used, and can be degraded by increasing patient size or bone demineralization (44). Finally, virtual fluoroscopy has been shown to reduce fluoroscopic radiation exposure (55) although it requires some degree of radiation exposure to the operative staff (44).
Intraoperative CT-based Navigation
Stereotaxis utilizing intraoperative CT (IoCT) represents the latest generation of advanced image guidance systems (IGS) to gain use in spinal fusion. IoCT systems utilize similar imaging technology to preoperative CT-based navigation, incorporating imaging guidance computer software, a stationary DRA, electro-optic camera, and specialized surgical hardware for stereotaxis (44). Because of these similarities, IoCT and preoperative CT-based navigations systems are easily confused in the literature The principle advancement in IoCT is the application of portable fluoroscopy units to obtain CT-like datasets within the operative field (15). Under general endotracheal anesthesia, patients are imaged while in the operative position. Two commonly used systems are the Iso-C (Siemens Healthcare USA, Malvern, PA, USA) and the O-arm (Medtronic Inc., Minneapolis, MN, USA) shown in Figure 4. The scan generally requires less than 2 minutes (44) and consists of either a 180° C-arm or 360° O-arm rotation. The image quality of CT images acquired in this fashion are acceptable for navigational purposes due to the intrinsic contrast differences between the dense osseous structures of the lumbosacral spine and the relatively low attenuation of surrounding regional soft tissues (56).
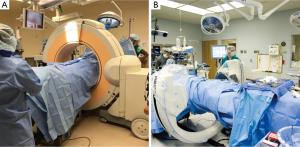
IoCT eliminates positional variances that degrade the accuracy of preoperative CT systems by imaging patients in the operative position. The electro-optic camera tracks and triangulates the DRA position during the initial CT scan, eliminating the time-consuming registration process. Images are automatically loaded into the image guidance computer system in a process that typically takes less than one minute (44). This technology provides a mechanism for repeated CT-imaging if the DRA or patient is moved significantly during the procedure (15,44), and 2D fluoroscopy remains available for use in intraoperative troubleshooting when necessary. These factors contribute to the increased accuracy of pedicle screw placement using IoCT when compared to other methods of image guidance. In a comparative study, Wood noted a 6.4% pedicle breach rate using preoperative CT-based 3D stereotaxis compared with a breach rate of 1.6% using IoCT stereotactic navigation (57). IoCT has also demonstrated improved rates of pedicle breach when compared directly to the conventional (Roy-Camille) method (58), 2-D fluoroscopic navigation (54,59) and virtual fluoroscopy (54).
Although IoCT navigation provides a number of unique advantages, it is not without drawbacks. This modality presents a learning curve for inexperienced operators and those less familiar with 3D anatomical representations (15). It should be noted, however, that a meta-analysis by Shin concluded that average operative times were similar among navigated and non-navigated groups (9). A primary concern with IoCT navigation is that of cost. The cost of an O-arm can significantly exceed that of a conventional fluoroscopy unit, even when not accounting for the necessary accompanying software and surgical instruments. Cost is likely a significant contributor to why IoCT stereotactic systems are predominantly located at tertiary referral centers, with relatively limited availability in Latin America and the Middle East (36). Further, accuracy of the stereotactic array diminishes with increasing distance, potentially requiring repositioning of the DRA and repeated CT imaging in multilevel procedures (15). Any movement of the reference array after the CT scan could result in misregistration and hardware malposition. This is underscored by Bourgeois et al, who reported pedicle breach rates below 1% in a large cohort of IoCT navigated patients. However, one patient in this series had bilateral pedicle breach presumably attributable to intraoperative movement of the reference array, with resulting nerve irritation requiring early reoperation (30).
Minimally invasive spine surgery and 3-D stereotaxis
Traditional management of lumbar stenosis entails open decompression via bilateral laminectomy, with or without facetectomy. While this methodology is effective in removing the inciting anatomic cause of spinal stenosis, removal of the posterior osseous and ligamentous structures can cause instability and result in prolonged back pain (60-62). Furthermore, open surgical decompression requiring a wide operative field requires incision though and retraction of a large volume of paraspinal musculature, potentially inducing muscle atrophy and further propagating instability. Minimally invasive spine surgery (MISS) refers to collective operative techniques aimed at reducing operative morbidity by decreasing the operative footprint. MISS surgical techniques include use of tubular access retractors introduced through small paramidline incisions, with or without endoscopic assistance, as well as limited decompression via partial facetectomy and microdiscectomy (63). While functional outcomes have been shown to be similar among conventional and minimally invasive techniques, MISS approaches are associated with reduced operative blood loss, reduced operative times, decreased rates of durotomy and fewer postoperative infections (60,64,65). These techniques also reduce postoperative hospitalization (60), and permit same-day discharge in some cases (66). Minimally invasive techniques are not limited to treatment of degenerative pathology and have been applied for treatment of spodylolysis (67), traumatic fractures (68,69), and malignancy (70).
The small access approaches used in MISS require much less surgical exposure of the lumbar spine, relative to traditional open techniques (Figure 5). Instead of placing pedicle screws via large open incisions, minimally invasive spine surgeons generally use a percutaneous approach. Percutaneous pedicle screw placement (PPSP) is challenging in that it removes bony palpable and visual landmarks that are typically used by the surgeon to guide hardware placement (30). This increases reliance on 3D navigation to visualize the bony anatomy of the pedicle and vertebral body to guide pedicle screw placement. Furthermore, MISS generally prohibits use of paired-point matching registration for preoperative CT-based stereotaxis, as the bony landmarks required for registration are not conventionally exposed. This requires use of another matching method if preoperative CT-based stereotactic navigation is to be used. These factors have contributed to a shift towards the use of the newest generation of advanced IGS, included those based on intraoperative CT with 3D stereotaxis. The need for advanced image guidance likely contributes to the limited availability of MISS.
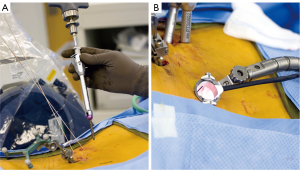
According to a recent global survey in 2013, only 15% of spine surgeons reported routine use of minimally invasive techniques for interbody fusion (15). This is at least in part attributable to the challenges of MISS, associated reliance on advanced IGS, and availability (or lack thereof) of navigation systems. However, recent data have validated the accuracy of PPSP using advanced image guidance. A recent study of 2,132 lumbosacral pedicle screws placed percutaneously under IoCT based 3D navigation in conjunction with MISS showed a breach rate of 0.33% (30). These data were compared to a cohort of 4,248 fluoroscopically-navigated pedicle screws which demonstrated a breach rate of 13.1% (71-80). The authors concluded that treatment of only 6 patients with IoCT navigational guidance would be required to prevent one pedicle breach. These data are among the lowest ever reported in a large patient series.
Radiation exposure from spinal surgery
All of the navigational systems discussed thus far require the use of some form of ionizing radiation. Radiation exposure can be a concern for the patient undergoing a procedure. Additionally, prolonged exposure to the operative staff can result in the accumulation of large absorbed doses over the course of a career. This has been the subject of a number of preclinical and in vivo studies. A phantom study published in 1999 showed increased phantom dose associated with the use of CT-based navigation systems, although the dose could be decreased 40% using an optimized protocol (81). However, subsequent cadaveric studies have shown decreased dose to the surgeon without differences in operative time among the fluoroscopy and navigated groups, although slightly increased patient radiation exposure was reported with CT-based navigation in one study (82). A 2008 study by Smith showed that navigation reduced mean dose to the surgeons’ torso when compared to conventional fluoroscopy, from 433±266 to 33±82 mGy (83). An in vivo examination of patient dose with and without computer-aided navigation corroborated the dose saving associated with navigation (84). In this study, Gebhard reported median absorbed dose of 1,091 mGy using conventional C-arm guidance, versus 664 mGy with virtual fluoroscopy and 432 mGy with CT-based fluoroscopic navigation (84). IoCT based guidance imparted the lowest radiation, with median dose of 152 mGy (84). It has also been shown that radiation exposure to the operative staff during the intraoperative CT is negligible when the staff members are at least 10 feet from the fluoroscopy unit (85). These studies demonstrate an overall dose reduction with advanced image guidance particularly IoCT based 3D stereotactic systems. MISS approaches alone have been shown to impart reduced radiation dose to the operator when compared to conventional approaches, although this likely reflects increasing use of advance image guidance systems with MISS (82,86).
Future horizons: robotic-assisted surgery
Robotic assisted surgery has considerably expanded since its earliest description in 1992 (87). The first surgical robot to gain FDA approval, the da Vinci, was originally designed for cardiac surgeries. It has since gained widespread use in a number of applications including prostatectomy, hysterectomy, and nephrectomy (88). Robotic surgery has further expanded in scope in recent years, having been applied to thyroidectomy (89), head and neck and lung cancer resection (90), colorectal surgeries (91), organ transplant (92), and endovascular procedures (93). Over the past decade, at least 18 robotic systems have also been used for surgical and percutaneous spine intervention (87). Robotic surgery in the lumbosacral spine has been primarily performed by three systems: the da Vinci surgical system (Intuitive Surgical Inc., Sunnyvale, CA, USA), SpineAssist (Mazor Robotics Ltd., Caesarea, Israel) and the Renaissance system (Mazor Robotics Ltd., Caesarea, Israel). The Renaissance system is currently the most widely used for spine intervention (88) (Figure 6).
The da Vinci consists of a robotic platform that has either 3 or 4 robotic arms that operate different endoscopic trocar systems (88). Each arm is controlled from the surgeon’s console, typically located in the same room as the patient. The da Vinci allows the operator to work from a seated position and offers excellent 3D visualization of the operative field. The da Vinci has also been applied to anterior lumbar interbody fusion (ALIF) in the lumbosacral spine. ALIF is generally performed in clinical settings in which rigid biomechanical stability is desired, either in the case of revision for pseudoarthrosis, spinal deformity, or instability (94). Anterior interbody fusion provides increased anterior and middle vertebral column stabilization although access to the central canal for decompression is limited. This surgical method requires a transperitoneal approach which places the patient at risk for significant morbidity, including neurovascular and ureteral injury (88,95). Lee reported two cases in which patients underwent ALIF at L5-S1 for correction of degenerative disease in which the da Vinci was used to avoid the presacral nerve plexus, achieving technical and clinical success (95). While these results show promise, current literature evaluating the da Vinci is limited to small patient series given its infrequent use in spine surgery (95).
The SpineAssist is the first robotic system dedicated solely to spine intervention. It was originally referred to as the MARS, or MinAture Robot for Surgical procedures (87). It was approved for use in 2004 under the commercial name SpineAssist. The newest generation of SpineAssist is known as the Renaissance Robotic System, offering better ergonomics, a smaller profile, and an enhanced software platform (88). Both of these systems are considerably smaller than the da Vinci, approximating a soda can in size and weight (5 cm base diameter and 8 cm height with weight of 250 g) (87). Via a dedicated computer workstation, the SpineAssist or Renaissance aids positioning of surgical hardware and instruments according to predetermined trajectories (15). Operative planning occurs in a similar fashion to that of preoperative CT-based stereotaxis, requiring a dedicated preoperative CT scan. The robot is mounted to either a bony landmark on the patient or to the operative table. Matching to the pre-operative CT dataset occurs using a fluoroscopy matching algorithm with a conventional C-arm.
SpineAssist and Rennaissance have been implemented worldwide in over 2,500 procedures (87) in more than 25 medical centers (88). Their use has been the subject of at least one large, multicenter analysis using PPSP. This report of 840 cases showed a total pedicle breach rate of 10.7% using the SpineAssist, although only 1.7% breached the pedicle cortex by more than 2 mm (96). Neurologic complications were found in only 4 of the 840 patient series, and the deficits were transient in each instance. These data illustrate the potential benefits of robotic systems over conventional methods. However, it should be noted that these rates of pedicle screw placement are inferior to previously discussed studies evaluating PPSP accuracy using IoCT stereotaxis (30). This phenomenon could result from slight inaccuracies in the registration process, a phenomenon well-described in stereotactic navigation based on preoperative CT (87). Regardless, spinal robotics remains in the early stages of development. The future may hold a role for widespread use of robotic navigation systems, as well as implementation of robotics in laminectomy and percutaneous spine intervention.
Conclusions
Spine surgery is a rapidly evolving field, which has changed significantly over the past century with recent major advances in operative technique and image guidance systems designed to increase the accuracy and decrease the morbidity of hardware placement. The pedicle screw construct has served as the primary means of rigid internal stabilization for promoting spinal fusion since the 1970’s. Accurate placement of pedicle screws increases biomechanical stability and reduces operative and postoperative complications.
A number of imaging methods and image guidance systems have been used to aid transpedicular screw placement. Of these, stereotactic navigation based on intraoperative CT is a promising modality offering the benefits of highly accurate pedicle screw placement, reduced operative radiation exposure, and seamless integration into minimally invasive spine surgery. Robotic-assistance systems represent an emerging modality that may also facilitate high accuracy of hardware placement.
Acknowledgements
The authors would like to acknowledge William S. Reid, MD formerly of the University of Tennessee Medical Center and Fort Sanders Regional Medical Center departments of Neurosurgery for sharing his operative photos and providing input from his vast surgical experience.
Disclosure: The authors declare no conflict of interest.
References
- Albee FH. Transplantation of a portion of the tibia into the spine for Pott's disease: a preliminary report 1911. Clin Orthop Relat Res 2007;14-6. [PubMed]
- Hibbs RA. An Operation for Progressive Spinal Deformities. Clin Orthop Relat Res 1964;4-8. [PubMed]
- Ishikawa Y, Kanemura T, Yoshida G, et al. Clinical accuracy of three-dimensional fluoroscopy-based computer-assisted cervical pedicle screw placement: a retrospective comparative study of conventional versus computer-assisted cervical pedicle screw placement. J Neurosurg Spine 2010;13:606-11. [PubMed]
- Kabins MB, Weinstein JN. The History of Vertebral Screw and Pedicle Screw Fixation. Iowa Orthop J 1991;11:127-36.
- Hoover NW. Methods of lumbar fusion. J Bone Joint Surg [Am] 1968;50:194-210.
- Thompson WA, Ralston EL. Pseudarthrosis following spine fusion. J Bone Joint Surg Am 1949;31A:400-5. [PubMed]
- Boucher HH. A method of spinal fusion. J Bone Joint Surg Br 1959;41-B:248-59. [PubMed]
- Gaines RW. The Use of Pedicle-Screw Internal Fixation for the Operative Treatment of Spinal Disorders. J Bone Joint Surg Am 2000;82-A:1458. [PubMed]
- Shin BJ, James AR, Njoku IU, et al. Pedicle screw navigation: a systematic review and meta-analysis of perforation risk for computer-navigated versus freehand insertion. J Neurosurg Spine 2012;17:113-22. [PubMed]
- Amiot LP, Lang K, Putzier M, et al. Comparative results between conventional and computer-assisted pedicle screw installation in the thoracic, lumbar, and sacral spine. Spine (Phila Pa 1976) 2000;25:606-14. [PubMed]
- Gaines RW, Carson WL, Satterlee CC, et al. Experimental evaluation of seven different spinal fracture internal fixation devices using nonfailure stability testing. The load-sharing and unstable-mechanism concepts. Spine (Phila Pa 1976) 1991;16:902-9. [PubMed]
- Roy-Camille R, Saillant G, Mazel C. Internal fixation of the lumbar spine with pedicle screw plating. Clin Orthop Relat Res 1986;7-17. [PubMed]
- Steffee AD, Biscup RS, Sitkowski DJ. Segmental spine plates with pedicle screw fixation. A new internal fixation device for disorders of the lumbar and thoracolumbar spine. Clin Orthop Relat Res 1986;45-53. [PubMed]
- Steffee AD, Biscup RS, Sitkowski DJ. Segmental spine plates with pedicle screw fixation. A new internal fixation device for disorders of the lumbar and thoracolumbar spine. Clin Orthop Relat Res 1986;45-53. [PubMed]
- Ringel F, Villard J, Ryang YM, et al. Navigation, robotics, and intraoperative imaging in spinal surgery. Adv Tech Stand Neurosurg 2014;41:3-22. [PubMed]
- Weinstein JN, Rydevik BL, Rauschning W. Anatomic and technical considerations of pedicle screw fixation. Clin Orthop Relat Res 1992;34-46. [PubMed]
- Merloz P, Troccaz J, Vouaillat H, et al. Fluoroscopy-based navigation system in spine surgery. Proc Inst Mech Eng H 2007;221:813-20. [PubMed]
- Brasiliense LB, Theodore N, Lazaro BC, et al. Quantitative analysis of misplaced pedicle screws in the thoracic spine: how much pullout strength is lost?: presented at the 2009 Joint Spine Section Meeting. J Neurosurg Spine 2010;12:503-8. [PubMed]
- Zindrick MR, Wiltse LL, Widell EH, et al. A biomechanical study of intrapeduncular screw fixation in the lumbosacral spine. Clin Orthop Relat Res 1986;99-112. [PubMed]
- Krag MH, Beynnon BD, Pope MH, et al. An internal fixator for posterior application to short segments of the thoracic, lumbar, or lumbosacral spine. Design and testing. Clin Orthop Relat Res 1986;75-98. [PubMed]
- An HS, Benoit PR. Saline injection technique to confirm pedicle screw path: a cadaveric study. Am J Orthop (Belle Mead NJ) 1998;27:362-5. [PubMed]
- Frank EH. The use of small malleable endoscopes to assess pedicle screw placement: technical note. Minim Invasive Neurosurg 1998;41:10-2. [PubMed]
- Calancie B, Donohue ML, Moquin RR. Neuromonitoring with pulse-train stimulation for implantation of thoracic pedicle screws: a blinded and randomized clinical study. Part 2. The role of feedback. J Neurosurg Spine 2014;20:692-704. [PubMed]
- Wang H, Liao X, Ma X, et al. Solid and hollow pedicle screws affect the electrical resistance: A potential source of error with stimulus-evoked electromyography. Indian J Orthop 2013;47:352-6. [PubMed]
- Kotani Y, Abumi K, Ito M, et al. Improved accuracy of computer-assisted cervical pedicle screw insertion. J Neurosurg 2003;99:257-63. [PubMed]
- Youkilis AS, Quint DJ, McGillicuddy JE, et al. Stereotactic navigation for placement of pedicle screws in the thoracic spine. Neurosurgery 2001;48:771-8;discussion 778-9. [PubMed]
- Tian NF, Xu HZ. Image-guided pedicle screw insertion accuracy: a meta-analysis. Int Orthop 2009;33:895-903. [PubMed]
- Han W, Gao ZL, Wang JC, et al. Pedicle screw placement in the thoracic spine: a comparison study of computer-assisted navigation and conventional techniques. Orthopedics 2010;33. [PubMed]
- Lee GY, Massicotte EM, Rampersaud YR. Clinical accuracy of cervicothoracic pedicle screw placement: a comparison of the "open" lamino-foraminotomy and computer-assisted techniques. J Spinal Disord Tech 2007;20:25-32. [PubMed]
- Bourgeois AC, Faulkner AR, Bradley YC, et al. Improved Accuracy of Minimally Invasive Transpedicular Screw Placement in the Lumbar Spine with Three-dimensional Stereotactic Image Guidance: A Comparative Meta-analysis. J Spinal Disord Tech 2014. [Epub ahead of print]. [PubMed]
- Odgers CJ 4th, Vaccaro AR, Pollack ME, et al. Accuracy of pedicle screw placement with the assistance of lateral plain radiography. J Spinal Disord 1996;9:334-8. [PubMed]
- Whitecloud TS, Skalley TC, Cook SD, et al. Roentgenographic measurement of pedicle screw penetration. Clin Orthop Relat Res 1989;57-68. [PubMed]
- Weinstein JN, Spratt KF, Spengler D, et al. Spinal pedicle fixation: reliability and validity of roentgenogram-based assessment and surgical factors on successful screw placement. Spine (Phila Pa 1976) 1988;13:1012-8. [PubMed]
- Kosmopoulos V, Schizas C. Pedicle screw placement accuracy: a meta-analysis. Spine (Phila Pa 1976) 2007;32:E111-20. [PubMed]
- Berlemann U, Heini P, Müller U, et al. Reliability of pedicle screw assessment utilizing plain radiographs versus CT reconstruction. Eur Spine J 1997;6:406-10. [PubMed]
- Härtl R, Lam KS, Wang J, et al. Worldwide survey on the use of navigation in spine surgery. World Neurosurg 2013;79:162-72. [PubMed]
- Lau D, Terman SW, Patel R, et al. Incidence of and risk factors for superior facet violation in minimally invasive versus open pedicle screw placement during transforaminal lumbar interbody fusion: a comparative analysis. J Neurosurg Spine 2013;18:356-61. [PubMed]
- Parker SL, McGirt MJ, Farber SH, et al. Accuracy of free-hand pedicle screws in the thoracic and lumbar spine: analysis of 6816 consecutive screws. Neurosurgery 2011;68:170-8; discussion 178. [PubMed]
- Schulze CJ, Munzinger E, Weber U. Clinical relevance of accuracy of pedicle screw placement. A computed tomographic-supported analysis. Spine (Phila Pa 1976) 1998;23:2215-20; discussion 2220-1. [PubMed]
- Galloway RL, Maciunas RJ. Stereotactic neurosurgery. Crit Rev Biomed Eng 1990;18:181-205. [PubMed]
- Barnett GH, Kormos DW, Steiner CP, et al. Use of a frameless, armless stereotactic wand for brain tumor localization with two-dimensional and three-dimensional neuroimaging. Neurosurgery 1993;33:674-8. [PubMed]
- Kalfas IH, Kormos DW, Murphy MA, et al. Application of frameless stereotaxy to pedicle screw fixation of the spine. J Neurosurg 1995;83:641-7. [PubMed]
- Gebhard F, Weidner A, Liener UC, et al. Navigation at the spine. Injury 2004;35 Suppl 1:S-A35-45. [PubMed]
- Holly LT, Foley KT. Intraoperative spinal navigation. Spine (Phila Pa 1976) 2003;28:S54-61. [PubMed]
- Ughwanogho E, Flynn JM. Current Navigation Modalities in Spine Surgery. UPOJ 2010;20:65-9.
- Holly LT, Bloch O, Johnson JP. Evaluation of registration techniques for spinal image guidance. J Neurosurg Spine 2006;4:323-8. [PubMed]
- Papadopoulos EC, Girardi FP, Sama A, et al. Accuracy of single-time, multilevel registration in image-guided spinal surgery. Spine J 2005;5:263-7; discussion 268. [PubMed]
- Taecholarn C, Montriwiwatchai P, Tongkon T, et al. Three-dimensional frameless stereotactic-guided pedicle screw fixation of the spine: early experiences in King Chulalongkorn Memorial Hospital. J Med Assoc Thai 2006;89:217-23. [PubMed]
- Laine T, Lund T, Ylikoski M, et al. Accuracy of pedicle screw insertion with and without computer assistance: a randomised controlled clinical study in 100 consecutive patients. Eur Spine J 2000;9:235-40. [PubMed]
- Kotani Y, Abumi K, Ito M, et al. Accuracy analysis of pedicle screw placement in posterior scoliosis surgery: comparison between conventional fluoroscopic and computer-assisted technique. Spine (Phila Pa 1976) 2007;32:1543-50. [PubMed]
- Ito H, Neo M, Yoshida M, et al. Efficacy of computer-assisted pedicle screw insertion for cervical instability in RA patients. Rheumatol Int 2007;27:567-74. [PubMed]
- Richter M, Cakir B, Schmidt R. Cervical pedicle screws: conventional versus computer-assisted placement of cannulated screws. Spine (Phila Pa 1976) 2005;30:2280-7. [PubMed]
- Sakai Y, Matsuyama Y, Nakamura H, et al. Segmental pedicle screwing for idiopathic scoliosis using computer-assisted surgery. J Spinal Disord Tech 2008;21:181-6. [PubMed]
- Mason A, Paulsen R, Babuska JM, et al. The accuracy of pedicle screw placement using intraoperative image guidance systems. J Neurosurg Spine 2014;20:196-203. [PubMed]
- Yang BP, Wahl MM, Idler CS. Percutaneous lumbar pedicle screw placement aided by computer-assisted fluoroscopy-based navigation: perioperative results of a prospective, comparative, multicenter study. Spine (Phila Pa 1976) 2012;37:2055-60. [PubMed]
- Grass M, Koppe R, Klotz E, et al. Three-dimensional reconstruction of high contrast objects using C-arm image intensifier projection data. Comput Med Imaging Graph 1999;23:311-21. [PubMed]
- Wood M, Mannion R. A comparison of CT-based navigation techniques for minimally invasive lumbar pedicle screw placement. J Spinal Disord Tech 2011;24:E1-5. [PubMed]
- Silbermann J, Riese F, Allam Y, et al. Computer tomography assessment of pedicle screw placement in lumbar and sacral spine: comparison between free-hand and O-arm based navigation techniques. Eur Spine J 2011;20:875-81. [PubMed]
- Waschke A, Walter J, Duenisch P, et al. CT-navigation versus fluoroscopy-guided placement of pedicle screws at the thoracolumbar spine: single center experience of 4,500 screws. Eur Spine J 2013;22:654-60. [PubMed]
- Wong AP, Smith ZA, Lall RR, et al. The microendoscopic decompression of lumbar stenosis: a review of the current literature and clinical results. Minim Invasive Surg 2012;2012:325095.
- Bassewitz H, Herkowitz H. Lumbar stenosis with spondylolisthesis: current concepts of surgical treatment. Clin Orthop Relat Res 2001;54-60. [PubMed]
- Mulholland RC. Degenerative lumbar spondylolisthesis: a meta-analysis of literature 1970-1993. Spine (Phila Pa 1976) 1995;20:1957-8. [PubMed]
- Nellensteijn J, Ostelo R, Bartels R, et al. Transforaminal endoscopic surgery for symptomatic lumbar disc herniations: a systematic review of the literature. Eur Spine J 2010;19:181-204. [PubMed]
- Anand N, Baron EM. Minimally invasive approaches for the correction of adult spinal deformity. Eur Spine J 2013;22:S232-41. [PubMed]
- Mezger U, Jendrewski C, Bartels M. Navigation in surgery. Langenbecks Arch Surg 2013;398:501-14. [PubMed]
- Eckman WW, Hester L, McMillen M. Same-day discharge after minimally invasive transforaminal lumbar interbody fusion: a series of 808 cases. Clin Orthop Relat Res 2014;472:1806-12. [PubMed]
- Widi GA, Williams SK, Levi AD. Minimally invasive direct repair of bilateral lumbar spine pars defects in athletes. Case Rep Med 2013;2013:659078.
- Barbagallo GM, Yoder E, Dettori JR, et al. Percutaneous minimally invasive versus open spine surgery in the treatment of fractures of the thoracolumbar junction: a comparative effectiveness review. Evid Based Spine Care J 2012;3:43-9. [PubMed]
- Yang WE, Ng ZX, Koh KM, et al. Percutaneous pedicle screw fixation for thoracolumbar burst fracture: a Singapore experience. Singapore Med J 2012;53:577-81. [PubMed]
- Molina CA, Gokaslan ZL, Sciubba DM. A systematic review of the current role of minimally invasive spine surgery in the management of metastatic spine disease. Int J Surg Oncol 2011;2011:598148.
- Kim MC, Chung HT, Cho JL, et al. Factors affecting the accurate placement of percutaneous pedicle screws during minimally invasive transforaminal lumbar interbody fusion. Eur Spine J 2011;20:1635-43. [PubMed]
- Lau D, Terman SW, Patel R, et al. Incidence of and risk factors for superior facet violation in minimally invasive versus open pedicle screw placement during transforaminal lumbar interbody fusion: a comparative analysis. J Neurosurg Spine 2013;18:356-61. [PubMed]
- Luther N, Iorgulescu JB, Geannette C, et al. Comparison of Navigated Versus Non-Navigated Pedicle Screw Placement in 260 Patients and 1434 Screws: Screw Accuracy, Screw Size, and the Complexity of Surgery. J Spinal Disord Tech 2013. [Epub ahead of print]. [PubMed]
- Nakashima H, Sato K, Ando T, et al. Comparison of the percutaneous screw placement precision of isocentric C-arm 3-dimensional fluoroscopy-navigated pedicle screw implantation and conventional fluoroscopy method with minimally invasive surgery. J Spinal Disord Tech 2009;22:468-72. [PubMed]
- Oh HS, Kim JS, Lee SH, et al. Comparison between the accuracy of percutaneous and open pedicle screw fixations in lumbosacral fusion. Spine J 2013;13:1751-7. [PubMed]
- Raley DA, Mobbs RJ. Retrospective computed tomography scan analysis of percutaneously inserted pedicle screws for posterior transpedicular stabilization of the thoracic and lumbar spine: accuracy and complication rates. Spine (Phila Pa 1976) 2012;37:1092-100. [PubMed]
- Ravi B, Zahrai A, Rampersaud R. Clinical accuracy of computer-assisted two-dimensional fluoroscopy for the percutaneous placement of lumbosacral pedicle screws. Spine (Phila Pa 1976) 2011;36:84-91. [PubMed]
- Schizas C, Michel J, Kosmopoulos V, et al. Computer tomography assessment of pedicle screw insertion in percutaneous posterior transpedicular stabilization. Eur Spine J 2007;16:613-7. [PubMed]
- Smith ZA, Sugimoto K, Lawton CD, et al. Incidence of lumbar spine pedicle breach after percutaneous screw fixation: a radiographic evaluation of 601 screws in 151 patients. J Spinal Disord Tech 2014;27:358-63. [PubMed]
- Waschke A, Walter J, Duenisch P, et al. CT-navigation versus fluoroscopy-guided placement of pedicle screws at the thoracolumbar spine: single center experience of 4,500 screws. Eur Spine J 2013;22:654-60. [PubMed]
- Slomczykowski M, Roberto M, Schneeberger P, et al. Radiation dose for pedicle screw insertion. Fluoroscopic method versus computer-assisted surgery. Spine (Phila Pa 1976) 1999;24:975-82;discussion 983. [PubMed]
- Kim CW, Lee YP, Taylor W, et al. Use of navigation-assisted fluoroscopy to decrease radiation exposure during minimally invasive spine surgery. Spine J 2008;8:584-90. [PubMed]
- Smith HE, Welsch MD, Sasso RC, et al. Comparison of radiation exposure in lumbar pedicle screw placement with fluoroscopy vs computer-assisted image guidance with intraoperative three-dimensional imaging. J Spinal Cord Med 2008;31:532-7. [PubMed]
- Gebhard FT, Kraus MD, Schneider E, et al. Does computer-assisted spine surgery reduce intraoperative radiation doses? Spine (Phila Pa 1976) 2006;31:2024-7;discussion 2028. [PubMed]
- Nottmeier EW, Bowman C, Nelson KL. Surgeon radiation exposure in cone beam computed tomography-based, image-guided spinal surgery. Int J Med Robot 2012;8:196-200. [PubMed]
- Yu E, Khan SN. Does less invasive spine surgery result in increased radiation exposure? A systematic review. Clin Orthop Relat Res 2014;472:1738-48. [PubMed]
- Bertelsen A, Melo J, Sánchez E, et al. A review of surgical robots for spinal interventions. Int J Med Robot 2013;9:407-22. [PubMed]
- Onen MR, Naderi S. Robotic systems in spine surgery. Turk Neurosurg 2014;24:305-11. [PubMed]
- Chai YJ, Lee KE, Youn YK. Can robotic thyroidectomy be performed safely in thyroid carcinoma patients? Endocrinol Metab (Seoul) 2014;29:226-32. [PubMed]
- Ambrogi MC, Fanucchi O, Melfi F, et al. Robotic surgery for lung cancer. Korean J Thorac Cardiovasc Surg 2014;47:201-10. [PubMed]
- Pucci MJ, Beekley AC. Use of robotics in colon and rectal surgery. Clin Colon Rectal Surg 2013;26:39-46. [PubMed]
- Oberholzer J, Giulianotti P, Danielson KK, et al. Minimally invasive robotic kidney transplantation for obese patients previously denied access to transplantation. Am J Transplant 2013;13:721-8. [PubMed]
- Rolls AE, Riga CV, Bicknell CD, et al. Robot-assisted uterine artery embolization: a first-in-woman safety evaluation of the Magellan System. J Vasc Interv Radiol 2014;25:1841-8. [PubMed]
- Rao PJ, Loganathan A, Yeung V, et al. Outcomes of anterior lumbar interbody fusion surgery based on indication: a prospective study. Neurosurgery 2015;76:7-23; discussion 23-4. [PubMed]
- Lee JY, Bhowmick DA, Eun DD, et al. Minimally invasive, robot-assisted, anterior lumbar interbody fusion: a technical note. J Neurol Surg A Cent Eur Neurosurg 2013;74:258-61. [PubMed]
- Devito DP, Kaplan L, Dietl R, et al. Clinical acceptance and accuracy assessment of spinal implants guided with SpineAssist surgical robot: retrospective study. Spine (Phila Pa 1976) 2010;35:2109-15. [PubMed]


