Primary hepatic leiomyoma: unusual cause of an intrahepatic mass
Introduction
Primary hepatic leiomyomas (PHLs) are rare. Few cases have been reported in surgical literature (1). Originating from the smooth muscle layer, leiomyomas are benign lesions, with a malignant potential and are a relatively common occurrence within the gastrointestinal and genitourinary tracts (1,2). Of the few cases reported in literature an association with immunodeficiency and Epstein-Barr virus (EBV) infection have been proposed as a possible etiology (1,2). We herewith present an unusual case of a PHL in a young healthy female. She presented with upper abdominal pain and discomfort. A laparoscopic left lateral sectionectomy was performed. Final histology confirmed it to be leiomyoma. Given the rarity of the case and the unusual diagnosis, we herewith report the same.
Case history
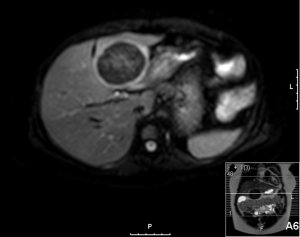
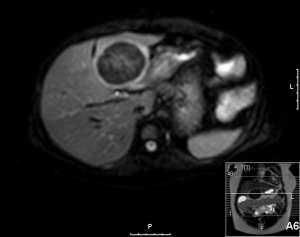
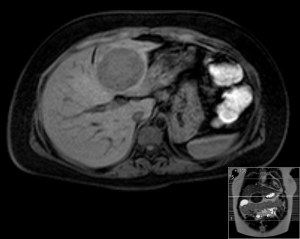
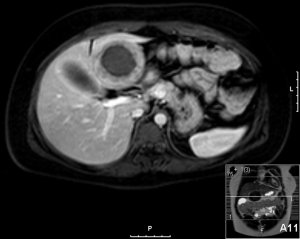
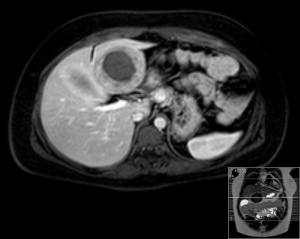
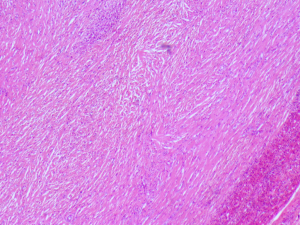
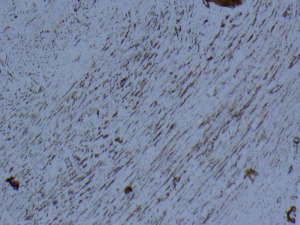
A 20-year-old female was referred to us from her local hospital, with a 6-month history of upper abdominal pain. There were a few episodes of vomiting within the last month of referral. She had been empirically treated with proton pump inhibitors and was referred to a tertiary care facility, upon failure of resolution of symptoms. An initial ultrasound (US) performed showed a 7 cm hypoechoic solid lesion with the left lateral segment of the liver. MRI confirmed the presence of a single solid 8 cm lesion within segment 3 of the liver (Figures 1-4). The lesion did not have the classical features of an adenoma or a focal nodular hyperplasia (FNH). It did not appear malignant on imaging. The lesion showed a very low signal on T2 sequences (Figures 1,2), with an intermediate signal on T1 (Figure 3) and exhibited peripheral rim enhancement after gadolinium (Figure 4). On MRI there was mainly peripheral enhancement in the arterial phase and some enhancement in the portal phase and delayed phase as well (Figure 5). An atypical adenoma was thought of as a possible diagnosis. The background liver was normal with no evidence of chronic liver disease. There was no previous history of medical liver disease, previous history of medical liver disease and all blood tests including liver function and tumour markers (AFP, CEA and CA 19-9) were within normal limits. However, given the radiological appearance, presence of symptoms and the possible pressure effects on the stomach, we decided to go ahead and resect this 8 cm symptomatic liver mass. She underwent an uneventful laparoscopic left lateral segmentectomy. Apart from a 12 mm optical port at the umbilicus, three other ports were used, 5 mm ports were used through the epigastrium and left upper quadrant. A 12 mm port was used through the right hypochondrium. After a laparoscopic US, the left triangular ligament was divided. Parenchymal transection was performed using UltraCision Harmonic Scalpel (Ethicon Endosurgery, Cincinnati, Ohio, USA). Echelon Flex Endopath (Ethicon Endo Surgery, Cincinnati, Ohio, USA) was used for transection of the left hepatic vein and Glissonian pedicles within the liver. We used an Endocatch (Covidean, Dublin, Ireland) to deliver the surgical specimen through a Pfannensteil incision. Gross examination revealed an 80 mm × 60 mm × 54 mm well circumscribed subcapsular lesion. Histology confirmed smooth muscle and spindle cell proliferation with elongated nuclei and eosinophilic cytoplasm in keeping with a leiomyoma. There was abundant fibro vascular stroma. There was no evidence of any nuclear atypia. Immunohistochemistry (IHC) confirmed positivity for alpha-smooth muscle actin (SMA) (Figures 6,7). Special staining also showed no excess iron or alpha-1 antitrypsin globules. The patient is well at 18 months follow up, with no radiological or clinical evidence of any recurrence.
Discussion
PHL is an uncommon condition with very few tumors reported in surgical literature (1,2). Smooth muscle leiomyomas, arising from the muscularis layer are a relatively common occurrence in the gastrointestinal and genitourinary tracts (3). In one of the earliest reports on PHL, Hawkins proposed criteria for the diagnosis of PHL—that apart from the presence of leiomyocytes within the liver lesion in question, there should be no evidence of a smooth muscle tumour elsewhere within the body (4). In our patient also, there was no clinical or radiological evidence of any smooth muscle tumour elsewhere in the body. Given the nature of the lesion within the liver and symptoms, resection was the only definitive treatment. Thus left lateral segmentectomy in our patient was a diagnostic cum therapeutic procedure. Radiologically the lesion was not typical for any of the more common benign neoplasms within the liver—FNH or a typical hepatic adenoma. There are no typical diagnostic radiological findings for a hepatic leiomyoma (5). Radiologically hypervascularity within the tumour may be suggestive but not diagnostic of a liver leiomyoma (5). Malignant potential of benign leiomyomas increases with size and differentiation becomes more difficult as the size increases (5). Increasing size, along with high cellularity, nuclear pleomorphism, a high mitotic index and degenerative features are more suggestive of malignant change within a leiomyoma (2). However presence of distant metastases is diagnostic for malignancy (1,2). Generally, for smooth muscle tumours, presence of mitoses >10/10 high power field (HPF), prominent cellular atypia and areas of coagulative necrosis, are more likely to be associated with a malignant smooth muscle tumour (1,6). Histologically the tumour may need differentiation from gastro intestinal stromal tumours (GIST). However, in contrast to GIST, on IHC leiomyomas are negative for GIST markers—CD117 and CD34 (7). It is assumed hepatic leiomyomas arise within the smooth muscle cells from the lining of either the blood vessels or biliary tree within the liver parenchyma (2,4). Smooth muscle tumours, are seen with an increasing prevalence in immunosuppressed patients (2). This may be related to an increased incidence of EBV infection in immunocompromised patients (1), as EBV has been implicated in the pathogenesis of smooth muscle tumours (2,8,9). In patients who are not immunosuppressed, the lesion is more likely to be symptomatic as opposed to immunocompromised states, wherein PHL may be a radiological incidentaloma (1). Our patient did not have any evidence of immunosuppression and she was not on any immunosuppressants either. On CT scan, these lesions demonstrate brisk, mainly peripheral enhancement in the arterial phase and persistent enhancement in the portal venous and delayed phases as well (10). However, there are no specific defined imaging findings, pathognomonic for the condition. Given the rarity of the condition, it is not always possible to predict the condition radiologically (2). Liver resection has become a relatively standardized and safe procedure over the last decade, with most high volume centers reporting an operative mortality below 5% among all comers and less than 1% in non-cirrhotic livers (11). Furthermore, liver resections can be safely performed laparoscopically, without compromising on margins (12,13). Perini et al. reported the first case of laparoscopic liver resection for a PHL (1). In a case similar to ours, they performed a laparoscopic left lateral sectorectomy (1). The first instance of PHL was reported by Demel in 1926. Luo et al. in a recent publication, reviewed the literature on the subject and summarized 28 cases of PHL resected before their case (7).
This report happens to be the 30th reported case of PHL in medical literature. Including this case and the case reported by Luo, two-thirds of the PHL have occurred in women (7).
Given these disease demographics, the rare nature of the condition, its predominance among women and its reported association with immunosuppression and EBV infection, establishment of an international PHL registry with tissue banking and analysis, may provide better insights and collaboration into understanding of this rare disease.
When a solid lesion is discovered within the liver, differential diagnosis includes FNH, adenoma, hemangioma as well as hepatocellular carcinoma (HCC). Hence when an accurate radiological diagnosis is not always possible, resection is indicated as a diagnostic cum therapeutic procedure. This is particularly true when the diagnosis of an incidental lesion being benign is impossible to be established with certainty on imaging. In our case the presence of a significant sized symptomatic solid lesion within the liver was a clear indication for surgery. The symptoms in our patient correlated well with the location of the lesion. Furthermore there was a complete resolution of her symptoms post-surgery. In keeping with the standard diagnostic algorithm for a liver mass, since the patient was symptomatic-resection was indicated, there was no necessity to obtain a pre-operative tissue diagnosis.
Conclusions
PHL is a very rare condition with select isolated instances reported. The presence of significant symptoms along with the presence of a solid lesion within the liver, was a sufficient clinical indication to proceed with a resection. Although rare, the possibility of PHL should be borne in mind. Pre-operatively imaging studies should be carried out to rule out coexisting conditions or other causes of such a lesion. Once a diagnosis of PHL is suspected or established (following histology), as mentioned by Hawkins appropriate and complete imaging studies should be carried out (4). Absence of any such coexisting leiomyomas will confirm and finally establish the diagnosis of PHL. Appropriate staining and IHC for smooth muscle antibody and staining should be done to confirm the diagnosis.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Perini MV, Fink MA, Yeo DA, et al. Primary liver leiomyoma: a review of this unusual tumour. ANZ J Surg 2013;83:230-3. [PubMed]
- Santos I, Valls C, Leiva D, et al. Primary hepatic leiomyoma: case report. Abdom Imaging 2011;36:315-7. [PubMed]
- Choi HS, Jung CW, Cho SY, et al. Primary myxoid leiomyoma of the liver. Korean J Pathol 2014;48:54-7. [PubMed]
- Hawkins EP, Jordan GL, McGavran MH. Primary leiomyoma of the liver. Successful treatment by lobectomy and presentation of criteria for diagnosis. Am J Surg Pathol 1980;4:301-4. [PubMed]
- Urizono Y, Ko S, Kanehiro H, et al. Primary leiomyoma of the liver: report of a case. Surg Today 2006;36:629-32. [PubMed]
- Valenti MT, Azzarello G, Vinante O, et al. Differentiation, proliferation and apoptosis levels in human leiomyoma and leiomyosarcoma. J Cancer Res Clin Oncol 1998;124:93-105. [PubMed]
- Luo XZ, Ming CS, Chen XP, et al. Epstein-Barr virus negative primary hepatic leiomyoma: case report and literature review. World J Gastroenterol 2013;19:4094-8. [PubMed]
- Sclabas GM, Maurer CA, Wente MN, et al. Case report: hepatic leiomyoma in a renal transplant recipient. Transplant Proc 2002;34:3200-2. [PubMed]
- Kanazawa N, Izumi N, Tsuchiya K, et al. A case of primary leiomyoma of the liver in a patient without evidence of immunosuppression. Hepatol Res 2002;24:80. [PubMed]
- Marin D, Catalano C, Rossi M, et al. Gadobenate dimeglumine-enhanced magnetic resonance imaging of primary leiomyoma of the liver. J Magn Reson Imaging 2008;28:755-8. [PubMed]
- Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg 2002;236:397-406; discussion 406-7. [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825-30. [PubMed]
- Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg 2009;250:831-41. [PubMed]