Selective right middle and lower lobar blockade for minimally invasive cardiac surgery: a prospective, single-center, randomized controlled study
Introduction
Minimally invasive cardiac surgery (MICS) through right lateral mini-thoracotomy may be an alternative to conventional surgery for patients with mitral valve disease (1). Compared with open surgery, MICS has greater advantages, such as fewer blood transfusion and shorter hospital stay (2), and increasingly more cardiac centers are now performing this surgery as a routine procedure (3).
MICS via the right chest typically requires lung isolation for surgical field exposure, which can be achieved by a double-lumen endotracheal tube (DLT) or a single-lumen endotracheal tube with a bronchial blocker (BB). However, hypoxemia is associated with one-lung ventilation (OLV) after cardiopulmonary bypass (CPB) (4). The surgical position of MICS patients also increases intrapulmonary shunt due to the smaller ventilation volume by the left lung (5). Moreover, CPB induces hemodilution, lung interstitial edema, and lung ischemia–reperfusion injury, which impact on pulmonary function and are associated with a significant reduction in oxygenation after CPB (6). Interventions of hypoxemia during OLV include recruitment maneuver, positive end-expiratory pressure (PEEP) to the ventilated lung, and continuous positive airway pressure (CPAP) to the remaining lung. In some patients, hypoxemia cannot be reversed by these methods, nor with FiO2 1.0. Therefore, anesthesiologists often choose double-lung ventilation (DLV) to maintain oxygenation and the surgical procedure is interrupted.
Patients who had previously undergone contralateral lung lobectomy might be at risk of hypoxemia during OLV due to the limited ventilation volume. For these patients, BB’s selective lobar blockade (SLB) should be the preferred ventilation strategy (7). Mini-thoracotomy, which is a MICS, is performed in the fourth intercostal space, where the middle and lower lobes of the right lung are located. In the present study, we present a novel modified lung isolation strategy by blocking the right middle and lower lobes and preserving the right upper lobe. We compared this modified lung isolation strategy with conventional OLV by DLT.
We present the following article following the CONSORT reporting checklist (available at http://dx.doi.org/10.21037/atm-20-986).
Methods
Research population
The present study was a prospective, randomized controlled trial. Patients were enrolled between August 2018 and February 2019. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Committee of Zhongshan Hospital, Fudan University (approval number: B2016-022R). All patients provided signed informed consent. The study has been registered on the Clinical trials website (registration number: NCT03505242).
Inclusion and exclusion criteria
The inclusion criteria were as follows: (I) 18–70 years of age; (II) European System for Cardiac Operative Risk Evaluation II 0–5; and (III) scheduled to undergo elective MICS through right lateral mini-thoracotomy and femoral cannulation for CPB.
The exclusion criteria were as follows: (I) body mass index >30 kg/m2; (II) coronary artery disease; (III) chronic obstructive pulmonary disease or asthma; (IV) previous thoracic or cardiac surgery; (V) infectious endocarditis; and (VI) predicted difficult airway.
Patient allocation
Enrolled patients were randomly divided into 2 groups (computerized random number, https://www.randomizer.org) 1 day before surgery: a conventional lung isolation strategy group (group C) and a modified lung isolation strategy group (group M). DLTs were used for lung isolation in group C, while BBs were used in group M to block the bronchus intermedius for the right middle and lower lobes collapse during lung isolation.
Anesthesia
Patients were monitored with an electrocardiogram and pulse oximetry; a central vein catheter and radial artery catheter were then cannulated. Sufentanil, propofol, and rocuronium were used for induction, following tracheal intubation with a Macintosh laryngoscope (Truphatek International Ltd., Netanya, Israel). Intraoperative transesophageal echocardiography was used for all patients. Sevoflurane inhalation (propofol infusion during CPB) was adopted to maintain intraoperative amnesia, along with sufentanil and rocuronium for analgesia and muscle relaxation.
Airway management strategy
Lung isolation method
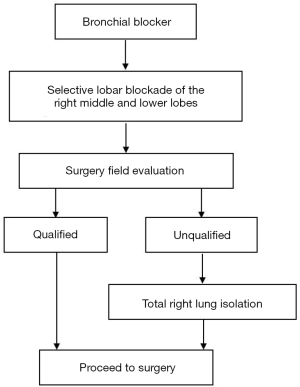
In group C, the left main bronchus diameter was measured by preoperative chest computed tomography. The 35F left DLT was selected when the diameter ≤11 mm, the 37F DLT, was used for 11–12 mm, and the 39F DLT was used for >12 mm. After intubation, pediatric fiberoptic bronchoscopy (FOB) was applied for appropriate position confirmation. In group M, the ID 8-mm tracheal tube was selected for male patients, and the ID 7.5-mm tracheal tube was selected for female patients. After intubation, FOB was applied to measure the tracheal tube’s depth to ensure that the tip of the tube was placed 3–4 cm away from the main carina. The BB’s cuff was placed in the bronchus intermedius with the guidance of FOB (Figure 1). The cuff position was reconfirmed by auscultation in both groups. All procedures were performed by an attending anesthesiologist who had experience in 10 cases for each method. Also, we recorded the time between the start of the tracheal intubation to the end of lung isolation confirmation.

For pleurectomy to commence, apnea had to last for 1 min in both groups, and the bronchial cuffs in both groups were then inflated to start lung isolation. After the retractor was fixed between the ribs, group M’s surgical field exposure was evaluated (Figure 2). If the inflated right upper lobe affected the ascending aorta and aortic root’s surgical exposure, mechanical ventilation was suspended for pericardial traction (if hypoxemia occurred during this period, manually controlled DLV with a small tidal volume was applied to assist the pericardial traction). The surgical field exposure was reassessed after restarting mechanical ventilation. If the exposure was still unsatisfactory, the SLB was changed to right lung isolation (Figure 3). Times of intraoperative dislodgement or loss of seal were also recorded.

Intraoperative mechanical ventilation
After tracheal intubation, volume-controlled mechanical ventilation mode without PEEP was initiated at FiO2 1.0, with oxygen flow at 2 L/min. During DLV, Tidal volume was 8 mL/kg (ideal body weight), and the initial respiratory rate was 10/min. Tidal volume during lung isolation was 6 mL/kg (ideal body weight), and the initial respiratory rate was 12/min. The inspiratory pause was set to 20% of the total inspiratory time, and the respiratory rate was adjusted to maintain end-tidal CO2 at 40±5 mmHg. Mechanical ventilation was stopped during intracardiac surgical procedures in CPB. Ventilation with lung isolation continued after the aortic unclamping, and the bilateral lung recruitment (pressure 40 cmH2O for 20 s, the same standard as follows) was performed before CPB weaning. The bloody secretion from the airway suction was defined as airway bleeding. When the surgery was completed, patients in group C were reintubated with a single-lumen endotracheal tube. At the same time, BB was removed in group M. After bilateral lung recruitment, the patients were transferred to the intensive care unit (ICU) for continued respiratory support.
Interventions for hypoxemia
Hypoxemia was defined as SpO2 <90%. If hypoxemia occurred during lung isolation, a recruitment maneuver (same standard as described above) was performed to the ventilated lung, and then ventilation under lung isolation continued with 5 cmH2O PEEP. DLV was implemented if hypoxemia had not resolved. Lung isolation was restarted when SpO2 >95%, and then CPAP of 5 cmH2O was applied to the remaining lung. DLV was directly implemented if hypoxemia occurred in the next lung isolation period.
Surgery and CPB procedure
The patient was placed in the supine position with a wedge under the right chest. The femoral artery and vein were cannulated to establish CPB. After incision at the fourth intercostal space and pericardium traction, CPB commenced. The priming solution contained 1,500 mL Lactated Ringer’s injection, 500 mL Gelofusine, and 100 mL of 20% albumin. A target body temperature of 32 °C and non-pulsating perfusion utilizing a roller pump was adopted.
Furthermore, α state was used for blood gas management. After aortic clamping and antegrade perfusion of blood-containing del Nido cardioplegia (8), surgery was performed through the interatrial groove or the right atrial incision. After rewarming, norepinephrine, dobutamine, and milrinone were transfused to assist weaning from CPB if necessary. Conventional ultrafiltration was performed during CPB with a target amount of priming fluid, cardioplegia, and surgical rinsing saline. Residual blood in the surgical field and circuit were collected in an autotransfusion system (Cell Saver Elite; Haemonetics). After the hemostasis was completed, a chest tube was placed, and the incision was closed.
Data collection
The respiratory rate, peak airway pressure (Ppeak), and plateau pressure (Pplat) during DLV and lung isolation were recorded in both groups before and after CPB. Blood gas analysis and PaO2/FiO2 were collected 4 times. T0 indicated data before anesthesia induction (FiO2 0.21), T1 indicated data at 10 min after weaning from CPB (if DLV were performed due to hypoxemia within 10 min, the sample would be collected when the lowest SpO2 occurred in the next lung isolation period), T2 indicated data after bilateral lung recruitment at the end of the surgery, and T3 indicated data 24 h after surgery. The operation duration, CPB, aortic clamping, and surgical hemostasis (time between weaning from CPB to the end of surgery), along with volumes, including ultrafiltration, fluid infusion, blood transfusion, and urine, were recorded. The minimum hemoglobin level and the maximum creatinine and cardiac troponin T levels after surgery were also recorded.
Primary and secondary endpoints
The primary endpoint was to determine the number of patients who required recruitment maneuvers on the ventilated lung due to hypoxemia during lung isolation after CPB.
The secondary endpoints were to determine the number of patients who required DLV due to hypoxemia during lung isolation after CPB; the time between the start of tracheal intubation to definite confirmation of the position of the lung isolation device, airway bleeding, and dislodgement; the differences in Ppeak and Pplat during DLV and lung isolation; PaO2/FiO2 at T0, T1, T2, and T3 and differences between groups; and evaluation of surgical field exposure [as scored by the surgeon after surgery (1–5 points, 1 indicating worst exposure and 5 indicating best exposure)].
Sample size calculation
Approximately 40% of patients suffered hypoxemia during lung isolation with DLTs after CPB (unpublished data). However, according to our clinical experience, hypoxemia rarely occurs after CPB with the successful application of an SLB. Assuming that the modified lung isolation strategy could reduce hypoxemia by 75%, 29 patients were required in each group when simulating the probability of α=0.05 and β=0.2. Considering a 20% loss, the number of selected patients in each group was 37.
Statistical analysis
Statistical analysis was performed using SPSS version 25.0 software (IBM, Armonk, NY, USA). Data with normal distribution were expressed as mean ± standard deviation, and those with non-Gaussian distribution were expressed as median and interquartile range. Discrete data were analyzed using the χ2-test or Fisher’s exact test. Continuous data were compared using an independent sample t-test or Mann-Whitney U-test. P<0.05 was considered statistically significant.
Results
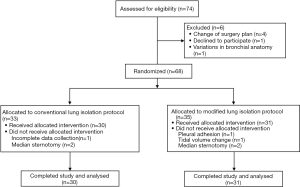
A total of 74 patients were included in the present study. Of these, 6 were excluded and 68 were randomized; 33 cases were assigned to the group C, and 3 were excluded, and 35 cases were assigned to group M, and 4 cases were excluded (Figure 4). No statistical differences were observed for baseline characteristics, comorbidities, preoperative evaluations, and surgery types (Table 1).

Full table
The number of cases in which lung recruitment was performed due to hypoxemia during lung isolation after CPB in group C (50%, 15/30) was significantly greater than in group M (16.1%, 5/31, P=0.005). The number of cases requiring DLV in group C (36.7%, 11/30) was notably greater when compared with group M (6.5%, 2/31) (P=0.004). The time for intubation and position confirmation in group M was prominently longer than group C {390 [344–480] s vs. 221 [179-278] s}. Furthermore, dislodgements in both groups were similar (6 vs. 7). There were 4 cases of airway bleeding in group C and 3 in group M. Surgical field exposure was 5 [4.3–5] in group C, which was higher than that of group M {4 [4–5], P=0.012} (Table 2).

Full table
Five patients in group M were changed to right lung isolation due to poor surgical field exposure. However, after excluding these cases, we also compared patients who successfully underwent SLB in group M (n=26) with group C. The statistical significance of lung isolation-related parameters was the same as the previous result (Table 2). There were significant increases in the value of Ppeak and Pplat during lung isolation in group C, which were higher than that of group M (n=26) before and after CPB (P=0.002 and P<0.001 vs. P=0.008 and P<0.001, respectively) (Table 3).

Full table
Surgical hemostasis time was 47.5 [45–67] min in group C and 55 [47.5–63.5] min in group M (P=0.294). Also, there was no significant difference in the following parameters: time durations of surgery, CPB, and aortic clamping and in the volumes of fluid infusion, ultrafiltration, and urine output. The minimum hemoglobin level measured in group C was 103.7±16.3 g/L, which was not significantly different from 103.9±12.6 g/L in group M (P=0.957). There was no perioperative allogeneic blood transfusion in either group. We found no significant differences in levels of postoperative maximum serum creatinine and cardiac troponin T and in the duration of postoperative mechanical ventilation, ICU discharge, and hospitalization between both groups (Table 4).

Full table
When comparing patients who successfully underwent SLB in group M (n=26) with group C, no significant difference in PaO2/FiO2 at T0, T2, and T3 (P=0.605, P=0.642, and P=0.801) was observed. PaO2/FiO2 at T1 in group C was 93.9±31.4 mmHg, which lower than group M (119.2±52.3 mmHg, P=0.038). PaO2/FiO2 at T1, T2, and T3 were inferior to T0 in both groups (P<0.01) (Table 5).

Full table
Discussion
In the present study, we found that PaO2/FiO2 was markedly lower than the preoperative baseline after CPB, even with DLV. Hypoxemia occurred in 50% of patients undergoing right lung isolation, while 36.7% required DLV to maintain oxygenation. Accordingly, satisfactory field exposure based on adequate oxygenation poses a challenge for anesthesiologists. The modified lung isolation strategy reduced the incidence of hypoxemia during lung isolation after CPB.
Although hypoxemia did not occur in all cases, the modified lung isolation strategy appeared to be more beneficial for patients with poor preoperative respiratory function and those at risk of hypoxemia during lung isolation after CPB. At the same time, SLB provides continuous lung isolation, which may be more appropriate for unskilled surgeons (9), as they can operate without the possibility of hypoxemia affecting the patient’s safety during surgery.
BB was previously used for lung isolation in MICS (10), with only the right main bronchus blocked to achieve the right lung collapse. This might fail to treat possible hypoxemia after CPB, whereas SLB can notably increase the oxygenation index (11). In the case of robot-assisted coronary artery bypass grafting, the patient can encounter severe hypoxemia when the BB is used to isolate the left lung. Subsequently, BB’s cuff was placed forward into the left upper lobe bronchus for SLB, after which hypoxemia was resolved and it was possible to proceed with the operation (12). We first attempted to apply SLB in MICS through right lateral mini-thoracotomy with BB blocking the bronchus intermedius (Figure 5).

With the ventilated right upper lobe, PaO2/FiO2 during lung isolation after CPB was significantly higher in group M than group C; however, it was quite low. Besides the impact of CPB, it might be due to rare lung recruitment in group M, FiO2 1.0, and relatively low tidal volume during lung isolation without PEEP, all of which resulted in atelectasis and affected oxygenation (13). With the increased volume of the right upper lobe in group M, Ppeak and Pplat values in group M were remarkably lower than those in group C under the same tidal volume setting, reducing the collapsed lung volume and incidence of lung injury (14).
Field exposure was important for surgical hemostasis, along with no difference in postoperative minimum hemoglobin level and hemostasis time between the 2 groups in our study. The surgical field evaluation score was lower in group M than group C. This could be due to surgeons feeling more comfortable operating in the cavity with the collapsed whole right lung and the original narrow vision becoming increasingly squeezed by the inflated right upper lobe, even when the operation targets were not covered. Also, for 5 cases in group M, the right upper lobe occluded the field at both inspiratory and expiratory phases, indicating that SLB might not be suitable for all patients. However, the exposure significantly improved after the BB cuff was retracted to the right main bronchus.
The advantages of DLT for lung isolation include absolute lung separation, easy suction, convenient and reliable conversion between OLV, and DLV. The disadvantages include difficulty selecting the appropriate size, unsuitability for difficult airways, and possible tracheobronchial injury. BB’s advantages include easy recognition of the carina anatomy through the trachea, suitability for difficult airway, and unnecessity to replace a tracheal tube after surgery. The disadvantages include difficult suction and potential dislodgement or loss of seal (15). It takes more time for BB to acquire a suitable position for lung isolation (16). Although lung isolation by the BB is a basic skill for anesthesiologists, the option may not be routine in clinical practice and requires a learning curve (17). When SLB is implemented, the time for positioning inevitably increases. The operation time is relatively shortened without replacing DLT with a single-lumen endotracheal tube after surgery. Airway edema can also occur after cardiac surgery, leading to the risk of replacing the endotracheal tube, where the BB has a prominent advantage (18). Also, we found no differences in airway bleeding and dislodgement, further supporting the modified lung isolation strategy’s safety and efficacy.
The present study has some limitations. First, the surgeons were not blinded to the interventions. Furthermore, the evaluation for surgical field exposure was based on subjective feelings and lacked objective evidence for lung collapse (19). This could have biased the evaluation. Second, a previously published study showed no difference in the time taken to replace a DLT with a single-lumen endotracheal tube using an exchange catheter compared to the BB, which was related to operation room efficiency (10). We did not collect related time data in our study, so lacking of the data to analyses the difference between two study groups. Finally, a recent study showed that the incidence of postoperative sore throat and hoarseness in patients using the BB were lower than DLT (20). In our study, patients in group C were reintubated with an accustomed single-lumen tracheal tube with a smaller size than that of group M (7.5# for males, 7.0# for females), so no relevant follow-up was performed.
Conclusions
The novel modified lung isolation strategy reduced hypoxemia during lung isolation after CPB in patients who underwent right lateral mini-thoracotomy, a MICS.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-986
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-986
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-986). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The present study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Committee of Zhongshan Hospital, Fudan University (approval number: B2016-022R). All patients provided signed informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Falk V, Cheng DCH, Martin J, et al. Minimally invasive versus open mitral valve surgery: a consensus statement of the international society of minimally invasive coronary surgery (ISMICS) 2010. Innovations 2011;6:66-76. [Crossref] [PubMed]
- Downs EA, Johnston LE, LaPar DJ, et al. Minimally invasive mitral valve surgery provides excellent outcomes without increased cost: a multi-institutional analysis. Ann Thorac Surg 2016;102:14-21. [Crossref] [PubMed]
- Schmitto JD, Mokashi SA, Cohn LH. Minimally-invasive valve surgery. J Am Coll Cardiol 2010;56:455-62. [Crossref] [PubMed]
- Kottenberg-Assenmacher E, Kamler M, Peters J. Minimally invasive endoscopic port-access intracardiac surgery with one lung ventilation: impact on gas exchange and anaesthesia resources. Anaesthesia 2007;62:231-8. [Crossref] [PubMed]
- Campos JH, Feider A. Hypoxia during one-lung ventilation-a review and update. J Cardiothorac Vasc Anesth 2018;32:2330-8. [Crossref] [PubMed]
- Clark SC. Lung injury after cardiopulmonary bypass. Perfusion 2006;21:225-8. [Crossref] [PubMed]
- Campos JH. Update on selective lobar blockade during pulmonary resections. Curr Opin Anaesthesiol 2009;22:18-22. [Crossref] [PubMed]
- Ad N, Holmes SD, Massimiano PS, et al. The use of del Nido cardioplegia in adult cardiac surgery: A prospective randomized trial. J Thorac Cardiovasc Surg 2018;155:1011-8. [Crossref] [PubMed]
- Sakaguchi T, Totsugawa T, Kuinose M, et al. Minimally invasive mitral valve repair through right minithoracotomy- 11-Year single institute experience. Circ J 2018;82:1705-11. [Crossref] [PubMed]
- Grocott HP, Darrow TR, Whiteheart DL, et al. Lung isolation during port-access cardiac surgery: double-lumen endotracheal tube versus single-lumen endotracheal tube with a bronchial blocker. J Cardiothorac Vasc Anesth 2003;17:725-7. [Crossref] [PubMed]
- Campos JH. Effects on oxygenation during selective lobar versus total lung collapse with or without continuous positive airway pressure. Anesth Analg 1997;85:583-6. [Crossref] [PubMed]
- Agrawal DR, Nambala S, Fartado A. Selective lobar blockade in minimally invasive coronary artery bypass grafting: A technical advantage in patients with low respiratory reserve that precludes one-lung ventilation. Ann Card Anaesth 2016;19:542-4. [Crossref] [PubMed]
- Minkovich L, Djaiani G, Katznelson R, et al. Effects of alveolar recruitment on arterial oxygenation in patients after cardiac surgery: a prospective, randomized, controlled clinical trial. J Cardiothorac Vasc Anesth 2007;21:375-8. [Crossref] [PubMed]
- O'Gara B, Talmor D. Perioperative lung protective ventilation. BMJ 2018;362:k3030. [Crossref] [PubMed]
- Campos JH. Which device should be considered the best for lung isolation: double-lumen endotracheal tube versus bronchial blockers. Curr Opin Anaesthesiol 2007;20:27-31. [Crossref] [PubMed]
- Clayton-Smith A, Bennett K, Alston RP, et al. A comparison of the efficacy and adverse effects of double-lumen endobronchial tubes and bronchial blockers in thoracic surgery: a systematic review and meta-analysis of randomized controlled trials. J Cardiothorac Vasc Anesth 2015;29:955-66. [Crossref] [PubMed]
- Rispoli M, Zani G, Bizzarri F, et al. Bronchial blocker positioning: learning curve and confidence in its use. Minerva Anestesiol 2018;84:1254-60. [Crossref] [PubMed]
- Vernick WJ, Woo JY. Anesthetic considerations during minimally invasive mitral valve surgery. Semin Cardiothorac Vasc Anesth 2012;16:11-24. [Crossref] [PubMed]
- Bussieres JS, Somma J, Del Castillo JL, et al. Bronchial blocker versus left double-lumen endotracheal tube in video-assisted thoracoscopic surgery: a randomized-controlled trial examining time and quality of lung deflation. Can J Anaesth 2016;63:818-27. [Crossref] [PubMed]
- Zhang C, Yue J, Li M, et al. Bronchial blocker versus double-lumen endobronchial tube in minimally invasive cardiac surgery. BMC Pulm Med 2019;19:207. [Crossref] [PubMed]
(English Language Editors: R. Scott and J. Chapnick)