Robotic-assisted left upper lobectomy
Clinical data
Medical history
The patient, a 53-year-old woman, was admitted due to “lesion in the left lung found during health check-up 2 years ago” and “a space-occupying lesion in the upper lobe of left lung”. Two years ago, chest CT during health check-up showed a ground-glass opacity (GGO) at the edge of the upper lobe of left lung. However, no special treatment was given. Ten days ago, the patient visited our hospital due to spinal joint conditions. Chest CT showed a space-occupying lesion in the upper lobe of left lung. The lesion has slightly irregular border and unclear margin, with mild pleural retraction. The lesion was slightly enlarged compared with that in the CT image 2 years ago. The patient’s complaints did not include cough/expectoration, chest tightness, shortness of breath, low fever, night sweats, nausea, vomiting, abdominal distension, diarrhoea, heart palpitations, or discomfort of precordial area. His mental status, physical performance, appetite, and sleep were normal, and the body weight did not obviously change. Urination and defecation were normal.
Physical examination
Physical examinations upon admission showed no obviously positive signs. The cervical and supraclavicular lymph nodes were not abnormally enlarged.
Auxiliary examination
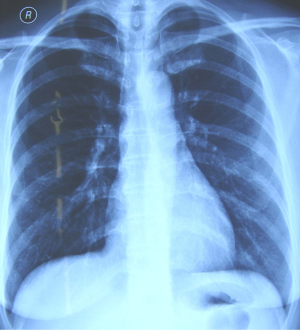
Chest X-ray had no abnormal findings in both lungs (Figure 1).
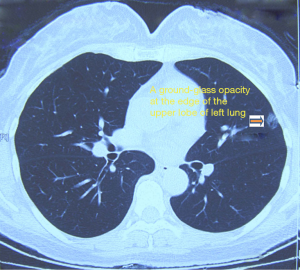
Chest CT showed a space-occupying GGO sized 1.0 cm in the upper lobe of left lung (near the pleural membrane). The lesion has slightly irregular border and unclear margin, with mild pleural retraction. The mediastinal lymph nodes were slightly swollen (Figure 2).
Epigastric ultrasound and bone ECT did not find the evidence of remote metastasis. Other surgical contraindications including thyroid nodules and breast nodules were ruled out after multidisciplinary consultations.
No obvious abnormality was found in ECG, echocardiography, pulmonary function test, blood gas analysis, and other biochemical tests.
Pre-operative preparation
Based on the imaging results, “a space-occupying lesion in the upper lobe of left lung” was considered, and the lesion showed no change during the follow-up visits; however, the possibility of malignancy could not be ruled out. Lesion inspection and wedge resection were planned. The subsequent surgical protocol was determined based on the results of intraoperative frozen section biopsy. (If the lesion was found to be malignant in the frozen biopsy, resection of the upper lobe of left lung and lymph node dissection would be performed). The surgery was planned to be completed using da Vinci robotic system. Since the lesion was small and thus difficult to locate during the surgery, CT-guided puncture of the lesion was performed before the surgery, and methylene blue solution was injected at the pleural membrane to assist lesion-locating. The patient was directly sent to the operation room after the lesion was located.
Procedures
Anesthesia and body position
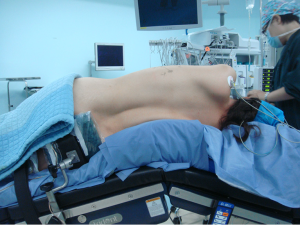
After the induction of general anesthesia, the patient was under double-lumen endotracheal intubation and directly underwent left-sided one-lung ventilation, so as to avoid the aggravation of pneumothorax caused by puncture. With the patient lying on her right side and with her hands put in front of head, he was fixed in a Jackknife position with single-lung (right) ventilation (Figure 3).
Surgical procedures
Incisions
A 1.5-cm camera port was created in the 8th intercostal space at left posterior axillary line, and two 1.0-cm working ports were separately made in the 5th intercostal space at left anterior axillary line and the 9th intercostal space at scapular line. A 4-cm auxiliary port was made in the 7th intercostal space at midaxillary line (Figure 4).
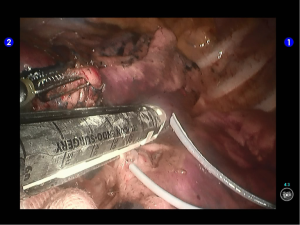
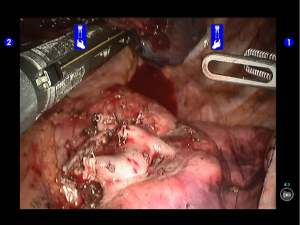
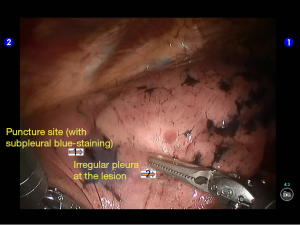
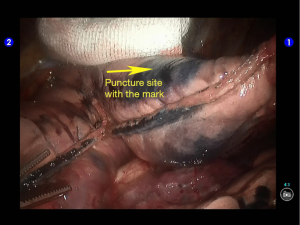
During the thoracic cavity inspection, the camera was inserted via the camera port and found no obvious adhesion in the thoracic cavity; however, a small amount of coagulated bloody fluid was visible, which might be caused by puncture. The puncture site was located at the lateral side of the upper lobe (near the oblique fissure), which was clearly visible after subpleural blue-staining (Figure 5).
The robot Patient Cart were connected over the patient’s head. A 12-mm trocar was placed at the camera port in the 8th intercostal space at posterior axillary line to be attached with the camera arm. The robot metal trocars were respectively attached to the 2# arm (left hand) and 1# arm (right hand) at the incisions in the 5th ICS at anterior axillary line and the 9th ICS at scapular line. Incision protector was applied in the auxiliary port.
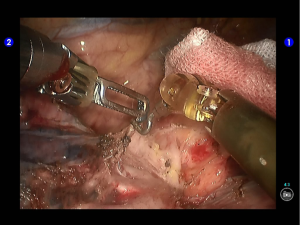
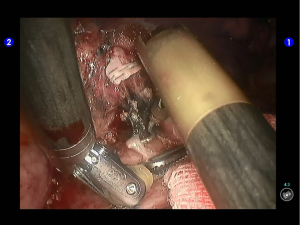
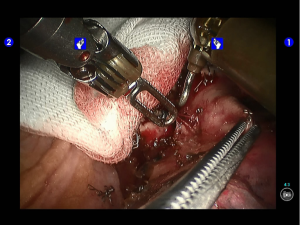
Pulmonary wedge resection: After the puncture site and lesion were located, the lesion was found to be with small size and good mobility and without external invasion. Wedge resection was decided. An endoscopic linear cutter/stapler (Ethicon Echelon Flex 60) was inserted via the auxiliary port. Wedge resection of the lingular segment of the upper lobe of left lung was performed using two blue reloads 2 cm away from the tumor (Figure 6). An endoscopic retriever was inserted via the auxiliary port to harvest the divided specimen (Figure 7), which was a soft mass, gray-white in color and sized about 1cm. It was immediately sent for frozen pathology.
Quick frozen pathology indicated that it was an atypical alveolar type II epithelial cell hyperplasia; cancer.
Lobectomy
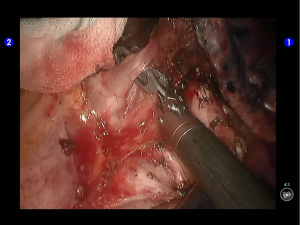
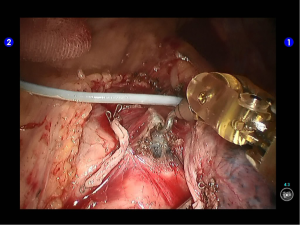
The right arm was re-connected with unipolar cautery hook. After the inferior pulmonary ligament was divided till the inferior lung vein level, the lymph nodes at the pulmonary ligament were removed (Figures 8,9).
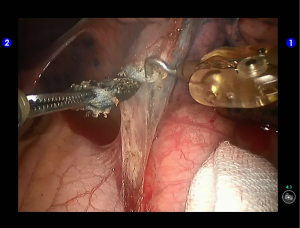
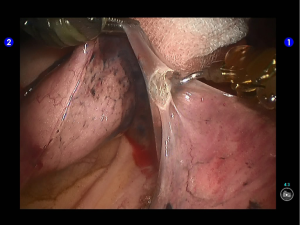
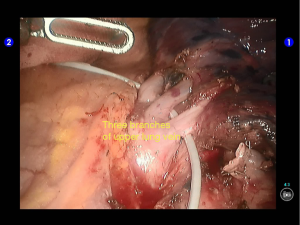
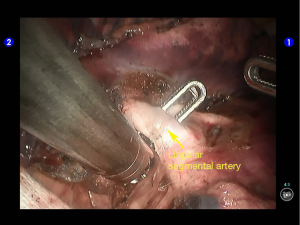
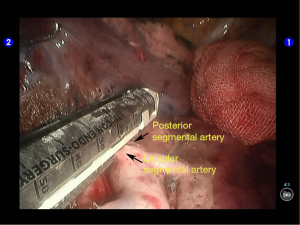
Inspection showed that the oblique fissure was well developed. Open the oblique fissure with the cautery hook, dissociate several branches of the pulmonary artery (two in lingular segments and two in posterior segments), and remove the lymph nodes among fissures (Figures 10-13).
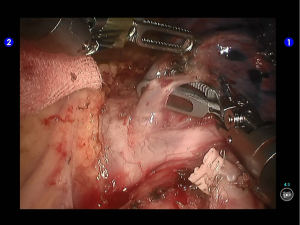
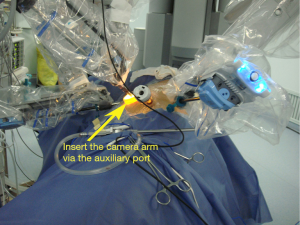
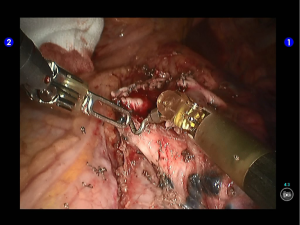
Dissociate the upper pulmonary vein (3 branches) (Figures 14-16), which was further suspended and pulled with elastic cuffs (Figure 17). Move and insert the camera arm via the auxillary port (Figure 18). The stapler was inserted through the camera port, and the vein was transected using a white reload (Figure 19).
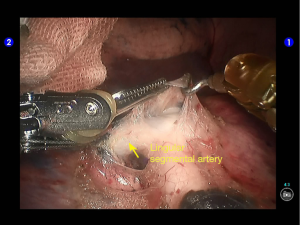
Move the camera arm back to the camera port. Insert the cutter/stapler via the auxiliary port. Transect one branch of the sublingular artery using a white reload, and then remove the lymph nodes behind the vessel (Figures 20-22).
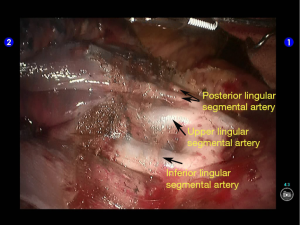
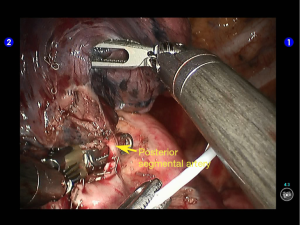
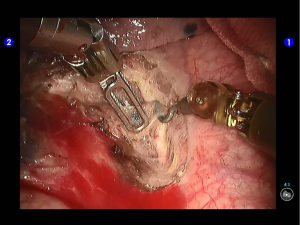
The superior lingular segmental artery, together with the inferior branch of the posterior segmental artery, was transected with a white reload (Figures 23,24), and the superior branch of the posterior segmental artery was transected with a white reload (Figures 25,26).
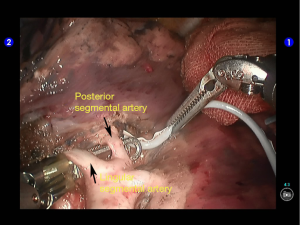
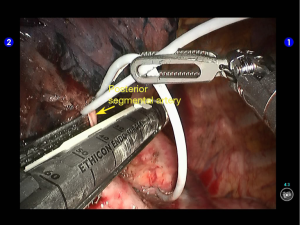
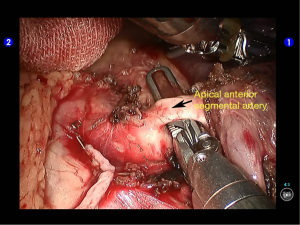
Dissociate the apical anterior segmental artery, and then transect it using a white reload (Figures 27,28).
Remove lymph nodes (Figures 29-32).
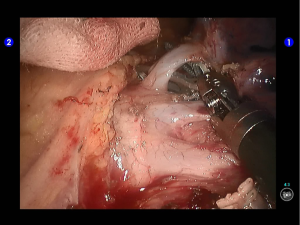
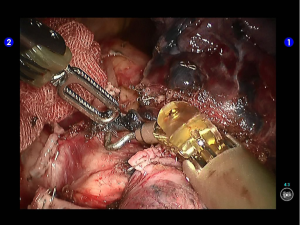
Clamp the upper lobe bronchus using a golden reload. An anesthesiologist was asked to suction sputum and ventilate the operated lung. After CXR revealed good expansion of the lower lobe, transect the bronchus and then divide the upper lobe. An endoscopic retriever was inserted via the auxiliary port to harvest the divided specimen (Figures 33,34).
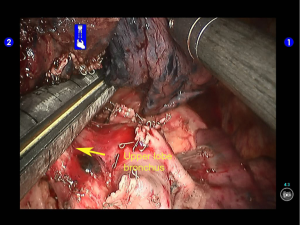
Open the posterior mediastinal pleura to remove the subcarinal and subaortic lymph nodes. Remove the interlobar lymph nodes. No swollen lymph node was found during aorta inspection (Figures 35,36).
When no obvious bleeding was observed at all the trauma surfaces, wash the thoracic cavity. If no air leakage was observed during lung recruitment and the inferior lobe was well expanded, suction all the rinsing water.
The hilar trauma surfaces and the subcarinal area were sprayed and covered with the sol of TISTAT absorbable hemostatic gauze.
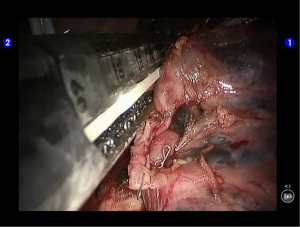
The thoracic drainage tube was indwelled at the working port at the 5th ICS and at the camera port, respectively. Close the chest after lung recruitment (Figure 37).
Postoperative treatment
Postoperative treatment is similar to that after the conventional open lobectomy. The thoracic drainage tube was withdrawn 7 days after the surgery. The post-operative pathological stage was pT1aN0M0. Currently the patient was under follow-up visits.
Pathological diagnosis
Well-differentiated adenocarcinoma in the upper lobe of left lung, sized about 1 cm. No cancer cell was detected at the bronchial stump or the hilar/mediastinal lymph nodes.
Comment
Resection of the upper lobe of left lung is the most difficult procedure in lobectomy. The vessels in this area have multiple branches and variations. Thus, resection of the left upper lobe is particularly challenging either under endoscope or using the robotic system. A successful surgery is often based on the factors including proper one-lung ventilation, appropriate body position and incision selection, clear exposure and anatomic relationships, as well as the skills and teamwork of operator and assistants. In our current case, the patient had well developed lung fissures. Thus, the vessels were dissociated firstly and then handled one by one. In patients with poorly developed lung fissures, the dissection of lung fissures and vessels will be difficult. A single direction procedure then can be adopted, during which the upper lung vein, apical and anterior segmental branches of lung artery, and upper lobe bronchus were transected one by one, followed by the handling of the lung fissures and the remaining arteries. Notably, during the handling of the bronchus, the cutter/stapler may hurt the pulmonary trunk or posterior segmental artery by mistake because the posterior segmental artery is not transected and the gap behind the bronchus is small. Clear exposure is particularly important to avoid such unnecessary injuries. In addition, the cutter/stapler can easily handle the oblique fissure, arteries, and bronchus via the auxiliary port created in the 7th intercostal space at midaxillary line. However, since there is a large angle in handling the upper lung vein, we need to move the camera to the auxiliary port and then insert the cutter/stapler via the camera port. This is a routine step in endoscopic surgeries but seems a bit complicated in the robotic surgeries. Thus, a skillful assistant who is familiar with the performances of the robotic arms is critically important.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflict of interest to declare.