Neuroprotective effects of porphyran derivatives against 6-hydroxydopamine-induced cytotoxicity is independent on mitochondria restoration
Introduction
Parkinson’s disease (PD) is a common neurodegenerative disease characterized by the progressive degeneration of the nigral dopaminergic neurons, leading to dopamine depletion in the striatum and various clinical symptoms. PD is associated with several risk factors including aging, genetic susceptibility or environmental factors, which could act synergistically in promoting degeneration of dopaminergic neurons (1-5). Among them, oxidative stress and free radicals are one of the most common mechanisms in the pathogenesis of PD (6,7). Therefore, natural components that exhibit antioxidant properties are drawing great attention as potential candidates for the treatment of PD.
Polysaccharides which are the main components of algae have been demonstrated to serve as free-radical scavengers and antioxidants against oxidative damage in living organisms (8,9) Some modifications, such as acetylation and phosphorylation, could enhance its biological functions (10,11). Porphyra haitanensis is an important economic alga in southern China. We previously reported that acetylated and phosphorylated derivatives of porphyran [acetylated porphyran (AP) and phosphorylated porphyran (PP)] extracted from Porphyra haitanensis exhibit antioxidant activity. The data suggested that AP showed more significant effect on superoxide radical and reducing power than porphyran, while phosphorylated derivatives had much stronger hydroxyl radical scavenging activities than porphyran in cell-free system (12). In the present study, we aim to study whether porphyran and its two derivatives could exert neuroprotective effects on dopaminergic cells against 6-OHDA-induced neurotoxicity, which are commonly used in inducing PD models. MES23.5 cells were used as the experimental neuronal model, a dopaminergic cell line hybridized from murine neuroblastoma-glioma N18TG2 cells with rat mesencephalic neurons exhibiting several properties similar to the primary neurons originated in the substantia nigra (13). The data presented here using 6-OHDA-treated MES23.5 cells as a model for PD suggest that two derivatives of porphyran, AP and PP, could antagonize the weak toxicity of 6-OHDA on MES23.5 dopaminergic cells, possessing minor neuroprotective effects independent of mitochondria restoration.
Materials and methods
Materials
Porphyran, AP and PP were prepared as described previously (14). Dulbecco’s modified Eagle’s medium Nutrient Mixture-F12 (Ham; DMEM/F12) was from Gibco (Gibco, Grand Island, NY, USA). MES23.5 cells was a dopaminergic cell line hybridized from murine neuroblastoma-glioma N18TG2 cells with rat mesencephalic neurons (13). It was kindly provided by Dr. Weidong Le (Department of Neurology and Molecular Physiology and Biophysics, Baylor College of Medicine, Houston, Texas, USA). All other chemicals and regents were of Sigma Chemical Co (St Louis, Missouri, USA) or the highest grade available from local commercial sources.
Cell culture
MES23.5 cells were cultured in DMEM/F12 containing Sato’s components growth medium supplemented with 5% fetal bovine serum (FBS), 100 units/mL penicillin and 100 μg/mL streptomycin at 37 °C, in a humid 5% CO2, 95% air environment. For experiments, cells were seeded at a density of 1×105/cm2 in the plastic flasks. Porphyran, AP and PP were dissolved in serum-free DMEM/F12 to a final concentration of 10 mg/mL, stored at −20 °C. Then they were diluted with DMEM/F12 without serum to different concentrations (1, 10−1, 10−2 mg/mL) before usage. To study the protective effects of porphyran, AP and PP, they were added to the medium with 6-OHDA (25 or 75 μmol/L) for 24 hours, respectively.
Cell viability assay
Cell viability was determined by the conventional 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. MES23.5 cells were seeded in a 96-well plate at a density of 1×104 cells/well. After attachment, cells were treated with different concentrations of porphyran, AP, PP alone, or together with 25/75 μmol/L 6-OHDA in DMEM/F12 without serum supplement for 24 hours, respectively. After incubation, cells were incubated in MTT (5 mg/mL) for 4 hours at 37 °C, the medium was removed and 100 μL of DMSO was added to each well and the formazan dye crystals were solubilized. The absorbance was measured at 570 nm by colorimetric assay (Spectra Max M5, Molecular Devices).
Mitochondrial transmembrane potential (ΔΨm) measurement
Changes in the ΔΨm with various treatment in MES23.5 cells were measured by rhodamine123 using flow cytometry (Becton Dickinson, USA) as described before (15). The uptake of rhodamine123 into mitochondria is an indicator of the ΔΨm. Fluorescent intensity was recorded at 488 nm excitation and 525 nm emission wavelength [fluorescence (FL1)]. The gated region M1 was set automatically as a threshold when cells without rhodamine123 incubation were analyzed. That means most of the cells without rhodamine123 fluorescence should be located in M1 region and most of the normal cells with rhodamine123 fluorescence should be located in M2 region. Cells treated as described above were incubated in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)-buffered saline (HBS) containing rhodamine123 in a final concentration of 5 μmol/L for 30 minutes at 37 °C and followed by washing twice with HBS. For analysis, data was collected as the percentage of cells which were rhodamine123 fluorescence-positive using CellQuest Software.
Statistical analysis
Data were presented as means ± SEM. Normal distribution of data and homogeneity of variance were verified by normality test and homogeneity test (P>0.05). One-way analysis of variance (ANOVA) followed by Turkey’s test was use to compare the differences between different groups. A probability value of P<0.05 was considered to be statistically significant.
Results
Effects of porphyran, AP and PP on cell viability in MES23.5 dopaminergic cells
Using MTT assay, we detected the changes in cell viability of MES23.5 cells treated with different concentrations of porphyran, AP and PP. When treated with porphyran, AP and PP lower than 1 mg/mL respectively, cell viability was unchanged compared with that of control (Figure 1). However, a significant reduction in cell viability was observed when cells were treated with 3 mg/mL or 10 mg/mL porphyran (Figure 1A), AP (Figure 1B) and PP (Figure 1C).

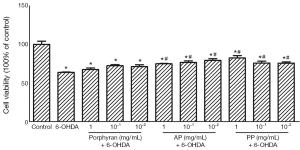
AP and PP rather than porphyran antagonized 25 µmol/L 6-OHDA-induced cytotoxicity in MES23.5 cells
The neuroprotective effects of porphyran and its derivatives against 6-OHDA-induced cytotoxicity were assessed in cultured MES23.5 cells by MTT assay. As shown in Figure 2, the cell viability was reduced to 63.7%±1.8% in 25 μmol/L 6-OHDA-treated group. When treated with 1, 10−1, 10−2 mg/mL AP or PP, the cell viability could be observed a slightly increase compared with that of control, indicating that they possess a certain neuroprotective property. However, raw porphyran did not have any protective effect (Figure 2).
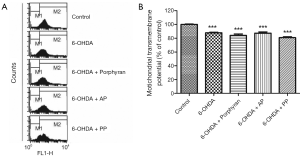
AP and PP could not prevent 6-OHDA-induced mitochondrial transmembrane potential (ΔΨm) collapse
ΔΨm is the marker of mitochondria function, which is involved in a variety of key events in oxidative stress. Next in order to explore the mechanisms underlying the neuroprotective effects of AP and PP, we measured the changes in ΔΨm of the above groups. Unfortunately, neither AP nor PP pretreatment could antagonize 6-OHDA-induced ΔΨm collaps (Figure 3).
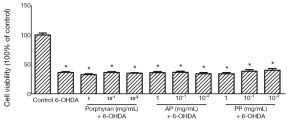
Porphyran, AP and PP could not antagonize 75 µmol/L 6-OHDA-induced cytotoxicity in MES23.5 cells
The neuroprotective effects of porphyran and its derivatives against 6-OHDA-induced cytotoxicity were further assessed in a high dose of 6-OHDA treatment. The cell viability was dramatically reduced to 37% with 75 μmol/L 6-OHDA treatment. Unfortunately, none of them showed cytoprotective effects against 6-OHDA-induced reduction in cell viability (Figure 4).
Discussion
In this study we demonstrated that two derivatives of porphyran (acetylated and phosphorylated forms) could exert neuroprotective effects against a lower dose of 6-OHDA-induced neurotoxicity. However, these effects were weak when compared with the classical antioxidant N-acetylcysteine (NAC) or other natural derivatives (such as curcumin, Rg1, myricetin et al.) reported before in our laboratory (15-18). We also demonstrated that both AP and PP could not antagonize 6-OHDA induced ΔΨm collapse, indicating this neuroprotection was independent of mitochondria restoration.
Oxidative stress was implicated in the pathogenesis of PD (19). 6-OHDA lesioning is a well established method for preparing PD models (20,21) which is related to both intracellular or extracellular oxidation, and it was also shown that 6-OHDA could inhibit the mitochondrial respiratory chain through inhibiting Complex I, uncoupling oxidative phosphorylation and collapsing mitochondrial membrane (22). In the present study, 6-OHDA was chosen as a neurotoxin to induce oxidative damage in MES23.5 dopaminergic cells. We previously reported porphyran had strong antioxidant activity (23). However, it may not be a simple stoichiometric reaction and the function of porphyran was biphasic with higher doses causing damage as shown in Figure 1. In that cell-free system, both raw material and certain derivatives exerted scavenging effects for superoxide/hydroxyl radical and reducing power, with derivatives being more powerful. In accordance with these results, we revealed in the present study that both AP and PP, but not porphyran itself, effectively antagonized 25 μmol/L 6-OHDA-induced cytotoxicity. To explore the underlying mechanisms, we further measured the mitochondrial transmembrane potential in 25 μmol/L 6-OHDA-treated cells and 10-2 mg/mL AP or PP co-treated cells. However, both AP and PP could not prevent 6-OHDA-induced ΔΨm collapse, indicating that the protective mechanism of AP and PP might be due to the antioxidative effects as what they showed in the previous publication (12), however, irrelevant to mitochondria function. Moreover, the protective effect could only be achieved in a low concentration (25 μmol/L) of 6-OHDA-treated cells but not be observed in cells with dramatically decreased cell viability due to a higher concentration (75 μmol/L) of 6-OHDA treatment.
In summary, the present study demonstrates that two derivatives, AP and PP, exert minor cytoprotective effects, even if they have stronger antioxidant activities than raw material. Mitochondrial restoration was not involved in this process.
Acknowledgements
We thank Dr. Wei-Dong Le for giving us the MES23.5 cell line.
Funding: This work was supported by National Natural Science Foundation of China (81171207, 31471114) and Excellent Innovative Team of Shandong Province and Taishan Scholars Construction Project.
Authors’ contributions: W. Wang performed cell viability assay and flow cytometric measurement of mitochondrial transmembrane potential. N. Song performed cell viability assay and analyzed data. F. Jia performed cell viability assay. J. Xie participated in the experiment design and drafted the manuscript. Q. Zhang participated in the experiment design. Hong Jiang participated in the experiment design, analyzed data, and drafted the manuscript.
Disclosure: The authors declare no conflict of interest.
References
- Reeve A, Simcox E, Turnbull D. Ageing and Parkinson's disease: why is advancing age the biggest risk factor? Ageing Res Rev 2014;14:19-30. [PubMed]
- Sulzer D. Multiple hit hypotheses for dopamine neuron loss in Parkinson's disease. Trends Neurosci 2007;30:244-50. [PubMed]
- Trinh J, Farrer M. Advances in the genetics of Parkinson disease. Nat Rev Neurol 2013;9:445-54. [PubMed]
- Goldman SM. Environmental toxins and Parkinson's disease. Annu Rev Pharmacol Toxicol 2014;54:141-64. [PubMed]
- Shulman JM, De Jager PL, Feany MB. Parkinson's disease: genetics and pathogenesis. Annu Rev Pathol 2011;6:193-222. [PubMed]
- Gaki GS, Papavassiliou AG. Oxidative stress-induced signaling pathways implicated in the pathogenesis of Parkinson's disease. Neuromolecular Med 2014;16:217-30. [PubMed]
- Smeyne M, Smeyne RJ. Glutathione metabolism and Parkinson's disease. Free Radic Biol Med 2013;62:13-25. [PubMed]
- Hu JF, Gen MY, Zhang JT, et al. An in vitro study of the structure-activity relationships of sulfated polysaccharide from brown algae to its antioxidant effect. J Asian Nat Prod Res 2001;3:353-8. [PubMed]
- Rupérez P, Ahrazem O, Leal JA. Potential antioxidant capacity of sulfated polysaccharides from the edible marine brown seaweed Fucus vesiculosus. J Agric Food Chem 2002;50:840-5. [PubMed]
- Liang ZY, Mao CY, Zhang YS. Influence of chemical-modified structures on antitumor activity of polysaccharides from clitopilus caespitosus. Chin Pharm J 1996;30:613-5.
- Chen XM, Zhang J, Tian GY. Studies on Synthesis and Antitumor Activity of Phosphorylated Achyranthes bidentata Polysaccharide (P-AbPS). Chinese J Chem 2002;20:1406-10.
- Zhang ZS, Zhang QB, Wang J, et al. In vitro antioxidant activities of acetylated, phosphorylated and benzoylated derivatives of porphyran extracted from Porphyra haitanensis. Carbohyd Polym 2009;78:449-53.
- Crawford GD Jr, Le WD, Smith RG, et al. A novel N18TG2 x mesencephalon cell hybrid expresses properties that suggest a dopaminergic cell line of substantia nigra origin. J Neurosci 1992;12:3392-8. [PubMed]
- Zhang Z, Zhang Q, Wang J, et al. Preparation of the different derivatives of the low-molecular-weight porphyran from Porphyra haitanensis and their antioxidant activities in vitro. Int J Biol Macromol 2009;45:22-6. [PubMed]
- Jiang H, Song N, Xu H, et al. Up-regulation of divalent metal transporter 1 in 6-hydroxydopamine intoxication is IRE/IRP dependent. Cell Res 2010;20:345-56. [PubMed]
- Wang J, Du XX, Jiang H, et al. Curcumin attenuates 6-hydroxydopamine-induced cytotoxicity by anti-oxidation and nuclear factor-kappa B modulation in MES23.5 cells. Biochem Pharmacol 2009;78:178-83. [PubMed]
- Xu H, Jiang H, Wang J, et al. Rg1 protects iron-induced neurotoxicity through antioxidant and iron regulatory proteins in 6-OHDA-treated MES23.5 cells. J Cell Biochem 2010;111:1537-45. [PubMed]
- Zhang K, Ma Z, Wang J, et al. Myricetin attenuated MPP(+)-induced cytotoxicity by anti-oxidation and inhibition of MKK4 and JNK activation in MES23.5 cells. Neuropharmacology 2011;61:329-35. [PubMed]
- Sorce S, Krause KH, Jaquet V. Targeting NOX enzymes in the central nervous system: therapeutic opportunities. Cell Mol Life Sci 2012;69:2387-407. [PubMed]
- Ungerstedt U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur J Pharmacol 1968;5:107-10. [PubMed]
- Singhal NK, Srivastava G, Agrawal S, et al. Melatonin as a neuroprotective agent in the rodent models of Parkinson's disease: is it all set to irrefutable clinical translation? Mol Neurobiol 2012;45:186-99. [PubMed]
- Blum D, Torch S, Lambeng N, et al. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson's disease. Prog Neurobiol 2001;65:135-72. [PubMed]
- Zhao T, Zhang Q, Qi H, et al. Extension of life span and improvement of vitality of Drosophila melanogaster by long-term supplementation with different molecular weight polysaccharides from Porphyra haitanensis. Pharmacol Res 2008;57:67-72. [PubMed]


