Rate of early onset Alzheimer’s disease: a systematic review and meta-analysis
Introduction
Early onset Alzheimer’s disease (EOAD), with onset of symptoms at a young age (1,2), usually has a higher prevalence of atypical manifestations with earlier multi domain cognitive impairment when compared to late-onset Alzheimer’s disease (LOAD) cases (3). As we all know, Alzheimer’s disease (AD) is the most common neurodegenerative dementia (4,5), and it would turn devastated when it occurs at a young age. EOAD disproportionately impacts daily life. In addition, the psychological and medical toll to treat EOAD patients is also significantly.
There is no definitive definition for EOAD. And the cutoff age of EOAD is not definitive, either. EOAD is generally accepted as AD patients with onset before 65 years of age (1). This cutoff point, 65 years old, is generally regarded as a sociological partition according to employment and retirement age. However, the cutoff point has no specific biological significance. But a range of disease features appear across this arbitrary divide. Hence, it is reasonable to choose 65 years of age as the cutoff point of EOAD.
Although more attention has been paid to pathophysiology and treatment of EOAD, epidemiological data for rate of EOAD is sparse. It is generally accepted that EOAD accounts for 1% to 2% of AD cases (6). However, the percentage needs to be affirmed further. Hence, we performed a systematic review and meta-analysis of all studies that presented original data to calculate a rate of EOAD in AD. Then analysis of the population based rate was undertaken to make a geographic comparison.
Methods
Systematic search
Electronic searches of Cochrane Library, Embase, Medline and PubMed databases were used to identify published articles and studies. Medical Subject Headings (MeSH) terms and keywords included “EOAD”, “Early Onset Alzheimer Disease”, “Presenile Alzheimer Dementia”, “Alzheimer Disease, Early Onset”, “incidence”, “prevalence” and “epidemiology” were used to look for related studies. Additional trials were searched from previous related reviews and reference lists of included papers. These included studies were published in the period from 1985 to 2013. When no information reported on the rate of EOAD in a population based study, we tried to contact the corresponding author to get data.
Study selection
The eligible studies for our meta-analysis from the initial search were all according to the following criteria: (I) Participants: the definition of EOAD is defined as AD patients with onset before 65 years of age. The diagnosis of AD can follow many criteria, such as the National Institute of Neurological Disorders and Stroke-Alzheimer Diseases and Related Disorders Association Working Group criteria (NINCDS-ADRDA) (7,8), the Diagnostic and Statistical Manual of Mental Disorders (DSM) (9), the Clinical Dementia Rating Scale (CDR) (10,11), or the International Classification of Diseases, 9th edition (ICD-9) (12), and Mini-Mental State Examination (MMSE) score (13). In addition, AD can be diagnosed by Clinical manifestations (14). Our diagnostic criteria of EOAD participants have two points: firstly, they were diagnosed as AD patients; secondly, they were younger than 65 years old. (II) Outcome: we included studies which presented original data with the count of EOAD and total AD cases. We excluded the studies that did not contain statistical information. The participants with front-temporal dementia, vascular dementia, or other rarer forms of dementia were also excluded. In addition, studies with non-random enrolment for EOAD participants were also excluded from our analysis.
Data extraction and quality assessment
Two reviewers independently read the appropriate articles and extracted data according to predefined criteria. The final valid statistics of each outcome were the count of EOAD and AD cases. Additionally, data for country of study origin and characteristics of participants (number, age, female, and diagnostic criteria) were also extracted. When conflicts appeared in inclusion, exclusion or data extraction, disagreement was resolved with the third author through review and discussion. The Agency for Healthcare Research and Quality (AHRQ) evaluation standard was a common tool for observational studies to assess the quality of prevalence studies in a meta-analysis (http://www.ncbi.nlm.nih.gov/books/NBK35156/). Our meta-analysis was assessed by AHRQ, which totally had 11 items to evaluate the quality of these included studies.
Statistical analysis
We use the Meta function of the Meta Analyst 3.13 software to combine proportions. Random effect model was performed in our meta-analysis. According to the heterogeneity, fixed or random effect model was then performed in our meta-analysis. The effect of heterogeneity was quantified using I2 =100% × (Q − df)/Q (15). When a significant I2-statistic (I2 >50%) appeared, heterogeneity was thought existed in studies, then meta-analysis was conducted in random effect model (15).
Results
Literature search and characteristics of included study
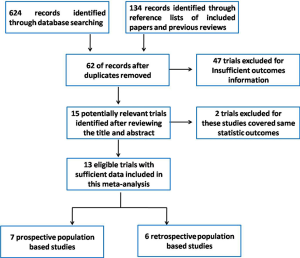
Finally, a total of 15 relevant articles seemed to fulfill the inclusion criteria after the application of search strategy, and the search strategy was presented in Figure 1. Among the 15 trials, two trials were from Zhang group and these two trials contained same amounts of EOAD and AD patients, hence we only included one in our meta-analysis. In addition, two studies performed by V. Chandra were conducted among the same cohort (a rural Hindi-speaking population in Ballabgarh in northern India) (16,17), we chose the one with higher quality in our meta-analysis (16). These included articles were published between 1985 and 2013. In addition, these included trials were all sporadic forms of EOAD. These included trials were conducted in Europe, America and Asia. Finally, our study included a total of 1,274 EOAD patients and 11,982 AD cases. Data details of the included studies are presented in Table 1.
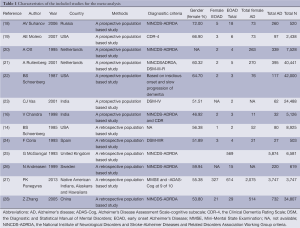
Full table
Methodological quality and data available for analysis
In our meta-analysis, we used AHRQ evaluation standard to assess the quality of included trials. There are 11 items to assess the quality of these included studies, and Table S1 showed these results by presenting the main statistical results on every measurement scale.
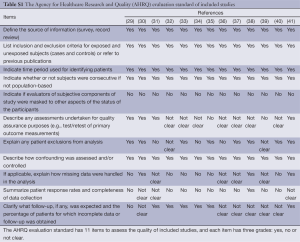
Full table
Meta-analysis
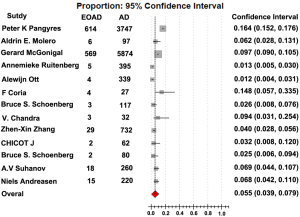
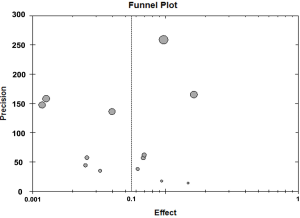
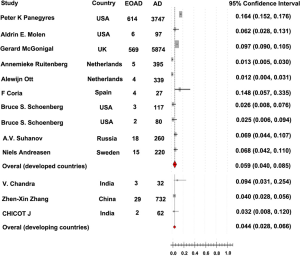
The rate of EOAD in AD, 5.5% [95% confidence interval (CI): 0.039-0.079, P<0.001], was generated after pooled analysis of all studies. We chose the outcome analyzed in random effect model, and the Forrest plot of the results in random effect model was demonstrated in Figure 2. The value of I2 is 0.486 indicating low heterogeneity exists in this study. In addition, the funnel plot of the results was seen in Figure S1. There were eight countries included in our meta-analysis. Among these six were developed countries, two were developing countries. After analysis, the rate of EOAD in AD is 6.9% (95% CI: 0.044-0.107) in Russia, 5.7% (95% CI: 0.019-0.160) in USA, 1.2% (95% CI: 0.006-0.023) in Netherlands, 6.8% (95% CI: 0.042-0.110) in Sweden, 14.8% (95% CI: 0.057-0.335) in Spain and 9.7% (95% CI: 0.090-0.105) in UK. The pooled analysis of the rate in developed country was 5.9% [95% CI: (0.040-0.085), P<0.001]. This result of the meta-analysis shows low heterogeneity (I2=0.487) about the rate of EOAD in developed countries. The rate of EOAD was 5.9% (95% CI: 0.020-0.159) in India and 4.0% (95% CI: 0.028-0.056) in China. The pooled analysis of the rate in developing countries was 4.4% [95% CI: 0.028-0.066, P<0.001]. In addition, the result of meta-analysis indicates low heterogeneity (I2=0.101) about the rate of EOAD in developing countries. The individual rate of EOAD for each country was demonstrated in Figure 3. In addition, we found P value of the rate of EOAD between developing countries and developed countries was less than 0.05, indicating that significant difference of the rate of EOAD exits between developing countries and developed countries.
Discussion
Numerous studies have paid attention to examine the incidence of LOAD. However, there seems to be a paucity of epidemiologic data about the frequency of EOAD, not to mention the epidemiological research which explored the rate of EOAD cases among AD cases. EOAD is a devastating condition for the patients and their families. Hence, more attention should be paid to EOAD and it is necessary to figure out the answer of the matter about the rate of EOAD cases in AD cases.
This systematic review and meta-analysis is made up of 13 studies. According to our meta-analysis based on population enriched studies, generally reported rates of EOAD (1-2%) from previous studies are likely biased. Since our study was conducted by population based studies in a defined geographic area during a given period of time, the final population based rate, 6.1%, is likely to represent more accurate estimation about the rate of EOAD.
As shown in Figure 3, we notice that the rate of EOAD varies among different countries. These geographic differences may result from variability in the underlying genetic structure. In addition, the pooled analyses of the rate in developed and developing countries are significant different. Moreover, the rate in developed countries consistent with the finally pooled analysis of all countries, and the rate in developed countries are relative higher than in developing countries. These outcomes may result from that developed countries have more robust basic medical care, hence, more EOAD population in developed countries would be discovered and diagnosed timely. Maybe the rate in developed counties is more close to the accurate prevalence of EOAD in world, and this is consistent with our outcomes.
Our meta-analysis still has several potential limitations. First, the account of the trials included in our meta-analysis was relatively small, and the included trials only covered eight countries. Therefore, it is a weak argument to reveal the accurate rate over the world. Second, APOE, age, and sex play a vital role in EOAD. Hence, more studies are needed to perform to explore the possible APOE-, age- and gender-dependent effect. Lastly, our included trials were all sporadic EOAD, we did not figure out the difference between sporadic and familial forms of EOAD.
In summary, our meta-analysis first offered some evidence of the potential rate of EOAD among AD cases. And the present rate (6.1%) is higher than the generally accepted rate (1-2%). The result of our meta-analysis draws more attention to EOAD. But our meta-analysis still has several limitations. Therefore, further trials with larger samples across more countries and careful design of experiment are required to confirm whether our findings are truly significant.
Acknowledgements
Funding: This work was supported by grants from the National Natural Science Foundation of China (81471309, 81371406, 81171209), the Shandong Provincial Outstanding Medical Academic Professional Program, Qingdao Key Health Discipline Development Fund, and Qingdao Outstanding Health Professional Development Fund.
Disclosure: The authors declare no conflict of interest.
References
- Rossor MN, Fox NC, Mummery CJ, et al. The diagnosis of young-onset dementia. Lancet Neurol 2010;9:793-806. [PubMed]
- Jiang T, Yu JT, Tan L. Novel disease-modifying therapies for Alzheimer's disease. J Alzheimers Dis 2012;31:475-92. [PubMed]
- Smits LL, Pijnenburg YA, Koedam EL, et al. Early onset Alzheimer's disease is associated with a distinct neuropsychological profile. J Alzheimers Dis 2012;30:101-8. [PubMed]
- Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med 2010;362:329-44. [PubMed]
- Jiang T, Yu JT, Tian Y, et al. Epidemiology and etiology of Alzheimer's disease: from genetic to non-genetic factors. Curr Alzheimer Res 2013;10:852-67. [PubMed]
- Sassi C, Guerreiro R, Gibbs R, et al. Exome sequencing identifies 2 novel presenilin 1 mutations (p.L166V and p.S230R) in British early-onset Alzheimer's disease. Neurobiol Aging 2014;35:2422.e13-6. [PubMed]
- Lopez OL, Swihart AA, Becker JT, et al. Reliability of NINCDS-ADRDA clinical criteria for the diagnosis of Alzheimer's disease. Neurology 1990;40:1517-22. [PubMed]
- Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol 2007;6:734-46. [PubMed]
- Gomez R, Harvey J, Quick C, et al. DSM-IV AD/HD: confirmatory factor models, prevalence, and gender and age differences based on parent and teacher ratings of Australian primary school children. J Child Psychol Psychiatry 1999;40:265-74. [PubMed]
- Miller JM, Pliskin NH. The clinical utility of the Mattis Dementia Rating Scale in assessing cognitive decline in Alzheimer's disease. Int J Neurosci 2006;116:613-27. [PubMed]
- Juva K, Sulkava R, Erkinjuntti T, et al. Usefulness of the Clinical Dementia Rating scale in screening for dementia. Int Psychogeriatr 1995;7:17-24. [PubMed]
- ICD-9-CM. International Classification of Diseases, 9th revision, Clinical Modification. 3d edition, volumes 1, 2 and 3. Official authorized addendum effective October 1, 1990--HCFA. J Am Med Rec Assoc 1990;61:suppl 1-35.
- Squitti R, Barbati G, Rossi L, et al. Excess of nonceruloplasmin serum copper in AD correlates with MMSE, CSF [beta]-amyloid, and h-tau. Neurology 2006;67:76-82. [PubMed]
- Schoenberg BS, Anderson DW, Haerer AF. Severe dementia. Prevalence and clinical features in a biracial US population. Arch Neurol 1985;42:740-3. [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [PubMed]
- Chandra V, Ganguli M, Pandav R, et al. Prevalence of Alzheimer's disease and other dementias in rural India: the Indo-US study. Neurology 1998;51:1000-8. [PubMed]
- Chandra V, Pandav R, Dodge HH, et al. Incidence of Alzheimer's disease in a rural community in India: the Indo-US study. Neurology 2001;57:985-9. [PubMed]
- Suhanov AV, Pilipenko PI, Korczyn AD, et al. Risk factors for Alzheimer's disease in Russia: a case-control study. Eur J Neurol 2006;13:990-5. [PubMed]
- Molero AE, Pino-Ramirez G, Maestre GE. High prevalence of dementia in a Caribbean population. Neuroepidemiology 2007;29:107-12. [PubMed]
- Ott A, Breteler MM, van Harskamp F, et al. Prevalence of Alzheimer's disease and vascular dementia: association with education. The Rotterdam study. BMJ 1995;310:970-3. [PubMed]
- Ruitenberg A, Ott A, van Swieten JC, et al. Incidence of dementia: does gender make a difference? Neurobiol Aging 2001;22:575-80. [PubMed]
- Schoenberg BS, Kokmen E, Okazaki H. Alzheimer's disease and other dementing illnesses in a defined United States population: incidence rates and clinical features. Ann Neurol 1987;22:724-9. [PubMed]
- Vas CJ, Pinto C, Panikker D, et al. Prevalence of dementia in an urban Indian population. Int Psychogeriatr 2001;13:439-50. [PubMed]
- Coria F, Gomez de Caso JA, Minguez L, et al. Prevalence of age-associated memory impairment and dementia in a rural community. J Neurol Neurosurg Psychiatry 1993;56:973-6. [PubMed]
- McGonigal G, Thomas B, McQuade C, et al. Epidemiology of Alzheimer's presenile dementia in Scotland, 1974-88. BMJ 1993;306:680-3. [PubMed]
- Andreasen N, Blennow K, Sjodin C, et al. Prevalence and incidence of clinically diagnosed memory impairments in a geographically defined general population in Sweden. The Pitea Dementia Project. Neuroepidemiology 1999;18:144-55. [PubMed]
- Panegyres PK, Chen HY. Differences between early and late onset Alzheimer's disease. Am J Neurodegener Dis 2013;2:300-6. [PubMed]
- Zhang ZX, Zahner GE, Roman GC, et al. Dementia subtypes in China: prevalence in Beijing, Xian, Shanghai, and Chengdu. Arch Neurol 2005;62:447-53. [PubMed]
- Schoenberg BS, Anderson DW, Haerer AF. Severe dementia. Prevalence and clinical features in a biracial US population. Arch Neurol 1985;42:740-3. [PubMed]
- Chandra V, Ganguli M, Pandav R, et al. Prevalence of Alzheimer's disease and other dementias in rural India: the Indo-US study. Neurology 1998;51:1000-8. [PubMed]
- Suhanov AV, Pilipenko PI, Korczyn AD, et al. Risk factors for Alzheimer's disease in Russia: a case-control study. Eur J Neurol 2006;13:990-5. [PubMed]
- Molero AE, Pino-Ramirez G, Maestre GE. High prevalence of dementia in a Caribbean population. Neuroepidemiology 2007;29:107-12. [PubMed]
- Ott A, Breteler MM, van Harskamp F, et al. Prevalence of Alzheimer's disease and vascular dementia: association with education. The Rotterdam study. BMJ 1995;310:970-3. [PubMed]
- Ruitenberg A, Ott A, van Swieten JC, et al. Incidence of dementia: does gender make a difference? Neurobiol Aging 2001;22:575-80. [PubMed]
- Schoenberg BS, Kokmen E, Okazaki H. Alzheimer's disease and other dementing illnesses in a defined United States population: incidence rates and clinical features. Ann Neurol 1987;22:724-9. [PubMed]
- Vas CJ, Pinto C, Panikker D, et al. Prevalence of dementia in an urban Indian population. Int Psychogeriatr 2001;13:439-50. [PubMed]
- Coria F, Gomez de Caso JA, Minguez L, et al. Prevalence of age-associated memory impairment and dementia in a rural community. J Neurol Neurosurg Psychiatry 1993;56:973-6. [PubMed]
- McGonigal G, Thomas B, McQuade C, et al. Epidemiology of Alzheimer's presenile dementia in Scotland, 1974-88. BMJ 1993;306:680-3. [PubMed]
- Andreasen N, Blennow K, Sjodin C, et al. Prevalence and incidence of clinically diagnosed memory impairments in a geographically defined general population in Sweden. The Pitea Dementia Project. Neuroepidemiology 1999;18:144-55. [PubMed]
- Panegyres PK, Chen HY. Differences between early and late onset Alzheimer's disease. Am J Neurodegener Dis 2013;2:300-6. [PubMed]
- Zhang ZX, Zahner GE, Roman GC, et al. Dementia subtypes in China: prevalence in Beijing, Xian, Shanghai, and Chengdu. Arch Neurol 2005;62:447-53. [PubMed]