Pacemaker insertion
Introduction
Artificial cardiac pacemakers (PMs) are small electronic devices, approximately the size of a matchbox and weight of 20-50 g that sense intrinsic heart rhythm and transmit electrical impulses, if indicated, to stimulate the heart and replace the defective natural PM, the sinus node.
Dr. Ake Senning was the first to implant a PM in a human being in 1958; it lasted for only a few hours. Since then, for more than 50 years, PMs have been the treatment for choice for bradyarrhythmia and heart block (1-4).
The rate of implantation is increasing annually. For PMs the 10-year average growth rate is 4.7% and for implantable cardioverter defibrillator (ICD) is 15.1% in the UK (5).
PMs can be either temporary or permanent. Temporary PMs are used for short-term heart problems, such as arrhythmias caused by myocardial infraction and also in emergencies. Permanent are for chronic cardiac rhythm dysfunction. In this chapter, permanent PMs are the ones to be discussed. There are three different kind of permanent cardiac pacing devices: (I) single-chamber PMs-VVI: one pacing lead is implanted in the right ventricle or right atrium; (II) dual-chamber PMs-DDD: two leads are implanted (in the right ventricle and in the right atrium); this is the most common type of implanted PM, (III) biventricular PMs-BiV, also called cardiac resynchronization therapy (CRT): in addition to single- or dual-chamber right heart pacing leads, a lead is advanced to the coronary sinus for left ventricular epicardial pacing. CRT-P includes pacing and CRT-D includes defibrillation. CRT is mainly implanted to patients with heart failure, improving symptoms and quality of life (3,4). Indications for implantation of permanent PMs divided into three classes, as defined by the ACC/AHA/HRS guidelines for device-based therapy of cardiac rhythm abnormalities (6-8). Absolute and relative indications are shown in Table 1.
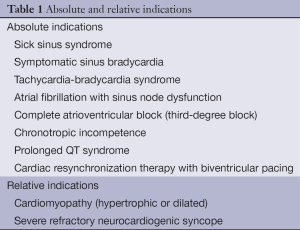
Full table
An ICD is recommended as primary therapy in survivors of cardiac arrest due to ventricular fibrillation or hemodynamically unstable ventricular tachycardia. ICD indications are secondary prophylaxis against sudden cardiac death and primary prophylaxis (9).
Technique of implantation
A PM consists of: (I) a pulse generator which contains all the computerized information to sense the intrinsic cardiac electric potentials and to stimulate cardiac contraction, and a battery; (II) leads, which are wires with electrodes at their tips. These leads connect the heart to the generator and transfer all the data between them (2).
Implantation of permanent PM is performed in a cardiac catheterization laboratory under local or less common general anesthesia and is considered to be a minimally invasive procedure. Transvenous access to the heart chambers is the preferable technique, commonly via a percutaneous approach of the subclavian vein, the cephalic vein (cut-down technique), or rarely the axillary vein, the internal jugular vein or the femoral vein (4). In some cases both subclavian vein and cephalic vein are punctured. The most common transvenous route is the left or right subclavian vein, entered at the junction of the middle and inner thirds, where the first rib and the clavicle are joined. The vein is usually blindly punctured, unless there are certain anatomical abnormalities, such as chest wall or clavicle deformation. In these cases an initial brief intravenous contrast injection-venography is attempted in the peripheral arm vein. After the puncture, a small incision 3.8-5.1 cm is made in the infraclavicular area and a subcutaneous pocket is created, where the generator will be implanted. After successful vein access, a guide wire is advanced and placed on the right atrium or the vena caval area under fluoroscopy. A second guide wire can be positioned, if necessary, via the same route either by a second puncture or by a double-wire technique in which two guide wires are inserted through the first sheath.
A sheath and dilator are advanced, and when sheath is set in the right place the guide wire and the dilator are retracted. Then the lead is inserted into the sheath and advanced under fluoroscopy to the appropriate heart chamber, where is attached to the endocardium either passively with tines or actively via screw-in leads. When implanting a DDD, the ventricular lead is the first to be placed. When leads are securely placed, then the sheath is removed. Specifics tests for sensing and pacing are held and to avoid stimulation of the diaphragm, pacing is set at 10 V. The lead is sewn with a nonabsorbable suture to the underlying tissue and afterwards, the generator is placed to the pocket and connected to the lead. Last, the incision is closed with absorbable sutures and an arm immobilizer is applied for 12-24 hours. The cut-down technique of the cephalic vein demands extensive skin and muscle dissection to visualize the vein. Occasionally, PM can be implanted surgically via a thoracotomy, and the generator is placed in the abdominal area. Antibiotic prophylaxis is compulsory for device implantation, routinely cefazolin 1 g i.v. 1 hour prior to the procedure, or alternatively 1 g vancomycin i.v. in case of allergy to penicillin and/or cephalosporins. The day following the implantation, a chest radiograph in standing position anteroposterior and lateral is performed, to confirm lead position and exclude the complication of pneumothorax (4,10-12).
Complications
From 1993 to 2009, 2.9 million patients received a primary PM in the US (13). Although implantation of PM is a minimal invasive procedure, there is the potential for complications during or after implantation (14-32). The rate for the early complications is 4-5% and for the late complications is 2.7% (14); however these rates can be presented within a wider range in literature due to difficulties in defining and identifying the complications in different studies, which could raise up to 12.6% (31). The technological progress and the increasing experience of the operators have resulted in a significant reduction in frequency of complications (15). These complications can be divided into early (postoperative, during hospitalization and within 30 days) and late [in literature short-term complications are also defined as those, which occur in <3 months (31)], according to implantation time and also procedure- and device-based, as seen on tables. Complications are related to venous access (e.g., pneumothorax), to leads (e.g., lead dislodgement) and the generator pocket (e.g., hematoma) and can be defined as major (e.g., death, cardiac perforation) and minor (e.g., drug reaction, hematoma). Mortality rarely occurs in a rate of 0.08-1.1% (18,24,29,30). Most frequent complications are those related to implantation procedure, such as lead dislodgement and pneumothorax. Implantation of dual chamber devices may be more challenging, however, the difference in complication rates between single and complex pacing is not consistent in all studies probably because of different use of technology and variable experience of operators (16-27). The most common complication is lead dislodgement (higher rate atrial dislodgment than ventricular dislodgment), followed by pneumothorax, infection, bleeding/pocket hematoma, and heart perforation, not necessarily in that order, depending on the study (15-29) (Tables 2,3).
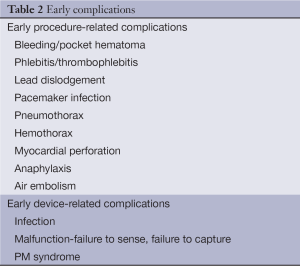
Full table

Full table
Pneumothorax
Pneumothorax is the presence of gas in the pleural space. A spontaneous pneumothorax is one that occurs without antecedent trauma to the thorax. A primary spontaneous pneumothorax occurs in the absence of underlying lung disease, whereas a secondary pneumothorax occurs in its presence. A traumatic pneumothorax results from penetrating or nonpenetrating chest injuries. A tension pneumothorax is a pneumothorax in which the pressure in the pleural space is positive throughout the respiratory cycle (33). Pneumothorax is a major complication of PM implantation, mainly after subclavian puncture technique that can cause patient morbidity and increase the cost of hospitalization (24). Pneumothorax usually develops during the implantation procedure or during the first 48 hours after the implantation.
The incidence of pneumothorax after subclavian vein access varies in the literature from low 0.6-1% to high 5.2% with an average of 2% (11,21,22,24,25,31,32,34-43); the upgrade of the PM involves greater risk than the primal procedure (44). The reasons for this variation are the small sample sizes, the exact definition and clinical recognition of pneumothorax and the identification and report of PM implantation complications (11,42). In a study of short-term implantation-related complications of cardiac rhythm management device therapy in Helsinki, Finland, pneumothorax was defined as “the absence of lung markings over the lung field ipsilateral to the PM pocket assessed from the predischarge X-ray” (31).
Pneumothorax, after subclavian vein puncture attempts, is usually ipsilateral. Contralateral pneumothorax is also reported in the literature, occurring due to the perforation caused by the endocardial atrial lead, which is a rare complication. The screw-in atrial leads increase the risk of perforation through the wall of the right atrial appendage. Operators must be very careful of the anatomy of the right atrial wall and avoid overs crewing the screw-in leds, so the complication of pneumothorax is eliminated (45-47). A pneumothorax could also be involved with pneumopericardium, pneumomediastinum and subcutaneous emphysema.
Risk factors
A population-based cohort study of 28,860 Danish patients (42) identified the risk factors for pneumothorax in cardiac pacing, treated with a chest tube. The most important risk factor appears to be the venous access; blind subclavian vein puncture attempt is of the highest risk followed by the utilization of both subclavian and cephalic cut-down technique. The risk of pneumothorax is higher in female patients (42,48,49), probably because of anatomical characteristics and in patients over 80 years old (18,42) irrelevant to the technique of venous access. Findings revealed an increased risk in 20 to 59 years old patients, because of higher preference in subclavian vein puncture (42). Dual chamber PM implantation is associated with higher rates of incidences of pneumothorax; however it is shown that CRT-P device implantation is not. That could be explained by the fact that high experienced operators perform CRT-P insertions (42). Dual-chamber pacing involves a second subclavian puncture, so that the passage of two leads is accomplished and that is the reason for the higher risk noticed (27). Medical history of chronic obstructive pulmonary disease (COPD) results in a higher risk of pneumothorax. Patients suffering from COPD, most times, demonstrate severe symptoms, so clinicians are more alert, which helps to a greater rate of pneumothorax identification and treatment with a chest tube. Furthermore, COPD itself can cause spontaneous pneumothorax (42,50-52). Longer procedure duration and implantation being performed in a non-university hospital, which probably means less experienced operators, can both influence the risk of pneumothorax and increase it (42). The risk of pneumothorax could be eliminated by puncturing the axillary vein or if the cut-down technique of the cephalic vein is used, however this technique is not the appropriate one for all cases and furthermore, it demands extensive skin and muscle dissection. A fluoroscopic guidance of the subclavian vein puncture instead of the blind one is also helpful and could reduce the risk. Good knowledge of the anatomy of the patient (aware of any deformation of the clavicle or chest abnormality) and careful handlings are essential for the safe accomplishment of the implantation.
Indications of pneumothorax
A chest radiography in standing and in two directions (anteroposterior and lateral), the day after the PM implantation, could prove if the patient has developed pneumothorax, however not all centres perform the radiography as a routine. When a patient demonstrates symptoms of pneumothorax, the chest radiography is obligatory. The chest radiographs should be reviewed for the presence of pneumothorax, preferably by a radiologist. There is a significant possibility that a pneumothorax is underdiagnosed by a chest radiography (11,53). Clinical signs that should mean an alert for a pneumothorax event could be shortness of breath, hypoxia, pleuritic pain and hypotension. If the pneumothorax occurs during the implantation procedure, then the symptoms are sudden; sudden chest pain, respiratory distress, air aspiration during subclavian vein puncture and sudden hypotension. If any of the former signs is present, then an urgent fluoroscopy of the upper lung and critical monitoring should be performed. If the saturation, measured from the fingertip, is less than 90% and/or patient presents hypotension, then the procedure should be terminated (11). Pneumothorax could also be asymptomatic and the only notification of it would be the routine chest radiography.
Treatment of pneumothorax
Treatment for pneumothorax varies from simple aspiration, chest tube drainage to thoracoscopy and thoracotomy. If a tension pneumothorax is developed, then an urgent treatment, mainly with a chest tube is necessary. A small pneumothorax, estimated to involve less than 10% of the lung parenchyma, with a normal physical examination except maybe from tachycardia, should be treated conservatively. A conservative treatment with simple aspiration could be also applied even when there is less than 30% reduction of the lung tissue, as long as there is no haemothorax or severe symptoms. Conservative therapy can reduce time of hospitalization and patient’s morbidity and by avoiding the invasive treatments of chest tube, thoracoscopy and thoracotomy, the complications of pneumothorax are reduced.
When a partial pneumothorax occurs, then the selective treatment is chest tube drainage (11,15,27,54-69). The rate of pneumothorax events, after PM implantation, that demand a chest tube is low (42,70-79) and so is the morbidity that pneumothorax causes (11). The invasive procedures rise the pain and delay rehabilitation, increase hospitalization duration, cost of therapy and radiography exposure (27,80-98).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- British Heart Foundation. Pacemakers. Available online: http://www.bhf.org.uk/heart-health/treatment/pacemakers.aspx
- American Heart Association. Available online: http://www.heart.org/HEARTORG/Conditions/Arrhythmia/PreventionTreatmentofArrhythmia/Artificial-Pacemaker
- NIH-National Heart, Lung and Blood Institute. What Is a Pacemaker? Available online: http://www.nhlbi.nih.gov/health/health-topics/topics/pace
- Yarlagadda C, Lange RA. Permanent Pacemaker Insertion Technique. Available online: http://emedicine.medscape.com/article/1839735-overview
- Heart rhythm devices. UK National Clinical Audit 2009. Available online: http://www. ccad.org.uk/device.nsf
- Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation 2008;117:e350-408. [PubMed]
- Tracy CM, Epstein AE, Darbar D, et al. 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 2012;126:1784-800. [PubMed]
- Gregoratos G, Cheitlin MD, Conill A, et al. ACC/AHA Guidelines for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices: Executive Summary--a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Pacemaker Implantation). Circulation 1998;97:1325-35. [PubMed]
- Beyerbach DM, Rottman JN. Pacemakers and Implantable Cardioverter-Defibrillators. Available online: http://emedicine.medscape.com
- Lange RA. Implantable Pacemakers. Available online: http://emedicine.medscape.com
- Res JCJ, de Priester JA, van Lier AA, et al. Pneumothorax resulting from subclavian puncture: a complication of permanent pacemaker lead implantation. Neth Heart J 2004;12:101-5. [PubMed]
- CardiologyHD. Global Community. Available online: http://www.cardiologyhd.com/
- Greenspon AJ, Patel JD, Lau E, et al. Trends in permanent pacemaker implantation in the United States from 1993 to 2009: increasing complexity of patients and procedures. J Am Coll Cardiol 2012;60:1540-5. [PubMed]
- Trohman RG, Kim MH, Pinski SL. Cardiac pacing: the state of the art. Lancet 2004;364:1701-19. [PubMed]
- Gul EE, Kayrak M. Common Pacemaker Problems: Lead and Pocket Complications, Modern Pacemakers - Present and Future, Prof. Mithilesh R Das (Ed.), ISBN: 978-953-307-214-2, InTech, 2011. Available online: http://www.intechopen.com/books/modern-pacemakers-present-and-future/commonpacemaker-problems-lead-and- pocket-complications
- Connolly SJ, Kerr CR, Gent M, et al. Effects of physiologic pacing versus ventricular pacing on the risk of stroke and death due to cardiovascular causes. Canadian Trial of Physiologic Pacing Investigators. N Engl J Med 2000;342:1385-91. [PubMed]
- Andersen HR, Thuesen L, Bagger JP, et al. Prospective randomised trial of atrial versus ventricular pacing in sick-sinus syndrome. Lancet 1994;344:1523-8. [PubMed]
- Link MS, Estes NA 3rd, Griffin JJ, et al. Complications of dual chamber pacemaker implantation in the elderly. Pacemaker Selection in the Elderly (PASE) Investigators. J Interv Card Electrophysiol 1998;2:175-9. [PubMed]
- Lamas GA, Lee KL, Sweeney MO, et al. Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N Engl J Med 2002;346:1854-62. [PubMed]
- Møller M, Arnsbo P, Asklund M, et al. Quality assessment of pacemaker implantations in Denmark. Europace 2002;4:107-12. [PubMed]
- Kiviniemi MS, Pirnes MA, Eränen HJ, et al. Complications related to permanent pacemaker therapy. Pacing Clin Electrophysiol 1999;22:711-20. [PubMed]
- Chauhan A, Grace AA, Newell SA, et al. Early complications after dual chamber versus single chamber pacemaker implantation. Pacing Clin Electrophysiol 1994;17:2012-5. [PubMed]
- Parsonnet V, Bernstein AD, Lindsay B. Pacemaker-implantation complication rates: an analysis of some contributing factors. J Am Coll Cardiol 1989;13:917-21. [PubMed]
- Tobin K, Stewart J, Westveer D, et al. Acute complications of permanent pacemaker implantation: their financial implication and relation to volume and operator experience. Am J Cardiol 2000;85:774-6, A9.
- Aggarwal RK, Connelly DT, Ray SG, et al. Early complications of permanent pacemaker implantation: no difference between dual and single chamber systems. Br Heart J 1995;73:571-5. [PubMed]
- Møller JE, Simonsen EH, Møller M. Impact of continuous quality improvement on selection of pacing mode and rate of complications in permanent pacing. Heart 1997;77:357-62. [PubMed]
- Bond R, Augustine D, Dayer M. Pacemaker complications in a district general hospital. Br J Cardiol 2012;19:90-4.
- Matthews RJ. Permanent pacing. Available online: http:// www. rjmatthewsmd.com/Definitions/permanent_pacing.htm
- Reynolds MR, Cohen DJ, Kugelmass AD, et al. The frequency and incremental cost of major complications among medicare beneficiaries receiving implantable cardioverter-defibrillators. J Am Coll Cardiol 2006;47:2493-7. [PubMed]
- van Rees JB, de Bie MK, Thijssen J, et al. Implantation-related complications of implantable cardioverter-defibrillators and cardiac resynchronization therapy devices: a systematic review of randomized clinical trials. J Am Coll Cardiol 2011;58:995-1000. [PubMed]
- Pakarinen S, Oikarinen L, Toivonen L. Short-term implantation-related complications of cardiac rhythm management device therapy: a retrospective single-centre 1-year survey. Europace 2010;12:103-8. [PubMed]
- van Eck JW, van Hemel NM, Zuithof P, et al. Incidence and predictors of in-hospital events after first implantation of pacemakers. Europace 2007;9:884-9. [PubMed]
- Right RW. Disorders of the Pleura and the Mediastinum. In: Longo DL, Kasper DL, Jameson JL, et al. Harrison’s Principles of Internal Medicine Volume 2. 18th Edition. New York, NY: McGrawHill, 2012:2181.
- Littleford PO, Parsonnet V, Spector SD. Method for the rapid and atraumatic insertion of permanent endocardial pacemaker electrodes through the subclavian vein. Am J Cardiol 1979;43:980-2. [PubMed]
- Miller FA Jr, Holmes DR Jr, Gersh BJ, et al. Permanent transvenous pacemaker implantation via the subclavian vein. Mayo Clin Proc 1980;55:309-14. [PubMed]
- Zwirner K. Insertion of permanent endocardial pacing electrodes through the subclavian vein: results of a five-year period (author's transl). Z Kardiol 1980;69:835-9. [PubMed]
- Furman S. Venous cutdown for pacemaker implantation. Ann Thorac Surg 1986;41:438-9. [PubMed]
- Fiorista F, Lazari M, Marzegalli M, et al. Use of the subclavian vein for permanent cardiac stimulation. Arch Inst Cardiol Mex 1986;56:309-13. [PubMed]
- Magney JE, Parsons JA, Flynn DM, et al. Pacemaker and defibrillator lead entrapment: case studies. Pacing Clin Electrophysiol 1995;18:1509-17. [PubMed]
- Parsonnet V, Roelke M. The cephalic vein cutdown versus subclavian puncture for pacemaker/ICD lead implantation. Pacing Clin Electrophysiol 1999;22:695-7. [PubMed]
- Calkins H, Ramza BM, Brinker J, et al. Prospective randomized comparison of the safety and effectiveness of placement of endocardial pacemaker and defibrillator leads using the extrathoracic subclavian vein guided by contrast venography versus the cephalic approach. Pacing Clin Electrophysiol 2001;24:456-64. [PubMed]
- Kirkfeldt RE, Johansen JB, Nohr EA, et al. Pneumothorax in cardiac pacing: a population-based cohort study of 28,860 Danish patients. Europace 2012;14:1132-8. [PubMed]
- Bartecchi CE. Bedside emergency transvenous cardiac pacing: experience in two community hospitals. JACEP 1976;5:169-73. [PubMed]
- Hildick-Smith DJ, Lowe MD, Newell SA, et al. Ventricular pacemaker upgrade: experience, complications and recommendations. Heart 1998;79:383-7. [PubMed]
- Ho WJ, Kuo CT, Lin KH. Right pneumothorax resulting from an endocardial screw-in atrial lead. Chest 1999;116:1133-4. [PubMed]
- Oginosawa Y, Abe H, Nakashima Y. Right pneumothorax resulting from an endocardial screw-in atrial lead in an implantable cardioverter defibrillator system. Pacing Clin Electrophysiol 2002;25:1278-9. [PubMed]
- Srivathsan K, Byrne RA, Appleton CP, et al. Pneumopericardium and pneumothorax contralateral to venous access site after permanent pacemaker implantation. Europace 2003;5:361-3. [PubMed]
- Peterson PN, Daugherty SL, Wang Y, et al. Gender differences in procedure-related adverse events in patients receiving implantable cardioverter-defibrillator therapy. Circulation 2009;119:1078-84. [PubMed]
- Nowak B, Misselwitz B, Erdogan A, et al. Do gender differences exist in pacemaker implantation?--results of an obligatory external quality control program. Europace 2010;12:210-5. [PubMed]
- Tanaka F, Itoh M, Esaki H, et al. Secondary spontaneous pneumothorax. Ann Thorac Surg 1993;55:372-6. [PubMed]
- MacDuff A, Arnold A, Harvey J. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii18-31. [PubMed]
- Guo Y, Xie C, Rodriguez RM, et al. Factors related to recurrence of spontaneous pneumothorax. Respirology 2005;10:378-84. [PubMed]
- Tocino IM, Miller MH, Fairfax WR. Distribution of pneumothorax in the supine and semirecumbent critically ill adult. AJR Am J Roentgenol 1985;144:901-5. [PubMed]
- Williams JL, Stevenson RT. Complications of Pacemaker Implantation, Current Issues and Recent Advances in Pacemaker Therapy. Dr. Attila Roka (Ed.), ISBN: 978-953-51-0703-3, InTech, 2012. Available online: http://www.intechopen.com/books/current-issues-and-recent-advances-in-pacemaker therapy /complications_ of_pacemaker_implantation
- Kioumis IP, Zarogoulidis K, Huang H, et al. Pneumothorax in cystic fibrosis. J Thorac Dis 2014;6:S480-7. [PubMed]
- Kuhajda I, Zarogoulidis K, Kougioumtzi I, et al. Tube thoracostomy; chest tube implantation and follow up. J Thorac Dis 2014;6:S470-9. [PubMed]
- Manika K, Kioumis I, Zarogoulidis K, et al. Pneumothorax in sarcoidosis. J Thorac Dis 2014;6:S466-9. [PubMed]
- Kuhajda I, Zarogoulidis K, Kougioumtzi I, et al. Penetrating trauma. J Thorac Dis 2014;6:S461-5. [PubMed]
- Visouli AN, Zarogoulidis K, Kougioumtzi I, et al. Catamenial pneumothorax. J Thorac Dis 2014;6:S448-60. [PubMed]
- Huang Y, Huang H, Li Q, et al. Transbronchial lung biopsy and pneumothorax. J Thorac Dis 2014;6:S443-7. [PubMed]
- Terzi E, Zarogoulidis K, Kougioumtzi I, et al. Acute respiratory distress syndrome and pneumothorax. J Thorac Dis 2014;6:S435-42. [PubMed]
- Boskovic T, Stojanovic M, Stanic J, et al. Pneumothorax after transbronchial needle biopsy. J Thorac Dis 2014;6:S427-34. [PubMed]
- Li Z, Huang H, Li Q, et al. Pneumothorax: observation. J Thorac Dis 2014;6:S421-6. [PubMed]
- Huang Y, Huang H, Li Q, et al. Approach of the treatment for pneumothorax. J Thorac Dis 2014;6:S416-20. [PubMed]
- Browning RF, Parrish S, Sarkar S, et al. Bronchoscopic interventions for severe COPD. J Thorac Dis 2014;6:S407-15. [PubMed]
- Machairiotis N, Kougioumtzi I, Dryllis G, et al. Laparoscopy induced pneumothorax. J Thorac Dis 2014;6:S404-6. [PubMed]
- Ouellette DR, Parrish S, Browning RF, et al. Unusual causes of pneumothorax. J Thorac Dis 2014;6:S392-403. [PubMed]
- Parrish S, Browning RF, Turner JF Jr, et al. The role for medical thoracoscopy in pneumothorax. J Thorac Dis 2014;6:S383-91. [PubMed]
- Terzi E, Zarogoulidis K, Kougioumtzi I, et al. Human immunodeficiency virus infection and pneumothorax. J Thorac Dis 2014;6:S377-82. [PubMed]
- Zarogoulidis P, Kioumis I, Pitsiou G, et al. Pneumothorax: from definition to diagnosis and treatment. J Thorac Dis 2014;6:S372-6. [PubMed]
- Tsakiridis K, Mpakas A, Kesisis G, et al. Lung inflammatory response syndrome after cardiac-operations and treatment of lornoxicam. J Thorac Dis 2014;6 Suppl 1:S78-98. [PubMed]
- Tsakiridis K, Zarogoulidis P, Vretzkakis G, et al. Effect of lornoxicam in lung inflammatory response syndrome after operations for cardiac surgery with cardiopulmonary bypass. J Thorac Dis 2014;6 Suppl 1:S7-20. [PubMed]
- Argiriou M, Kolokotron SM, Sakellaridis T, et al. Right heart failure post left ventricular assist device implantation. J Thorac Dis 2014;6 Suppl 1:S52-9. [PubMed]
- Madesis A, Tsakiridis K, Zarogoulidis P, et al. Review of mitral valve insufficiency: repair or replacement. J Thorac Dis 2014;6 Suppl 1:S39-51. [PubMed]
- Siminelakis S, Kakourou A, Batistatou A, et al. Thirteen years follow-up of heart myxoma operated patients: what is the appropriate surgical technique? J Thorac Dis 2014;6 Suppl 1:S32-8. [PubMed]
- Foroulis CN, Kleontas A, Karatzopoulos A, et al. Early reoperation performed for the management of complications in patients undergoing general thoracic surgical procedures. J Thorac Dis 2014;6 Suppl 1:S21-31. [PubMed]
- Nikolaos P, Vasilios L, Efstratios K, et al. Therapeutic modalities for Pancoast tumors. J Thorac Dis 2014;6 Suppl 1:S180-93. [PubMed]
- Koutentakis M, Siminelakis S, Korantzopoulos P, et al. Surgical management of cardiac implantable electronic device infections. J Thorac Dis 2014;6 Suppl 1:S173-9. [PubMed]
- Spyratos D, Zarogoulidis P, Porpodis K, et al. Preoperative evaluation for lung cancer resection. J Thorac Dis 2014;6 Suppl 1:S162-6. [PubMed]
- Porpodis K, Zarogoulidis P, Spyratos D, et al. Pneumothorax and asthma. J Thorac Dis 2014;6 Suppl 1:S152-61. [PubMed]
- Panagopoulos N, Leivaditis V, Koletsis E, et al. Pancoast tumors: characteristics and preoperative assessment. J Thorac Dis 2014;6 Suppl 1:S108-15. [PubMed]
- Visouli AN, Darwiche K, Mpakas A, et al. Catamenial pneumothorax: a rare entity? Report of 5 cases and review of the literature. J Thorac Dis 2012;4 Suppl 1:17-31. [PubMed]
- Zarogoulidis P, Chatzaki E, Hohenforst-Schmidt W, et al. Management of malignant pleural effusion by suicide gene therapy in advanced stage lung cancer: a case series and literature review. Cancer Gene Ther 2012;19:593-600. [PubMed]
- Papaioannou M, Pitsiou G, Manika K, et al. COPD assessment test: a simple tool to evaluate disease severity and response to treatment. COPD 2014;11:489-95. [PubMed]
- Boskovic T, Stanic J, Pena-Karan S, et al. Pneumothorax after transthoracic needle biopsy of lung lesions under CT guidance. J Thorac Dis 2014;6 Suppl 1:S99-107. [PubMed]
- Papaiwannou A, Zarogoulidis P, Porpodis K, et al. Asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): current literature review. J Thorac Dis 2014;6 Suppl 1:S146-51. [PubMed]
- Zarogoulidis P, Porpodis K, Kioumis I, et al. Experimentation with inhaled bronchodilators and corticosteroids. Int J Pharm 2014;461:411-8. [PubMed]
- Bai C, Huang H, Yao X, et al. Application of flexible bronchoscopy in inhalation lung injury. Diagn Pathol 2013;8:174. [PubMed]
- Zarogoulidis P, Kioumis I, Porpodis K, et al. Clinical experimentation with aerosol antibiotics: current and future methods of administration. Drug Des Devel Ther 2013;7:1115-34. [PubMed]
- Zarogoulidis P, Pataka A, Terzi E, et al. Intensive care unit and lung cancer: when should we intubate? J Thorac Dis 2013;5 Suppl 4:S407-12. [PubMed]
- Hohenforst-Schmidt W, Petermann A, Visouli A, et al. Successful application of extracorporeal membrane oxygenation due to pulmonary hemorrhage secondary to granulomatosis with polyangiitis. Drug Des Devel Ther 2013;7:627-33. [PubMed]
- Zarogoulidis P, Kontakiotis T, Tsakiridis K, et al. Difficult airway and difficult intubation in postintubation tracheal stenosis: a case report and literature review. Ther Clin Risk Manag 2012;8:279-86. [PubMed]
- Zarogoulidis P, Tsakiridis K, Kioumis I, et al. Cardiothoracic diseases: basic treatment. J Thorac Dis 2014;6 Suppl 1:S1. [PubMed]
- Kolettas A, Grosomanidis V, Kolettas V, et al. Influence of apnoeic oxygenation in respiratory and circulatory system under general anaesthesia. J Thorac Dis 2014;6 Suppl 1:S116-45. [PubMed]
- Turner JF, Quan W, Zarogoulidis P, et al. A case of pulmonary infiltrates in a patient with colon carcinoma. Case Rep Oncol 2014;7:39-42. [PubMed]
- Machairiotis N, Stylianaki A, Dryllis G, et al. Extrapelvic endometriosis: a rare entity or an under diagnosed condition? Diagn Pathol 2013;8:194. [PubMed]
- Tsakiridis K, Zarogoulidis P. An interview between a pulmonologist and a thoracic surgeon-Pleuroscopy: the reappearance of an old definition. J Thorac Dis 2013;5 Suppl 4:S449-51. [PubMed]
- Huang H, Li C, Zarogoulidis P, et al. Endometriosis of the lung: report of a case and literature review. Eur J Med Res 2013;18:13. [PubMed]


