
The effect of 1.9-mm versus 2.4-mm probes in transbronchial cryobiopsies for interstitial lung diseases: a prospective analysis
Introduction
Despite being frequently encountered in clinical practice, interstitial lung diseases (ILD) are difficult to diagnose. Transbronchial cryobiopsy (TBCB) is critical procedure in the assessment of patients with suspected ILD when an accurate diagnosis cannot be made solely based on clinical and radiological assessments. Recent reports have suggested that the diagnostic value of TBCB approaches that of surgical lung biopsy (SLB) (1,2). However, TBCB is also one of the most invasive diagnostic procedures (3-5), with an observed overall complication rate of 23.1% (6), and significant bleeding and pneumothorax reported in 14.2% and 9.4% of patients, respectively. Several procedural factors have been associated with the safety profile and diagnostic efficacy of TBCB, such as the cryoprobe-pleura distance, cryoprobe type, the freezing duration of biopsy, guidance method, and the number of cryobiopsies. Hagmeyer et al. identified procedural modifications that potentially minimized the risks associated with TBCB (7).
Cryoprobes with outer diameters of 1.9-mm and 2.4-mm are commonly used for TBCB. In a retrospective study (8), probes of both sizes were shown to yield specimens adequate for ILD diagnosis. However, the duration to freezing varied, with specimens requiring 4–6 s when extracted with a 2.4-mm probe, and 6–8 s with a 1.9-mm probe. Additionally, no difference in diagnostic yield was observed between cryoprobes of these two sizes. However, the study revealed that 1.9-mm probes were associated with a significantly reduced incidence of pneumothorax compared to 2.4-mm probes. Preferences for the 1.9-mm probe over 2.4-mm probe have also been cited in several guidelines and expert statements (4,5), based on ease of maneuverability in the airways and tactile feedback when extended to the pleura, which may reduce the risk of bleeding and pneumothorax. However, to date, prospective studies have not demonstrated the superiority of one probe over the other. Furthermore, no studies have compared the qualities of specimens obtained with different cryoprobes. In this study, we analyzed the differences between 1.9-mm and 2.4-mm probes based on a prospective cohort study in which cryoprobe placement was guided by three dimensional (3D) images acquired by cone beam computed tomography (CBCT) during TBCB. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4159).
Methods
Patients
This study was analyzed based on an updated single-center prospective cohort study concerning on the CBCT guided TBCB for ILD. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization. This study was reviewed and approved by the Ethics Committee of China-Japan Friendship Hospital (2017-25-1) and was registered at clnicaltrial.gov (NCT04047667). Written informed consent was obtained from all patients enrolled. Participant registration was carried out between September 2018 to January 2020.
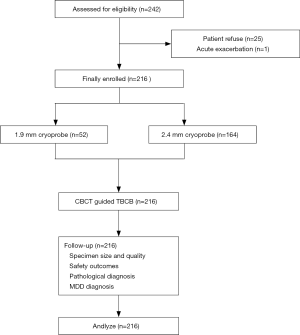
All patients diagnosed with ILD who met the following eligibility criteria were recommended to undergo TBCB under guidance by CBCT (Figure 1): aged >18 years old with evidence of diffuse parenchymal lung disease; a diagnosis of ILD that could not be established after integration of clinical data, laboratory tests, and high-resolution computed tomography (HRCT) features; forced vital capacity (FVC) >50%; and diffusing capacity of the lung for CO (DLCO) >35%. Patients who met any of the following criteria were excluded from this study: acute exacerbation in the previous 30 days; bleeding diathesis; anticoagulant therapy; current use of antiplatelet drugs; pulmonary hypertension; respiratory failure; liver or kidney dysfunction; cardiac insufficiency; or platelet count <50´109/L. Eventually, 216 patients were enrolled and divided into two groups according to the size of the cryoprobe used during their TBCB procedure.
TBCB procedure
All bronchoscopies were performed under general anesthesia in a hybrid CBCT operation room. An endotracheal tube or rigid bronchoscope was placed; then, a bronchial balloon blocker was positioned at the origin of the target lobe, from either the outside of the endotracheal tube or inside the rigid bronchoscope. Subsequently, a 1.9- or 2.4-mm cryoprobe (ERBE, Solingen, Germany) was advanced to the fullest extent possible into the target bronchial segment through the bronchoscope working channel. The cryoprobe was then retracted by 1 cm and the bronchoscope was affixed to a stand. The clinical team was then moved to the control room prior to performing CBCT imaging (Artis Zee III ceiling, Siemens AG, Munich, Germany).
Three-dimensional CT images were acquired at 90 kV for 6 s via digital chest tomosynthesis spanning a 200-degree rotational acquisition, with a frame rate of 0.5 degrees per frame (397 frames). Images were reviewed in axial, coronal, and sagittal planes to accurately assess the cryoprobe position within the lung parenchyma, relative to other thoracic structures. The minimum probe-to-pleura distance was measured. If the probe-to-pleura distance was <1 cm, the cryoprobe was retracted to achieve an appropriate distance. Similarly, if the distance was >1 cm, the cryoprobe was reinserted at a different location to ensure a probe-to-pleura distance of ~1 cm. Repeat CBCT images were not acquired. The total number of imaging acquisitions and whether cryoprobe was re-positioned were recorded for each patient.
Cryobiopsies were performed (6–8 s freeze time for 1.9-mm cryoprobe and 4–6 s for 2.4-mm cryoprobe) after probe positioning, using carbon dioxide as the cryogen. After each biopsy, a bronchial blocker was immediately applied to stop the bleeding. We aimed to perform 2–5 cryobiopsies per patient. CBCT imaging was not acquired for repeat cryobiopsies in the same lobe; instead, TBCB was conducted in the same or adjacent segment with the probe advanced at a consistent distance. Where cryobiopsies were performed in different lobes, the CBCT guidance procedure was repeated. Routine post-procedure CBCT or X-ray imaging was used to screen for acute pneumothorax. Bleeding severity was graded according to the following scale (9): no bleeding (traces of blood not requiring suctioning), mild bleeding (requiring suction to clear but no other endoscopic procedures), moderate bleeding (requiring endoscopic procedures such as bronchial occlusion-collapse and/or instillation of ice-cold saline), and severe bleeding (causing hemodynamic or respiratory instability, requiring tamponade or other surgical interventions, transfusions, or admission to the intensive care unit).
The long- and short-axis diameters were measured for each specimen. Gross specimen with a long-axis diameter ≥5 mm was considered to be qualified. The numbers of total, qualified, and unqualified specimens were recorded.
Follow-up and measures
For each patient, electrocardiogram, blood pressure, and oxygen saturation were monitored for 24 h after bronchoscopy. Chest X-rays were performed for patients who exhibited discomfort or disease progression without any other reason.
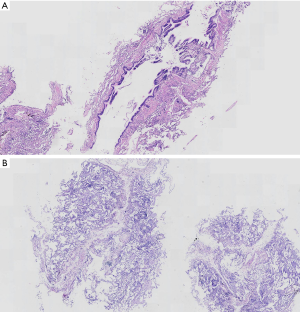
Histopathological diagnosis was conducted by an experienced pathologist. The microscopic quality of each specimen was assessed for all patients. If the prominent contents of all specimens were bronchial structures, this patient’s specimens were considered microscopically unqualified (Figure 2). The number of patients with microscopically unqualified specimens was recorded. Multidisciplinary discussions (MDD) based on clinical presentations, radiological findings, and TBCB histopathological features were conducted for each patient. All patients underwent a 30-day follow-up for intensive care unit admission, disease progression, and death after TBCB.
Endpoints and sample size
The primary endpoints were defined as the incidences of pneumothorax and moderate-severe bleeding. The second endpoints included diagnostic yields, specimen quality, cryoprobe re-position rates after CBCT guidance, and procedure duration. The estimated sample size was designed to have approximately 80% power for detecting a 6% decrease in the pneumothorax rate (from 9% to 3%), or a 10% decrease in the moderate-severe bleeding rate (from 20% to 10%) after CBCT guidance.
Statistical analysis
All patients competed 30-day follow-up and were included in this analysis. Anthropometric and lung function data were expressed as mean ± SD. All remaining results were presented as descriptive statistics, absolute numbers, and percentages. Continuous data were tested for normality using the Shapiro-Wilk test. Where normal distribution could be assumed, the two groups were compared using the independent samples t-test, otherwise the Mann-Whitney U test was applied. Differences in complication rates were analyzed for statistical significance using the chi-squared test. Multivariate logistic regression analyses were used to analyze the associations between safety outcomes and clinical features, as well as between diagnostic yields and clinical features. Statistical analyses were performed using STATA software (version 11, StataCorp, College Station, TX, USA), and a P value <0.05 was considered statistically significant.
Results
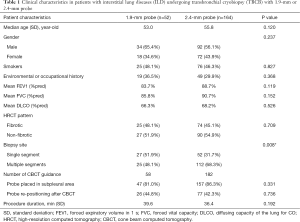
Of 242 patients eligible for enrolment in the study, 26 were excluded due to patient refusal (n=25) or acute exacerbation (n=1) prior to the procedure. Consequently, 216 patients were enrolled and underwent TBCB. Fifty-two and 164 patients underwent the procedure using 1.9-mm and 2.4-mm probes, respectively (Figure 1). All patients completed the 30-day follow-up. No significant differences were found between the 1.9- and 2.4-mm groups in terms of age, sex, smoking history, environmental or occupational history, lung functional impairment, or procedure duration (Table 1). However, significantly more patients in the 2.4-mm group underwent TBCB at multiple segments than in the 1.9-mm group (P=0.008).
Full table
Cryoprobe placement under CBCT guidance
CBCT 3D images were acquired to confirm the cryoprobe positions during 58 procedures in the 1.9-mm group and 182 procedures in the 2.4-mm group (Table 1). In the majority of procedures, both types of cryoprobes were advanced to the subpleural area, within 1 cm of the pleura (81.0% in the 1.9-mm group and 86.3% in the 2.4-mm group, P=0.331). Cryoprobe re-positioning after CBCT guidance was deemed necessary in 44.8% (26/58) of procedures in the 1.9-mm group and 42.3% (77/182) of procedures in the 2.4-mm group (P=0.736).
Specimen size and quality
Specimens obtained with the 2.4-mm probe were significantly larger than those acquired with the 1.9-mm probe (long-axis diameter: 5.5 mm in the 2.4-mm group vs. 5.2 mm in the 1.9-mm group, P<0.001; short-axis diameter: 4.3 vs. 4.1 mm, P<0.001; surface area: 24.6 vs. 22.0 mm2, P<0.001) (Table 2). An average of 3.5 specimens were obtained in both groups (P=0.875). The percentages of grossly and microscopically qualified specimens acquired with the 2.4-mm probe were significantly higher than those yielded from the 1.9-mm probe (grossly qualified: 80.1% vs. 66.7%, P<0.001; microscopically qualified: 99.4% vs. 90.4%, P=0.003) (Table 2). Multivariate analysis indicated that the pathological diagnostic yield in patients with microscopically qualified specimens was significantly higher than that in patients with microscopically unqualified specimens (OR =21.2, 95% CI: 2.8–162.2; P=0.003).

Full table
Safety and diagnostic efficacy
Pneumothorax was reported in 6 patients (2.8%) (3 men and 3 women; mean age, 59.5 years old, range 28–72 years old; mean FVC %predicted, 88.8%; mean DLCO %predicted, 59.7%). All of pneumothorax were observed exclusively in the 2.4-mm group. However, no statistically significant difference was found between the two groups (3.7% vs. 0.0%, P=0.360) (Table 3). Three of the six subjects who exhibited pneumothorax displayed fibrotic patterns via HRCT analysis. Additionally, three were diagnosed as hypersensitivity pneumonitis (HP) after MDD. One patient immediately developed pneumothorax post TBCB, in contrast to the remaining 5 patients, who displayed delayed onset. Five patients underwent TBCB in a single lobe, where an average of 3.8 specimens with a mean long-axis diameter of 5.0 mm were obtained. Pleura was identified in two patients under microscopic examination. Five patients required chest-tube drainage. All cases of pneumothorax fully resolved within a week. No significant difference was found in the incidence of moderate bleeding between the groups (P=0.612, Table 3), with 15 (9.1%) and 6 (11.5%) of patients in the 2.4-mm and 1.9-mm groups affected, respectively. No severe bleeding occurred in either group. Multivariate analysis revealed no differences between the groups.

Full table
The most common histopathological patterns across both groups included non-specific interstitial pneumonia (NSIP), usual interstitial pneumonia (UIP), HP, desquamative interstitial pneumonia or respiratory bronchiolitis-interstitial lung disease (DIP/RB-ILD), and organizing pneumonia (OP). The most common MDD diagnoses were connective tissue disease-related ILD (CTD-ILD), HP, idiopathic pulmonary fibrosis (IPF), NSIP, DIP/RB-ILD, cryptogenic organizing pneumonia (COP), and malignancy. Neither univariate nor multivariate analysis revealed a significant difference in pathological or MDD diagnostic yield between the 1.9- and 2.4-mm groups (Table 3).
Discussion
This is the first prospective study to compare the use of 1.9- and 2.4-mm cryoprobes for TBCB in patients with ILD. Our results offer new insights into the differences between the two sizes of cryoprobe in TBCB.
The larger cryoprobe (2.4-mm) was considered to be more difficult to maneuver in the airway compared to the smaller cryoprobe (1.9-mm), and had a higher likelihood of being obstructed by a carina upon retraction to the lung periphery (4,5). However, no previous studies report to date have confirmed this hypothesis. In this study, TBCB was conducted under the guidance of 3D CBCT images which were reviewed in axial, coronal, and sagittal planes to accurately assess the cryoprobe position within the lung parenchyma, relative to other thoracic structures. Measurement of the probe-to-pleura distances revealed that both types of cryoprobes easily advanced to the subpleural area, which was within 1 cm of the pleura in the majority of procedures (81.0% vs. 86.3%, P=0.331). Furthermore, both cryoprobes displayed similar rates of cryoprobe re-positioning to the appropriate biopsy site after CBCT guidance (44.8% vs. 42.3%, P=0.736).
A retrospective study of 699 patients conducted by Ravaglia et al. (8) reported a pneumothorax rate that was significantly higher when a 2.4-mm probe was used than when a 1.9-mm probe was used [21.2% (130/613) vs. 2.7% (2/73), P<0.0001]. However, no difference was noted in the risk of bleeding. The safety profile was, therefore, of great concern to the investigators. However, our study did not demonstrate any significant differences in the risk of pneumothorax or bleeding. The known risks of complications were significantly associated with the biopsy location (4). Probe-to-pleura distances of <1 cm are associated with a significantly increased risk of pneumothorax. The accuracy of guidance techniques plays an important role in the prevention of complications. TBCB was conducted routinely under fluoroscopy guidance. However, complications, particularly pneumothorax, did not substantially decrease. This was attributed to the lack of clarity during fluoroscopy when the probe path was not perpendicular to the P-A plane of the C-arm when determining the probe-to-pleura distance. Ravaglia’s study (8) reported the occurrence of pneumothorax in up to 19.2% of patients who underwent fluoroscopy-guided TBCB. In contrast, our study revealed an overall risk of pneumothorax of only 2.8% under CBCT guidance. The significant decrease in the influence of the probe location on the risk of pneumothorax (10,11) may be attributable to the accurate guidance provided by CBCT. This potentially explains the differences in findings across current and previous studies regarding the relationship between cryoprobe influence and pneumothorax risk. The small number of patients who exhibited pneumothorax may be another reason for failing to reveal the potential relationships.
No difference was found in pathological or MDD diagnostic yield between the two sizes of cryoprobe. However, the size and quality of the specimens obtained with the 2.4-mm probe were significantly superior to those of specimens acquired with the 1.9-mm probe. However, this finding requires more consideration, given its potential to influence diagnostic yield when using the 1.9-mm probe. Our results indicated that the pathological diagnostic yield with microscopically unqualified specimens was significantly lower than that with microscopically qualified specimens. Therefore, bronchoscopists should take some measures to improve the quality of specimens when using a 1.9-mm cryoprobe; these measures may include increasing freezing time, using nitrous oxide as the cryogen [which was shown to be more effective than carbon dioxide (4)], and maintaining the cryogen at a relatively higher-pressure level.
This study was limited by its unrandomized design. However, thanks to the study’s prospective nature, few patient selection biases existed between the two groups, which produced compelling results. Further investigations that incorporate other procedural factors are warranted.
Conclusions
The size and quality of specimens obtained with the 2.4-mm probe was markedly superior than those acquired with the 1.9-mm probe. The pathological diagnostic yield in patients with microscopically qualified specimens was significantly higher than that in patients with unqualified specimens. There were no significant differences in safety profile or diagnostic yield between the two different-sized probes used.
Acknowledgments
Funding: This work was supported by National Key Technologies R & D Program Precision Medicine Research [No. 2016YFC0901101 to HD]; CAMS Innovation Fund for Medical Sciences [CIFMS,No. 2018-12M-1-001 to HD]; and Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences [No. 2019PT320021 to HD].
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-4159
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-4159
Peer Review File: Available at http://dx.doi.org/10.21037/atm-20-4159
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4159). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization. This study was reviewed and approved by the Ethics committee of China-Japan Friendship Hospital (2017-25-1) and registered at clnicaltrials.gov (NCT04047667). All patients enrolled completed and signed the informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Troy LK, Grainge C, Corte TJ, et al. Diagnostic accuracy of transbronchial lung cryobiopsy for interstitial lung disease diagnosis (COLDICE): a prospective, comparative study. Lancet Respir Med 2020;8:171-81. [Crossref] [PubMed]
- Tomassetti S, Wells AU, Costabel U, et al. Bronchoscopic Lung Cryobiopsy Increases Diagnostic Confidence in the Multidisciplinary Diagnosis of Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med 2016;193:745-52. [Crossref] [PubMed]
- Babiak A, Hetzel J, Krishna G, et al. Transbronchial cryobiopsy: a new tool for lung biopsies. Respiration 2009;78:203-8. [Crossref] [PubMed]
- Hetzel J, Maldonado F, Ravaglia C, et al. Transbronchial Cryobiopsies for the Diagnosis of Diffuse Parenchymal Lung Diseases: Expert Statement from the Cryobiopsy Working Group on Safety and Utility and a Call for Standardization of the Procedure. Respiration 2018;95:188-200. [Crossref] [PubMed]
- Maldonado F, Danoff SK, Wells AU, et al. Transbronchial Cryobiopsy for the Diagnosis of Interstitial Lung Diseases: CHEST Guideline and Expert Panel Report. Chest 2020;157:1030-42. [Crossref] [PubMed]
- Sethi J, Ali MS, Mohananey D, et al. Are Transbronchial Cryobiopsies Ready for Prime Time?: A Systematic Review and Meta-Analysis. J Bronchology Interv Pulmonol 2019;26:22-32. [Crossref] [PubMed]
- Hagmeyer L, Theegarten D, Wohlschläger J, et al. Transbronchial cryobiopsy in fibrosing interstitial lung disease: modifications of the procedure lead to risk reduction. Thorax 2019;74:711-4. [Crossref] [PubMed]
- Ravaglia C, Wells AU, Tomassetti S, et al. Diagnostic yield and risk/benefit analysis of trans-bronchial lung cryobiopsy in diffuse parenchymal lung diseases: a large cohort of 699 patients. BMC Pulm Med 2019;19:16. [Crossref] [PubMed]
- Ernst A, Eberhardt R, Wahidi M, et al. Effect of routine clopidogrel use on bleeding complications after transbronchial biopsy in humans. Chest 2006;129:734-7. [Crossref] [PubMed]
- Braak SJ, van Strijen MJ, van Leersum M, et al. Real-Time 3D fluoroscopy guidance during needle interventions: technique, accuracy, and feasibility. AJR Am J Roentgenol 2010;194:W445-51. [Crossref] [PubMed]
- Steinfort DP, D'Agostino RD, Vrjlic I, et al. CT-Fluoroscopic Guidance for Performance of Targeted Transbronchial Cryobiopsy: A Preliminary Report. Respiration 2018;96:472-9. [Crossref] [PubMed]


