
The trend of indirect anastomosis formation in a 2-vessel occlusion plus encephalo-myo-synangiosis rat model
Introduction
Encephalo-myo-synangiosis (EMS), an indirect revascularization surgery, is a conventional method for the treatment of moyamoya disease and some other chronic ischemic cerebrovascular diseases (CICDs) (1). During surgery, chronically ischemic brain tissue is covered by capillary-rich temporal muscle (TM) tissue, and indirect anastomoses (new capillaries) are gradually formed between the TM and the brain (1,2). At 3 to 4 months after EMS, this kind of indirect anastomosis can have the desired effect of improving cerebral blood perfusion (CBP) and preventing stroke (3,4). Unfortunately, more than 50% of adult patients lack sufficient angiogenic potential to form sufficient anastomotic vessels between the TM and brain tissue (5,6). Therefore, the effects of surgery on these patients and their clinical prognoses are poor.
To improve the clinical efficacy of EMS surgery, some researchers have tried to simulate surgery in rats or mice to explore methods and mechanisms of promoting endothelial cell (EC) proliferation and anastomosis formation between the TM and brain (7-10). Although indirect anastomosis formation in these animal models has been revealed, when the anastomosis between the rat TM and brain peaks after EMS remains unclear. Sacrifice of the model rat before the peak anastomosis effect could result in missing potential significant results, whereas sacrifice of the model rat after the peak would undoubtedly prolong the experimental duration and waste experimental funds. Therefore, it is of great significance to determine the peak time of indirect anastomosis in the EMS rat model. Based on this need, we herein aimed to perform EMS on a 2-vessel occlusion (2VO) rat model of chronic cerebral ischemia to comparatively observe the expression of EC markers, the improvement of CBP and the improvement of cognitive function at different time points (different weeks after EMS) to explore the peak time of indirect anastomosis formation. These results will be extremely helpful for most basic research related to angiogenesis and indirect anastomosis formation. To the best of our knowledge, no similar reports have been published. We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-2936).
Methods
Animals and subgrouping
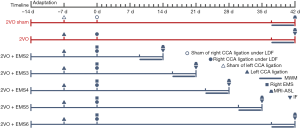
Adult male Wistar rats (280–320 g) were purchased from the animal center of Sun Yat-sen University, Guangdong, China. All animal experiments were approved by the Institutional Animal Ethics Committee of Sun Yat-sen University (No.: [2019]02-480-12), and treatment conformed to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (publication no. 80-23, revised 1996). Based on the different durations before sacrifice, rats were randomly divided into the (I) 2VO group (control group), (II) 2VO + EMS2 group, (III) 2VO + EMS3 group, (IV) 2VO + EMS4 group, (V) 2VO + EMS5 group, and (VI) 2VO + EMS6 group (Table 1), with eight rats in each group. Another 2VO sham group (n=8) was set to detect the baseline level of cerebral blood flow (CBF), CBP and cognitive function. The experimental schedule is presented in Figure 1.
Full table
Animal model of 2VO
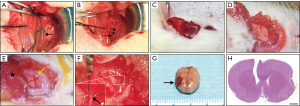
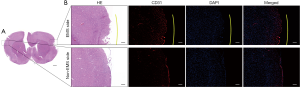
Two-vessel occlusion was performed to establish a chronic cerebral ischemia model (11). After being anesthetized by isoflurane inhalation, the temperature of each rat was maintained at 37 °C on a heating pad. A straight incision in the middle of the neck was made to expose the left common carotid artery (CCA). Once the left CCA was ligated with two 4-0 silk threads, the incision was closed (Figure 2A,B). Seven days after left CCA ligation, each rat was fixed on a stereotaxic instrument, and a burr hole was made with an electric drill in the right frontal region (0.8 mm posterior and 3.4 mm lateral to the bregma) after a midline scalp incision was made. In addition, a placement device fixed with cyanoacrylate adhesives for the contact probe was set. Then, the right CCA was exposed by the same procedures. After that, the right CCA was temporarily clamped twice (5 min each time) and finally ligated with two 4-0 silk threads. For the 2VO sham group, the same procedures were performed but without CCA ligation. The CBF before and after the procedure was measured by laser Doppler flowmetry, and the mean CBF values are expressed as a percentage of the baseline value. A decline in CBF to approximately 30% of the baseline before 2VO ligation was required to indicate successful 2VO model establishment (12).
Procedures for EMS surgery in rats
Immediately after ligation of the right CCA, EMS procedures were performed on the right temporal region of 2VO rats. Both the TM tissue and skin were reflected in a U-shape from the skull (Figure 2C,D), and a piece of the skull approximately 4 mm in diameter was removed from the temporoparietal region using an electric drill. After that, the dura mater (DM) was carefully opened with micro-tweezers under the microscope, taking care to avoid damaging the brain surface (Figure 2E). Then, the arachnoid membrane was carefully opened with a 1 ml syringe needle in multiple places. To ensure that the TM tissue can be constantly in close contact with the ischemic brain surface, the edge of the DM and TM tissue were stitched together with a 10-0 prolene suture (Figure 2F). Finally, the EMS area was pressed constantly for 15 s after the skin was sutured. The rat brain and TM tissue were harvested together on the day of sacrifice (Figure 2G,H). A schematic drawing showing the EMS procedure is presented in Figure 3.
Morris water maze (MWM) test
The MWM test was adopted to analyze cognitive improvement after EMS (13). A circular black pool 180 cm in diameter and 60 cm deep was filled with water at a temperature of 22±2 °C to a depth of 30 cm. The pool was divided into 4 equal quadrants. During the training stage, an escape platform was hidden in the center of quadrant III (10 cm × 10 cm, 1 cm below the water surface). The rats were first subjected to a place navigation test. The rats were gently placed in the water and released facing the wall of one of the four quadrants in a random order. They were allowed to find the underwater platform in 60 s, and both the latency to escape onto the platform and the swimming route were recorded. If a rat failed to find the platform, it was guided onto the platform with a stick, and its escape latency was recorded as 60 s. Each rat was allowed to stay on the escape platform for 10 s regardless of whether they found the platform. The training was conducted twice a day for 5 days, with intervals of approximately 20 min. On the same day that the place navigation test was finished, a 60-s spatial probe test was conducted during which the platform was removed. The time spent in the target quadrant and the cross platform area times were analyzed by a video surveillance system (SMART, Panlab SL, Barcelona, Spain). In addition, rat motor functions were also assessed by the recorded swimming speed.
Magnetic resonance imaging (MRI)
We compared the effects of EMS on CBP improvement in the different groups by MRI arterial spin labeling (MRI-ASL) sequence measurements. After being anesthetized by isoflurane inhalation, the rat brains and CBP were assessed by a 7.0-T MRI animal scanner (PharmaScan MRI with ParaVision 7 system, Bruker, Germany). The MRI parameters were set as follows: echo spacing =11 ms, echo time (TE) =33 ms, repetition time (TR) =2,500 ms, TR/TE =2,500/33 ms, matrix size =256×256, field of view (FOV) = 35×35 mm2, scan time =2 min 30 s, and section thickness =0.8 mm without gap. The regions of interest (ROIs) were set within the brain cortex in close contact with the TM tissue after EMS, with an area of 10 mm2 per ROI. In the 2VO and 2VO sham groups, the ROIs were set in similar locations to those in the other 2VO + EMS groups. The CBP value (mL/100 g·min) of each ROI was assessed and calculated using ParaVision software, and the ratio of the CBP on the EMS side to that on the contralateral side was also calculated and set as the “perfusion ratio”, which was used to represent the CBP improvement on the EMS side.
Immunofluorescence (IF)
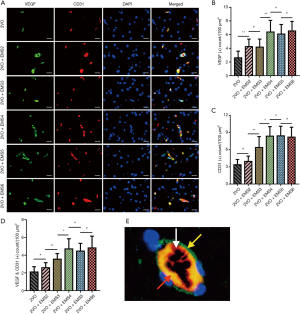
We used IF assays to determine the effects of EMS on EC proliferation. Briefly, brain and TM sections were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 5 min at room temperature, permeabilized, and blocked for 30 min with 1% bovine serum albumin and 0.1% Triton X-100. After that, the fixed sections were washed and incubated for 1 h with primary antibodies against CD31 (1:100; Santa Cruz Biotechnology, Dallas, TX, USA) or vascular endothelial growth factor (VEGF, 1:200; Cell Signaling Technology, Danvers, MA, USA) at 4 °C overnight. Thereafter, the sections were washed three times with PBS and incubated with fluorescence-conjugated species-specific secondary antibodies. Then, to identify the nuclei, the samples were counterstained with 4’,6-diamidino-2-phenylindole (DAPI, Beyotime Biotechnology, Shanghai, China), and a fluorescence microscope was used to acquire photographs. The quantities of VEGF- and CD31-positive cells indicated the distribution and proliferation of capillary ECs. The average numbers of positively stained cells in five random visual fields (100 µm × 100 µm) were counted. All experiments were repeated in triplicate.
Statistical analysis
The Statistical Program for Social Science (SPSS) version 22.0 was used to perform statistical analyses. The data are reported as the means ± standard deviations (SDs). A two-tailed unpaired Student’s t-test was adopted to analyze the MWM test, MRI-ASL and IF results. Differences were considered to be significant if P<0.05 and very significant if P<0.01.
Results
Mortality rate and effect on CBF changes in the 2VO + EMS model
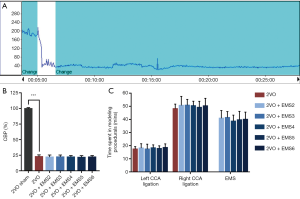
Of the 53 rats that were subjected to 2VO and 2VO + EMS, 5 (9.4%) died within 7 days after modeling. The remaining rats were randomly assigned to the experimental groups described above. No deaths occurred in the 2VO sham group. None of the brain surface damage was caused during dura opening, and no symptoms of hemiplegia were observed after modeling. An immediate and sharp decline in CBF was detected by laser Doppler flowmetry after bilateral CCA ligation in each rat from the baseline level of 186.67±13.51 to 42.23±8.95 PU (Figure 4A). The CBF decreased on average to 22.55%±4.26% of the baseline level before 2VO in this study (excluding the 2VO sham group) (Figure 4B). No significant change in CBF was detected when the EMS procedure was performed. The decrease rate in the 2VO group was significantly lower than that in the 2VO sham group (22.61%±2.90% vs. 99.66%±1.61%, P<0.001). No significant difference in the decrease rate was found between the 2VO group and the other 2VO + EMS groups. The operation time was not significantly different between the groups (Figure 4C).
The expression of VEGF and CD31 in the brain cortex of 2VO + EMS rats at different time points
IF was used to detect and quantify the expression of VEGF and CD31 in the EMS-side brain cortex of the 2VO + EMS rats at different time points (Figures 5,6). According to the IF results, the density of VEGF (+) cells was significantly higher in the 2VO + EMS2 group than in the 2VO group (4.25±1.10 vs. 2.61±0.98, P=0.0072), while the density of CD31 (+) cells in the 2VO + EMS2 group was not significantly different from that in the 2VO group (3.95±0.92 vs. 3.38±0.92, P=0.2330). The density of VEGF(+) cells in the 2VO + EMS3 group was not significantly different from that in the 2VO + EMS2 group (4.18±1.18 vs. 4.25±1.10, P=0.9872), whereas the density of CD31(+) cells in the 2VO + EMS3 group was significantly higher than that in the 2VO + EMS2 group (6.39±1.91 vs. 3.95±0.92, P=0.0058). Furthermore, both VEGF(+) and CD31(+) cells in the 2VO + EMS4 group were significantly denser than those in the 2VO + EMS3 group (VEGF: 6.39±1.70 vs. 4.18±1.18, P=0.0091; CD31: 8.36±1.66 vs. 6.39±1.91, P=0.0443). However, there were no significant differences in the densities of VEGF(+) and CD31(+) cells in the 2VO + EMS5 and 2VO + EMS4 groups (VEGF: 6.09±1.40 vs. 6.39±1.70, P=0.7060; CD31: 8.41±1.68 vs. 8.36±1.66, P=0.9530) or between the 2VO + EMS6 group and the 2VO + EMS5 group (VEGF: 6.54±1.40 vs. 6.09±1.40, P=0.5306; CD31: 8.20±1.73 vs. 8.41±1.68, P=0.8064).
CBP improvement in 2VO rats at different time points after EMS
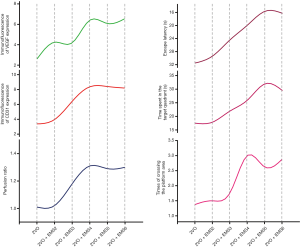
For each group, we used MRI-ASL sequences to determine the CBP values of the ROIs on both the EMS side and non-EMS side and used the perfusion ratio to indicate the degree of increased CBP on the EMS side (Figure 7). For the EMS side, there was no significant difference in the CBP values between the 2VO + EMS2 and 2VO groups (31.11±6.09 vs. 30.71±6.60 mL/100 g·min, P=0.9015). However, the 2VO + EMS3 group had a higher CBP value on the EMS side than the 2VO + EMS2 group (36.95±4.12 vs. 31.11±6.09 mL/100 g·min, P=0.0413). Furthermore, the CBP value on the EMS side in the 2VO + EMS4 group was significantly higher than that on the EMS side in the 2VO + EMS3 group (41.56±2.87 vs. 36.95±4.12 mL/100 g·min, P=0.0210). Nevertheless, there was no significant difference in the CBP values on the EMS side between the 2VO + EMS5 and 2VO + EMS4 groups (39.66±3.04 vs. 41.56±2.87 mL/100 g·min, P=0.2196) or between the 2VO + EMS6 and 2VO + EMS5 groups (40.61±3.38 vs. 39.66±3.04 mL/100 g·min, P=0.5638). On the other hand, there were no significant differences in the CBP values on the non-EMS side among all six groups. In addition, the rats in the 2VO sham group had a significantly higher CBP value in the right frontal lobe than the rats in the 2VO + EMS4 group (80.50±7.48 vs. 41.56±2.87 mL/100 g·min, P<0.001)
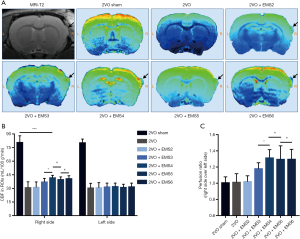
Accordingly, the perfusion ratio in the 2VO + EMS2 group was not significantly different from that in the 2VO group (1.02±0.07 vs. 1.01±0.11, P=0.8524). In addition, the 2VO + EMS3 group had a higher perfusion ratio than the 2VO + EMS2 group (1.18±0.08 vs. 1.02±0.07, P=0.0008). Furthermore, the perfusion ratio in the 2VO + EMS4 group was significantly higher than that in the 2VO + EMS3 group (1.31±0.10 vs. 1.18±0.08, P=0.0105). However, there was no significant difference in the perfusion ratio between the 2VO + EMS5 and 2VO + EMS4 groups (1.29±0.17 vs. 1.31±0.10, P=0.8056) or between the 2VO + EMS6 and 2VO + EMS5 groups (1.30±0.12 vs. 1.29±0.17, P=0.9867).
Pattern of cognitive function improvement in 2VO + EMS rats
MWM tests were performed to evaluate the cognitive function improvement in the 2VO rats after EMS (Figure 8). The escape latency 2 weeks after EMS surgery (2VO + EMS2 group) was not significantly different from that of the control group (29.38±5.37 vs. 31.50±5.83 s, P=0.4609). However, three weeks after EMS, the escape latency of 2VO + EMS rats began to shorten significantly (2VO + EMS3 vs. 2VO + EMS2: 24.38±3.62 vs. 29.38±5.37 s, P=0.0465). The escape latency in the fourth week (2VO + EMS4 group) was significantly shorter than that in the third week (2VO + EMS3 group) (19.86±4.26 vs. 24.38±3.62 s, P=0.0390). By the fifth week after EMS surgery, the escape latency was still significantly shortened (2VO + EMS5 vs. 2VO + EMS4: 15.63±2.88 vs. 19.86±4.26 s, P=0.0346). However, there was no significant difference in the escape latencies between the sixth and fifth weeks after EMS (2VO + EMS6 vs. 2VO + EMS5: 16.25±3.28 vs. 15.63±2.88 s, P=0.6916). In addition, the escape latency of the 2VO sham rats was significantly shorter than that of the 2VO + EMS5 group (9.13±1.81 vs. 15.63±2.88 s, P<0.001).
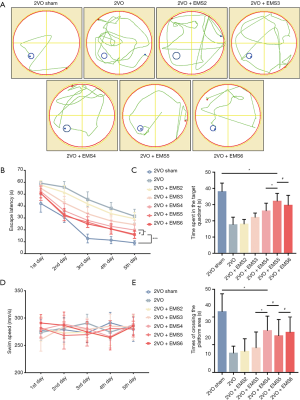
For the time spent in the target quadrant, the result in the 2VO + EMS2 group was not significantly different from that in the 2VO group (17.88±3.00 vs. 17.50±4.75 s, P=0.8529). Three weeks after EMS, the time spent in the target quadrant began to increase significantly (2VO + EMS3 vs. 2VO + EMS2: 22.00±2.83 vs. 17.88±3.00 s, P=0.0484). Furthermore, the rats in the 2VO + EMS4 group spent more time in the target quadrant than the rats in the 2VO + EMS3 group (26.00±4.90 vs. 22.00±2.83 s, P=0.0419), and the time spent in the target quadrant by the 2VO + EMS5 group rats was significantly longer than that spent by the 2VO + EMS4 group rats (32.02±4.96 vs. 26.00±4.90 s, P=0.0289). However, there was no significant difference in the time spent in the target quadrant between the 2VO + EMS6 and 2VO + EMS5 groups (29.50±6.23 vs. 32.02±4.96 s, P=0.3896). In addition, the time spent in the target quadrant of the 2VO sham rats was significantly longer than that of the 2VO + EMS5 group (37.88±5.38 vs. 32.02±4.96 s, P=0.0395). For the number of times crossing the platform area, there was no significant difference between the 2VO + EMS2 and 2VO groups (1.50±0.93 vs. 1.38±0.52, P=0.7438). However, the number of platform area crossings began to increase significantly from the third week after EMS (2VO + EMS3 vs. 2VO + EMS2: 1.75±1.16 vs. 1.50±0.93, P=0.0465). Furthermore, the number of times crossing the platform area was continuously more frequent at 4 weeks after EMS than at 3 weeks after surgery (2VO + EMS4 vs. 2VO + EMS3: 3.00±1.07 vs. 1.75±1.16, P=0.0431). Nevertheless, there was no significant difference in the number of times crossing the platform area between the 2VO + EMS5 and 2VO + EMS4 groups (2.63±1.19 vs. 3.00±1.07, P=0.5176) or between the 2VO + EMS6 and 2VO + EMS5 groups (2.88±1.13 vs. 2.63±1.19, P=0.6723). Furthermore, the number of times crossing the platform area of the 2VO sham rats was significantly greater than that of the 2VO + EMS4 group (4.38±1.30 vs. 3.00±1.07, P=0.0368).
In addition, there was no significant difference among groups in swimming speed during the experiments, indicating that the 2VO or 2VO + EMS procedures did not cause motor dysfunction in this study.
Discussion
In the present study, we revealed, for the first time, the trend in EC proliferation and indirect anastomosis formation induced and promoted by EMS surgery in the chronically ischemic brains of 2VO rats. We found that the degree of EC proliferation and CBP improvement on the EMS side of 2VO + EMS rat brains peaked in the fourth week after EMS, whereas cognitive improvement peaked in the fifth week after EMS. Undoubtedly, this finding is extremely helpful and illuminative for most basic studies related to angiogenesis and indirect anastomosis formation. Moreover, the novel method used to establish the 2VO + EMS rat model in this study had low mortality and was highly effective; thus, this method should be promoted. A discussion is presented in detail as follows.
The trends and significance of EC marker expression and CBP and cognitive improvement in the 2VO + EMS rat model
Unquestionably, EC proliferation is the basis of angiogenesis (14,15). Therefore, this study inferred the degree of angiogenesis by quantifying the expression of VEGF and CD31 (an EC marker). VEGF is a key angiogenesis-promoting cytokine that has an effect on increasing EC proliferation, migration and permeability (16-18). CD31 (platelet EC adhesion molecule-1, PECAM-1), which concentrates at the sites of interendothelial cell contact, is commonly used to detect the existence of ECs (19,20). In this study, the IF results showed that the expression of both VEGF and CD31 peaked at the fourth week after the EMS procedure. Thus, for basic studies performed using 2VO + EMS rat models, cytokine detection should optimally be performed at 4 weeks after EMS. On the one hand, this time point will optimally ensure that significant experimental results are obtained, and the difference between the experimental and control groups should be maximized. On the other hand, this time point could shorten the experimental period and save money in some cases. However, we also found that the expression of VEGF in 2VO + EMS rats was significantly higher at 2 weeks after EMS compared to that in the 2VO group, whereas the expression of CD31 was not significantly higher until the third week after EMS. This result probably indicates that VEGF is involved in the processes of EC proliferation and angiogenesis before CD31, and this finding could be explained by the different biological functions of these two cytokines (mentioned above).
In addition, to objectively and intuitively reflect the effect of EMS on promoting EC proliferation and angiogenesis, we used MRI-ASL sequences to quantify the perfusion ratio of CBP in each group. The perfusion ratio of 2VO + EMS rats increased slightly 2 weeks after EMS compared to that of 2VO rats, but the difference was not significant. However, the perfusion ratio increased significantly in the third week after EMS, peaked in the fourth week, and then entered the platform stage. The trend of CBP improvement on the EMS side was basically consistent with that of EC proliferation (especially the increased pattern of CD31 expression), which also confirmed the feasibility of using EC markers to represent the degree of angiogenesis.
Finally, we also assessed whether CBP improvement could improve the cognitive functions of 2VO + EMS rats by using the MWM test to evaluate cognitive function improvement over weeks after EMS. The results suggested that the cognitive function of 2VO + EMS rats was not significantly improved in the second week after EMS and began to show significant improvement beginning in the third week, continued to improve in the fourth week, and peaked in the fifth week after EMS (the escape latency and time spent in the target quadrant peaked in the fifth week, but the number of times crossing the platform area peaked in the fourth week). The improvement in cognitive function lagged slightly behind that of EC proliferation and CBP (which peaked in the fourth week) (Figure 9), but this lag is explainable. Taking patients with symptomatic CICD as an example, their recovery of affected neurological functions requires additional processes even if they undergo revascularization surgery (such as extracranial–intracranial bypass and middle cerebral artery recanalization) as soon as possible. In other words, after the formation of new capillaries, the repair of neurons needs to initiate another complex mechanism (21-23).
Advantages of and surgical techniques for the novel method for establishment of the 2VO + EMS rat model in this study
The traditional 2VO + EMS rat modeling method involves the simultaneous ligation of the bilateral CCAs and performance of EMS 1 week later (or at any given interval) (10,24). However, in this study, we first ligated the left CCA and then ligated the right CCA 1 week later and performed EMS simultaneously. Similar to the traditional method, our novel method also requires two anesthesia sessions and 1 week of experimental duration, but it has the following two advantages. First, the method of two separate unilateral CCA ligations allows the rat to adapt to the hypoperfusion state after unilateral CCA ligation (based on our prior experimental results, a decrease in CBF was not obvious when only one side of the CCA was ligated and soon returned to the baseline level). With this strategy, the sudden and sharp decrease in CBP caused by the simultaneous ligation of both CCAs can be avoided. In addition, clamping the right CCA repeatedly before ligating allows the rat to adapt to the hypoperfusion state after bilateral CCA ligation. Therefore, the novel modeling method described herein theoretically has a lower 7-day mortality rate than the traditional method. The 7-day mortality rate was 9.43% (5/53) in this study (the EMS procedure caused almost no brain damage, and its lethal risk was negligible), whereas the previously reported mortality rates of the traditional method ranged from 16% to 20% (12,25-28). Therefore, our novel method may have a lower mortality rate and be safer than the traditional method. Second, the immediate performance of indirect revascularization in a moyamoya patient before the natural establishment of adequate blood flow compensation generally leads to an ideal degree of anastomosis formation (for example, the effect of indirect revascularization in children with moyamoya disease is significantly better than that in adult patients) (29,30). However, the traditional method involves the performance of EMS 1 week after the establishment of 2VO. During this week, the affected anterior circulation blood flow of 2VO rats may be compensated to a certain degree (e.g., open posterior communicating artery), which has a negative effect on anastomosis establishment. In our modeling method, EMS was completed immediately after 2VO was established (when the ischemia was most severe). Therefore, compared with the traditional method, our new method can theoretically lead to better anastomosis formation, is more effective and is more conducive to experimental observation.
In addition, some useful surgical techniques can be utilized to ensure the safety and effectiveness of the 2VO + EMS model to the greatest extent. First, the rat DM is extremely soft and thin, and direct cutting with scissors or a scalpel will very likely damage the underlying brain tissues, resulting in dysfunction or death. We recommend gently scratching the dura with micro-tweezers to form a wrinkle and then tearing the wrinkled dura directly with two micro-tweezers, which can avoid damaging brain tissue. Second, the arachnoid membrane is thought to act as a barrier between the brain tissue and the TM, which can be a negative factor affecting the efficacy of indirect revascularization (31,32). Therefore, when modeling, a 1 mL syringe needle should be used to poke the arachnoid membrane in multiple places to promote anastomosis formation. Third, similar to the clinical EMS procedure (33), this method involves the suturing of the TM together with the edge of the DM (with 10-0 prolene) to ensure that the brain is covered tightly by TM. Fourth, after the skin is sutured, we recommend gently pressing the EMS area for 15 s to stop TM bleeding and to ensure that the brain is fully covered by TM. With all the above surgical techniques, anastomosis was observed in every 2VO + EMS rat in this study, and the mortality rate was very low; moreover, no cases of hemiplegia were observed (the lack of significant differences in the swimming speed between the groups also supports our point).
Limitations
The present study has two potential limitations. First, to reduce the number of rats needed, we did not establish more control groups to evaluate EC proliferation in the brain tissues of 2VO rats at the 2nd, 3rd, 4th, or 5th weeks. Second, our research results cannot clarify whether a part of EC proliferation was caused by physical exercise during the MWM test. Third, when we studied the effect of EMS surgery on CBP improvement, we used the CBF improvement in the ROIs (the regions with the best CBF improvement) to represent the CBF improvement in the whole cerebral hemisphere. Although this measure is representative of the overall CBF changes, it is not sufficiently rigorous. Therefore, in future work, we will further determine better methods to quantify the CBF improvement in the whole EMS-side hemisphere.
Conclusions
After establishing the 2VO + EMS rat model, the degree of EC proliferation and CBP improvement on the EMS side of the brain peaked at 4 weeks after EMS, whereas the cognitive improvement peaked in the fifth week after EMS. For basic experiments related to angiogenesis and indirect anastomosis formation, this finding will optimally ensure that the differences between groups are maximized and that significant experimental results are obtainable.
Acknowledgments
We thank Liying Zhang for her expert technical assistance.
Funding: This work was supported by the National Natural Science Foundation of China (Grant No. 81901211); the Guangdong Basic and Applied Basic Research Foundation (Grant No. 2019A1515010384); the Third Affiliated Hospital of Sun Yat-Sen University, Clinical Research Program (Grant No. QHJH201908); and the Medical Science and Technology Research Foundation of Guangdong Province (Grant No. A2019462).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-2936
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-2936
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-2936). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All experimental procedures were approved by the Animal Ethics Committee of Sun Yat-sen University (No.: [2019]02-480-12), and treatment conformed to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (publication no. 80-23, revised 1996).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pandey P, Steinberg GK. Neurosurgical advances in the treatment of moyamoya disease. Stroke 2011;42:3304-10. [Crossref] [PubMed]
- Acker G, Fekonja L, Vajkoczy P. Surgical management of moyamoya disease. Stroke 2018;49:476-82. [Crossref] [PubMed]
- Kuroda S, Houkin K. Moyamoya disease: current concepts and future perspectives. Lancet Neurol 2008;7:1056-66. [Crossref] [PubMed]
- Matsushima T, Inoue K, Kawashima M, et al. History of the development of surgical treatments for moyamoya disease. Neurol Med Chir (Tokyo) 2012;52:278-86. [Crossref] [PubMed]
- Chen C, Wang H, Hou B, et al. Surgical Revascularization for Children with Moyamoya Disease: A New Modification to the Pial Synangiosis. World Neurosurg 2018;110:e203-e211. [Crossref] [PubMed]
- Mizoi K, Kayama T, Yoshimoto T, et al. Indirect revascularization for moyamoya disease: is there a beneficial effect for adult patients? Surg Neurol 1996;45:541-8; discussion 548-9. [Crossref] [PubMed]
- Kusaka N, Sugiu K, Tokunaga K, et al. Enhanced brain angiogenesis in chronic cerebral hypoperfusion after administration of plasmid human vascular endothelial growth factor in combination with indirect vasoreconstructive surgery. J Neurosurg 2005;103:882-90. [Crossref] [PubMed]
- Kim HS, Lee HJ, Yeu IS, et al. The neovascularization effect of bone marrow stromal cells in temporal muscle after encephalomyosynangiosis in chronic cerebral ischemic rats. J Korean Neurosurg Soc 2008;44:249-55. [Crossref] [PubMed]
- Nakamura M, Imai H, Konno K, et al. Experimental investigation of encephalomyosynangiosis using gyrencephalic brain of the miniature pig: histopathological evaluation of dynamic reconstruction of vessels for functional anastomosis. Laboratory investigation. J Neurosurg Pediatr 2009;3:488-95. [Crossref] [PubMed]
- Hecht N, Pena-Tapia P, Vinci M, et al. Myoblast-mediated gene therapy via encephalomyosynangiosis--a novel strategy for local delivery of gene products to the brain surface. J Neurosci Methods 2011;201:61-6. [Crossref] [PubMed]
- Akinmoladun AC, Saliu IO, Olowookere BD, et al. Improvement of 2-Vessel Occlusion Cerebral Ischaemia/Reperfusion-Induced Corticostriatal Electrolyte and Redox Imbalance, Lactic Acidosis and Modified Acetylcholinesterase Activity by Kolaviron Correlates with Reduction in Neurobehavioural Deficits. Ann Neurosci 2018;25:53-62. [Crossref] [PubMed]
- Jiang T, Zhang L, Pan X, et al. Physical Exercise Improves Cognitive Function Together with Microglia Phenotype Modulation and Remyelination in Chronic Cerebral Hypoperfusion. Front Cell Neurosci 2017;11:404. [Crossref] [PubMed]
- D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev 2001;36:60-90. [Crossref] [PubMed]
- Goerke SM, Kiefer LS, Stark GB, et al. miR-126 modulates angiogenic growth parameters of peripheral blood endothelial progenitor cells. Biol Chem 2015;396:245-52. [Crossref] [PubMed]
- Li GJ, Yang Y, Yang GK, et al. Slit2 suppresses endothelial cell proliferation and migration by inhibiting the VEGF-Notch signaling pathway. Mol Med Rep 2017;15:1981-8. [Crossref] [PubMed]
- Park YS, Jeon YJ, Kim HS, et al. The role of VEGF and KDR polymorphisms in moyamoya disease and collateral revascularization. PLoS One 2012;7:e47158. [Crossref] [PubMed]
- Li N, Wang P, Ma XL, et al. Effect of bone marrow stromal cell transplantation on neurologic function and expression of VEGF in rats with focal cerebral ischemia. Mol Med Rep 2014;10:2299-305. [Crossref] [PubMed]
- Guo F, Liu J, Han X, et al. FBXO22 Suppresses Metastasis in Human Renal Cell Carcinoma via Inhibiting MMP-9-Mediated Migration and Invasion and VEGF-Mediated Angiogenesis. Int J Biol Sci 2019;15:647-56. [Crossref] [PubMed]
- Avari H, Rogers KA, Savory E. Quantification of Morphological Modulation, F-Actin Remodeling and PECAM-1 (CD-31) Re-distribution in Endothelial Cells in Response to Fluid-Induced Shear Stress under Various Flow Conditions. J Biomech Eng 2019. Epub ahead of print. [Crossref] [PubMed]
- Meza D, Shanmugavelayudam SK, Mendoza A, et al. Platelets modulate endothelial cell response to dynamic shear stress through PECAM-1. Thromb Res 2017;150:44-50. [Crossref] [PubMed]
- Shu J, He X, Li H, et al. The Beneficial Effect of Human Amnion Mesenchymal Cells in Inhibition of Inflammation and Induction of Neuronal Repair in EAE Mice. J Immunol Res 2018;2018:5083797. [Crossref] [PubMed]
- Klim JR, Williams LA, Limone F, et al. ALS-implicated protein TDP-43 sustains levels of STMN2, a mediator of motor neuron growth and repair. Nat Neurosci 2019;22:167-79. [Crossref] [PubMed]
- Gaudet AD, Mandrekar-Colucci S, Hall JC, et al. miR-155 Deletion in Mice Overcomes Neuron-Intrinsic and Neuron-Extrinsic Barriers to Spinal Cord Repair. J Neurosci 2016;36:8516-32. [Crossref] [PubMed]
- Huang TH, Lin YW, Huang CP, et al. Short-term auricular electrical stimulation rapidly elevated cortical blood flow and promoted the expression of nicotinic acetylcholine receptor alpha4 in the 2 vessel occlusion rats model. J Biomed Sci 2019;26:36. [Crossref] [PubMed]
- Bhuvanendran S, Bakar SNS, Kumari Y, et al. Improves the Spatial Memory and Hippocampal Long-Term Potentiation in a Rat Model of Chronic Cerebral Hypoperfusion. Sci Rep 2019;9:14507. [Crossref] [PubMed]
- Takahashi Y, Wakita H, Mizutani K, et al. Selective accumulation of adiponectin in the cerebral cortex under chronic cerebral hypoperfusion in the rat. Neuroreport 2020;31:148-55. [Crossref] [PubMed]
- Iwasaki Y, Ito S, Suzuki M, et al. Forebrain ischemia induced by temporary bilateral common carotid occlusion in normotensive rats. J Neurol Sci 1989;90:155-65. [Crossref] [PubMed]
- Ogata J, Fujishima M, Morotomi Y, et al. Cerebral infarction following bilateral carotid artery ligation in normotensive and spontaneously hypertensive rats: a pathological study. Stroke 1976;7:54-60. [Crossref] [PubMed]
- Abla AA, Gandhoke G, Clark JC, et al. Surgical outcomes for moyamoya angiopathy at barrow neurological institute with comparison of adult indirect encephaloduroarteriosynangiosis bypass, adult direct superficial temporal artery-to-middle cerebral artery bypass, and pediatric bypass: 154 revascularization surgeries in 140 affected hemispheres. Neurosurgery 2013;73:430-9. [Crossref] [PubMed]
- Piao J, Wu W, Yang Z, et al. Research Progress of Moyamoya Disease in Children. Int J Med Sci 2015;12:566-75. [Crossref] [PubMed]
- Baaj AA, Agazzi S, Sayed ZA, et al. Surgical management of moyamoya disease: a review. Neurosurg Focus 2009;26:E7. [Crossref] [PubMed]
- Adelson PD, Scott RM. Pial synangiosis for moyamoya syndrome in children. Pediatr Neurosurg 1995;23:26-33. [Crossref] [PubMed]
- Karasawa J, Kikuchi H, Furuse S, et al. A surgical treatment of "moyamoya" disease "encephalo-myo synangiosis". Neurol Med Chir (Tokyo) 1977;17:29-37. [Crossref] [PubMed]



