
Predictive factors of postoperative pancreatic fistula after laparoscopic pancreatoduodenectomy
Introduction
The establishment of safe and viable techniques in the recent and rapid advancement of laparoscopic pancreatoduodenectomy (LPD) has mainly been attributed to the innovation of laparoscopic instruments and the accumulation of laparoscopic experience (1-4). LPD has been found to be advantageous compared with open PD (OPD) with less intra-operative blood loss, shorter postoperative hospital stay, reduced pain, and faster recovery. Although the mortality rate after LPD has decreased to less than 5% in most of the high-volume centers, the incidence of postoperative complications remains high (1,5). Clinically relevant postoperative pancreatic fistula (CR-POPF) has been reported to be one of the most common complications, with an incidence rate of 5% to 20%. These results are strongly associated with other major complications, including intra-abdominal abscess, postoperative hemorrhage, and sepsis (1,3,6). Therefore, the early identification of patients at high risk of POPF is critical for postoperative management and improved prognosis after LPD.
Research examining the risk factors and indicators of POPF after LPD has lagged comparatively to that of OPD (7-9). In previous studies, risk factors including pancreatic texture, pancreatic duct size, obesity, operative time, intra-operative blood loss, and amylase value in drainage have been extensively studied (7,8,10,11). Among these factors, soft pancreatic texture was the most widely accepted risk factor of POPF after PD. Additionally, obesity has also demonstrated a close association with POPF after PD. The visceral fat area (VFA) and subcutaneous fat area (SFA) measured by computed tomography (CT) have been considered better indicators of obesity than body mass index (BMI) (12,13).
In this study, we comprehensively analyzed pre-, intra-, and postoperative variables derived from CT to investigate the risk factors of CR-POPF after LPD. We further assessed the relationship between obesity and the consistency of the pancreas. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-1411).
Methods
Study population
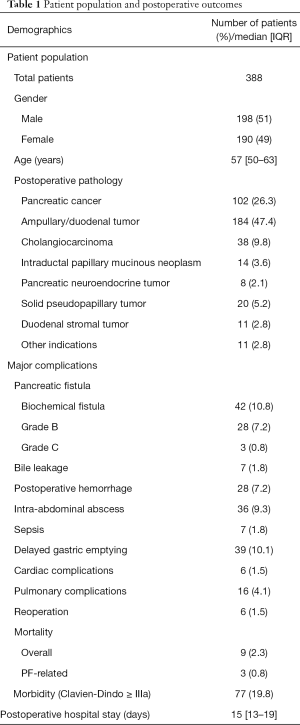
Between July 2014 and December 2018, 425 consecutive patients who underwent LPD at the Tongji Medical Hospital of Tongji Medical College of Huazhong University of Science and Technology were initially identified and enrolled in the study. The exclusion criteria of the study included the following: incomplete clinicopathological data, palliative resection, combined with other organ resections (such as hepatectomy or colectomy), combined vascular resection and converted to laparotomy. All cases included in this study were at phase III of the LPD learning curve, according to our previous report (1). Subsequently, 388 patients were finally included in the study, which consisted of 198 men (51.0%) and 190 women (49.0%), with a median age of 57 years (range, 50–63 years). Postoperative pathology included the following: pancreatic cancer in 102 patients (26.3%), ampullary/duodenal tumor in 184 patients (47.4%), cholangiocarcinoma in 38 subjects (9.8%), intraductal papillary mucinous neoplasm in 14 patients (3.6%), pancreatic neuroendocrine tumor in 8 patients (2.1%), duodenal stromal tumor in 11 patients (2.8%), solid pseudopapillary tumor of the pancreas in 20 patients (5.2%), and other indications in 11 (2.8%) patients. All LPD procedures were performed by two experienced hepatopancreatic and biliary surgeons (Qin R and Zhu F) at a single institution. The study was approved by the Institutional Review Board of Affiliated Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (approval ID: TJ-IRB20190418). The study was conducted according to the principles of the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from patients before enrolment. The patient population is presented in Table 1.
Full table
Operative technique and perioperative management
All patients in the study underwent a standard LPD by R. Q and F. Z.
Operative procedure
The pneumoperitoneum was established after the application of a 12-mm trocar, inferior to the umbilicus. Two 12-mm trocars were then placed on the right and left midclavicular lines, followed by the placement of two 5-mm trocars at the right and left infracostal arch on both sides of the anterior axillary line.
Organ resection
The Kocher maneuver was initially performed before the removal of the distal stomach, left of the pylorus. A tunnel was dissected posterior to the pancreatic neck and anterior to the superior mesenteric vein and portal vein. The pancreas was transected with ultrasonic shears, and the jejunum was transected approximately 15 cm from the ligament of Treitz. Cholecystectomy was performed, and the common hepatic duct was transected at the level of the cystic duct. Before digestive tract reconstruction, specimens from the abdominal cavity were removed, and the texture of the pancreas was examined.
Digestive tract reconstruction
Most patients underwent pancreatojejunostomy, and pancreatogastrostomy was only performed in a minority of cases. We performed pancreaticojejunostomy with the embedding method, as reported previously (14), using a stent tube for insertion into the pancreatic duct. Pancreatogastrostomies were performed by embedding the pancreatic remnant into the stomach. An end-to-side hepaticojejunostomy was performed with either running or interrupted Vicryl sutures. An antecolic side-to-side gastroenterostomy was performed with the staple technique, and two layers of running 3-0 Vicryl sutures were applied to close the gastric stump. Upon completion of the operation, two drains were routinely placed at the sites of the pancreatojejunostomy and hepaticojejunostomy, respectively.
Patients were admitted to the intensive care unit after surgery and subsequently transferred to the general ward. Routine hematology and biochemistry profiles were closely assessed the first few days post-surgery. The drain was removed in the absence of evident PF. Alternatively, the drain remained attached until the quantity of expelled fluid was less than 10 mL per 24 hours for at least 3 consecutive days if PF was evident. An abdominal CT scan was routinely performed 7 days post-surgery. All patients were administered octreotide for 1 week after surgery.
Clinical data collection
The following patient characteristics, preoperative laboratory data, preoperative CT parameters, intra-operative parameters, postoperative laboratory data, and postoperative outcomes were reviewed.
- Patient characteristics: age, gender, BMI, comorbidities (diabetes mellitus, high blood pressure (HPB), cardiovascular disease), pancreatitis history, abdominal surgery history, preoperative biliary drainage history, pathology (non-pancreatic cancer diseases, and pancreatic cancer diseases).
- Preoperative laboratory data: admission bilirubin, preoperative bilirubin, hemoglobin, serum albumin, alanine transaminase, γ-glutamyltranspeptidase, CA19-9, and CA125.
- Intraoperative parameters: estimated operative time, blood loss, transfusion, pancreatic texture assessed by surgeons, and type of pancreatoenterostomy.
- Postoperative blood tests: white blood cell count of postoperative day 1 (WBC POD1), white blood cell count of postoperative day 3 (WBC POD3), WBC (POD1-POD3), platelet count of postoperative day 1 (Plt POD1), Plt of postoperative day 3 (Plt POD3), Plt (POD1–POD3), serum albumin of postoperative day 1 (Alb POD1), serum albumin of postoperative day 3 (Alb POD3), and Alb of postoperative day 3 ((POD1–POD3).
- Postoperative outcomes: pancreatic fistula, bile leakage, postoperative hemorrhage, intra-abdominal abscess, sepsis, delayed gastric emptying, pulmonary complications, cardiac complications, reoperation, mortality, morbidity, postoperative hospital stay.
Preoperative CT parameters and measurement
Preoperative CT parameters: total fat area (TFA), visceral fat area (VFA), subcutaneous fat area (SFA), abdominal wall fat thickness, intra-abdominal fat thickness, main pancreatic duct width, and pancreas gland thickness. All patients underwent preoperative multiphasic (non-enhanced, arterial, and portal venous phases) multidetector CT examination within 1 month before surgery. The original DICOM image was transmitted to a dedicated workstation and reconstructed into the 5 mm image for further analysis. The measurements were performed by two experienced investigators (J.L.L. and Z.L.) of the department of radiology, who were blinded to clinical data and clinical outcomes. The open-source software FireVoxel (https://wp.nyu.edu/firevoxel) was used to quantify fat. The level of the umbilicus was selected for measurement of the TFA, SFA, VFA, abdominal wall fat thickness, intra-abdominal fat thickness.
TFA, SFA, VFA
The umbilicus level of the axial image was selected as the total region of interest (t-ROI). The TFA was obtained by using the threshold (−190 to −30 HU) segmentation function of the software to segment the t-ROI (Figure 1A) automatically. The range, of −190 to −30 HU can accurately include fat while excluding non-fat tissue. Additionally, SFA measurements were with the ROI of subcutaneous fat (s-ROI). The boundary of the abdominal wall muscles and paraspinal muscles were selected manually as the area of interest (Figure 1B). Finally, VFA was obtained by subtracting SFA from TFA.
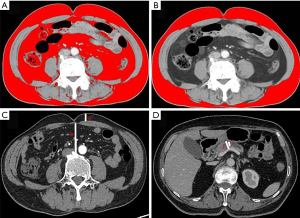
The thickness of abdominal- and intra-abdominal wall fat
The level of the umbilicus was selected for measurement of the abdominal wall thickness and intra-abdominal fat thickness. Abdominal wall thickness was obtained by measuring the anteroposterior distance between the skin and anterior rectus sheath. Intra-abdominal fat thickness (Figure 1C) was the anteroposterior distance between the linea alba and the posterior aortic wall (Figure 1C).
The main pancreatic duct width and pancreas gland thickness were measured at the left margin of the SMV/PV junction (Figure 1D).
Definition of major postoperative complications
Pancreatic fistula
The diagnosis of POPF was determined according to the definitions provided by the International Study Group of Pancreatic Surgery (ISGPS) (15): any measurable volume of drain fluid, on or after postoperative day 3 with an amylase level more than 3 times the upper limit of normal amylase was defined as PF.
POPF
Biochemical fistula is applied to the original grade A POPF, and is no longer considered a true pancreatic fistula or an actual complication; grade B PF is a fistula involving increased amylase activity in the fluid from any drain in association with a clinically relevant condition requiring a change in the management of the expected postoperative pathway; grade C PF is applied when a grade B POPF leads to organ failure or clinical instability, requiring a reoperation. CR-POPFs encompass grade B and grade C PF.
Delayed gastric emptying (DGE) (16) biliary leak (17), and postoperative hemorrhage (PPH) (18) were defined according to the International Study Group of Pancreatic Surgery. Postoperative morbidity was evaluated according to the Clavien-Dindo (CD) classification system (19).
Statistical methods
Continuous variables are expressed as median and interquartile range, (IQR), with P<0.05 dichotomized at the median. Categorical variables are expressed as frequencies and percentages. According to the distribution of variables, χ2 or Fisher’s exact tests were used to compare differences in discrete or categorical variables. The Mann-Whitney U-test was used for continuous variables. Univariate analysis was performed using patient characteristics, preoperative laboratory data, preoperative CT parameters, intraoperative parameters, and postoperative laboratory data potentially associated with CR-POPF. Variables with P<0.10 were entered into multivariable logistic regression models. A backward variable selection procedure was used to identify the independent predictive factors of CR-POPF. Odds ratios (OR) with 95% confidence intervals (CI) were given. To evaluate the predictive ability of the risk factor models, the area under the receiver operating characteristic curve (ROC) was determined. For all tests, differences with P<0.05 were considered statistically significant. All tests were two-sided. Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) version 22.0 (IBM SPSS, Armonk, NY, USA).
Results
Postoperative outcomes
Out of 388 patients, CR-POPF occurred in 31 patients (8.0%), including grade B POPF in 28 (7.2%), and grade C POPF in 3 (0.8%) patients. Biochemical fistula was observed in 42 patients (10.8%), and the morbidity incidence for the entire group was 19.8% (77/388). Bile leakage, postoperative hemorrhage, intra-abdominal abscess, sepsis, delayed gastric emptying, cardiac complications, pulmonary complications occurred in 7 (1.8%), 28 (7.2%), 36 (9.3%), 7 (1.8%), 39 (10.1%), 6 (1.5%), and 16 (4.1%) patients, respectively. The mortality rate was 2.3% (9/388) in the group, with three patients who died displaying CR-POPF and six (1.5%) patients requiring reoperation. The median postoperative hospital stay was 15 days (13-19). The postoperative outcomes are presented in Table 1.
Patient characteristics according to CR-POPF
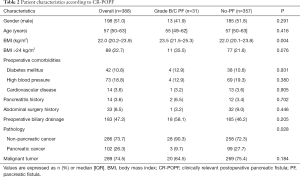
The comparison of patient characteristics with and without CR-POPF is shown in Table 2. No differences were observed between patients with or without CR-POPF across gender, age, comorbidities (diabetes mellitus, HPB, cardiovascular disease), pancreatitis history, abdominal surgery history, or preoperative biliary drainage. The median BMI was 22 kg/m2 (20.2–23.9). The occurrence of POPF was associated with BMI (P=0.004), with BMI >24 kg/m2 being the threshold for overweight classification in China. However, no correlation was noted between BMI >24 kg/m2 and the occurrence of CR-POPF (P=0.076). Also, statistical differences were noted between the two groups in pathological diagnoses: the PF group had more non-pancreatic cancer patients than the no-PF group (90.3% vs. 72.3%; P=0.028).
Full table
Preoperative CT parameters and laboratory data according to CR-POPF
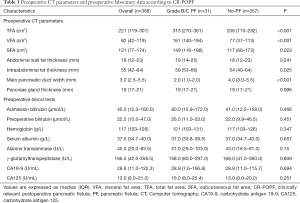
The comparison of preoperative CT parameters and laboratory data between patients with and without CR-POPF is shown in Table 3. In the whole population, the median VFA was 82 cm2 (range, 42–119 cm2) with a median SFA of 121 cm2 (range, 77–174 cm2) and a median TFA of 221 cm2 (range, 119–301 cm2); the median intra-abdominal fat thickness, main pancreatic duct width, and pancreas gland thickness were 55 mm (range, 42–64 mm), 3.0 mm (range, 2.5–5.5 mm), and 19 mm (range, 17–21 mm), respectively. The VFA, SFA, TFA, main pancreatic duct width, and intra-abdominal fat thicknesses were associated with the occurrence of CR-POPF. No differences between the two groups in abdominal wall fat thickness or pancreas gland thickness were observed. Additionally, patients did not exhibit any differences in preoperative blood tests, irrespective of CR-POPF occurrence.
Full table
Intraoperative data and postoperative laboratory data according to CR-POPF
The comparisons of intraoperative data and postoperative laboratory data between patients with and without CR-POPF are shown in Table 4. No differences were observed between the two groups in blood loss, transfusion, and type of pancreatoenterostomy. Soft pancreas was more common in the group with PF than in the group without PF (96.8% vs. 26.1%; P<0.001). The median operative time was 320 min (range, 289–360 min), and the occurrence of CR-POPF was significantly associated with operative duration (P<0.001). Postoperative blood tests showed no statistical differences between the two groups, except in Alb (POD1–POD3) (P=0.006).
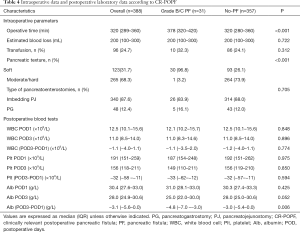
Full table
Univariate and multivariate analysis of potential predictors associated with CR-POPF
Continuous variables with P<0.05 were dichotomized at the median. Univariate analysis showed that the TFA >221 cm2 (P<0.001), SFA >121 cm2 (P=0.015), VFA >82 cm2 (P<0.001), main pancreatic duct width <3 mm (P<0.001), soft pancreas (P<0.001), operative time >320 min (P=0.002), non-pancreatic cancer diseases (P=0.028), and Alb (POD1–POD3) <−3.1 g/L (P=0.011) were significantly associated with a higher risk of CR-POPF. Variables with P<0.10 at univariate analysis were entered into multivariable logistic regression models, meaning BMI >24 kg/m2 (P=0.076) and intra-abdominal fat thickness >55 mm (P<0.059) were also included in multivariable logistic regression analysis. Also, as they are important clinical factors, gender and estimated blood loss, were also included in multivariable analysis. The results indicated that VFA >82 cm2 (OR =11.088; P=0.029), main pancreatic duct width <3 mm (OR =7.701; P=0.001), soft pancreatic texture (OR =12.543; P=0.022), and operative time >320 min (OR =6.061; P<0.001) were the independent risk factors of CR-POPF. The univariate and multivariate analysis of potential predictive factors associated with CR-POPF are presented in Table 5.
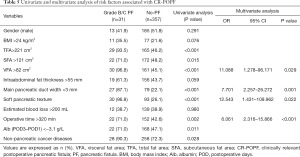
Full table
ROC analysis of predictive models associated with CR-POPF
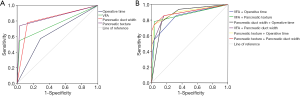
The multivariate analysis suggested that four factors (VFA ≥82 cm2, main pancreatic duct width ≤3 mm, pancreatic texture, and operative time >320 min) would be retained in the final model. Due to the small sample size of 31 CR-POPF patients, the model was limited to a maximum of two predictors. The pancreatic texture with an area under the curve (AUC) of 0.854 was the strongest single predictor, and a better indicator compared to VFA >82 cm2 (0.758), pancreatic duct width ≤3 mm (0.825), and operative time ≥320 min (0.642). When two predictors were combined, the greatest two-predictor model (pancreatic texture and pancreatic duct width <3 mm) resulted in an AUC of 0.904. The other combinations of predictive models are shown in Table 6 and Figure 2.
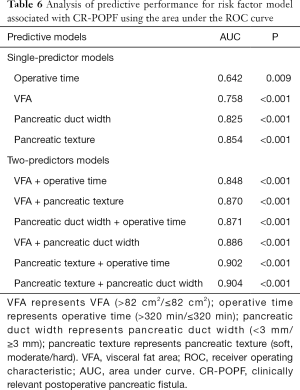
Full table
The relationship between the consistency of the pancreas and characteristics of patients, preoperative CT parameters
No differences were observed between the groups of patients with soft, moderate, or hard pancreas across gender, age, BMI, high blood pressure, diabetes mellitus, and abdominal wall fat thickness. Non-pancreatic cancer diseases (P=0.005), TFA >221 cm2 (P<0.001), SFA >121 cm2 (P<0.001), VFA >82 cm2 (P<0.001), main pancreatic duct width <3 mm (P<0.001), and intra-abdominal fat thickness >55 mm (P=0.037) were significantly associated with a soft pancreatic texture. In a multivariate analysis, TFA >221 cm2 (OR =8.637; P=0.001), VFA >82 cm2 (OR =7.009; P<0.001) and main pancreatic duct width <3 mm (OR =9.819; P<0.001) were the independent influence factors of a soft pancreatic texture. Pancreatic texture showed a significant correlation with obesity, as shown in Table 7.
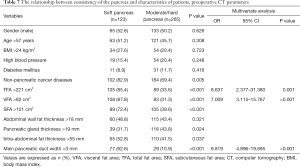
Full table
Discussion
In this retrospective cohort, 31 patients (8.0%) underwent LPD and displayed grade B/C POPF. Furthermore, we identified VFA >82 cm2, main pancreatic duct width <3 mm, soft pancreatic texture, and operative time >320 min as independent risk factors of CR-POPF after LPD. The combination of pancreatic texture and pancreatic duct width was the strongest predictor of CR-POPF (AUC =0.904).
Recently, the surgical efficacy of LPD has been consistently shown as an effective treatment for pancreatic and periampullary neoplasm. Nevertheless, the incidence of major postoperative complications is still relatively high, with CR-POPF being considered the most life-threatening and most common of these complications (1,5,6). Many studies have analyzed the risk factors of PF after PD (9,11,20). However, equivalent investigations of PF after LPD are rare. Therefore, it is of considerable significance to study the risk factors of PF to identify those patients who are likely to develop severe pancreatic fistula after LPD.
The predictive factors of PF have been studied extensively, but it is still difficult to accurately predict the occurrence of PF after PD. The risk factors that have been identified are mainly surgical, or disease- or host-related (8-10,12), with the presence of a soft pancreas widely considered to be the most critical risk factor. However, the relevant validation of risk factors for POPF after LPD remains unclear. In our study, the patient characteristics, preoperative laboratory data, preoperative CT parameters, intraoperative parameters, and postoperative laboratory data potentially influencing grade B or C POPF were comprehensively analyzed.
Obesity is a world health problem, and is closely linked to many chronic diseases like diabetes mellitus, coronary disease, and hypertension (21,22). Also, increasing evidence suggests that obesity increases the risk of PF after PD (12,13). BMI has often been used to measure obesity due to its simplicity and availability and is considered a risk factor for POPF. However, many contradictory reports within the current literature exist concerning whether or not BMI is an independent risk factor for POPF. For instance, Deng et al. (23) showed BMI >25 cm2 to be an independent risk factor of POPF, and this was corroborated by findings of Jang et al. (24). However, another study on 356 patients at the Memorial Sloan Kettering Cancer Center found no correlation between BMI and POPF (25). In the present study, multivariate logistic regression analysis revealed that a BMI >24 kg/m2 is not an independent risk factor of CR-POPF after LPD.
BMI only reflects the overall degree of obesity but fails to define body fat distribution, which forms the basis of many studies in identifying new ways to characterize these phenomena. Some authors have utilized intelligent systems on CT or magnetic resonance imaging (MRI) to quantify body fat distribution before identifying its correlation with POPF (7,20,26). Radiological variables mainly involve the visceral fat area (VFA), superficial fat area (SFA), retro-renal fat thickness, intraabdominal fat thickness, and abdominal wall fat thickness.
The research suggests that VFA is an independent risk factor of CR-POPF for PD (7,12,20). Kirihara et al. (7) demonstrated a clear relationship between body fat distribution and POPF in 173 consecutive patients who underwent PD. They observed that a higher visceral adipose tissue (VAT) area was the independent risk factor for POPF (C-index =0.860), and was a better predictor of PF than BMI (C-index =0.739). Meanwhile, the predictive ability of VAT was shown to be superior to that of subcutaneous adipose tissue (SAT). Compared to the SAT, the degree of visceral adiposity estimated by the VAT area was found to be a significant risk factor for PF after PD. Similarly, House et al. (25) reported that body fat distribution was an essential predictor of PF, where greater retro-renal fat thickness assessed by CT was significantly associated with POPF. In other recent research, visceral fat tissue was considered an endocrine organ, was associated with metabolic syndrome, and was found to participate in postoperative inflammatory reactions. These results correlate with the incidence of higher PF after PD when patients displayed more significant visceral fat (27,28). In our study, VFA >82 cm2 was an independent risk factor of PF after LPD (OR =11.088; P=0.029). TFA >221 cm2 and SFA >121 cm2 were risk factors of CR-POPF in univariate analysis, but not independent risk factors in multivariate logistic regression analysis. VFA showed a more significant correlation with pancreatic fistula than did SFA and TFA. LPD is more challenging to perform compared to OPD, due to excessive visceral fat in obese patients that significantly affects the operators’ laparoscopic field of view. Excessive visceral fat also increases the difficulty in exposing and separating the abdominal tissue, contributing to the challenges of LPD.
Fatty pancreas infiltration has been proposed as a significantly reliable and predictive factor of POPF. Gaujoux et al. (13) concluded that a fatty pancreas in the absence of pancreatic fibrosis was a more precise and objective prediction of PF than the consistency of pancreatic remnant. However, fatty infiltration could only be evaluated by postoperative histopathology and failed to predict PF at an early stage. Some researchers revealed a correlation between obesity and visceral fat with fatty pancreas infiltration (20). An increased VFA (>84 cm2), obtained on a preoperative CT scan, and fatty pancreas on pathological examination were linked to a heightened risk of CR-POPF. Also, VFA >84 cm2 was statistically associated with fatty pancreas infiltration and therefore used to predict the occurrence of PF. However, the relationship between VFA, fatty pancreas, and the texture of the pancreas was not described in detail.
Currently, a soft pancreatic remnant is the risk factor widely acknowledged by many investigators (13,24,29). Our study revealed soft pancreatic texture to be an independent risk factor (OR =12.543; P=0.022) and the strongest single-predictor (AUC =0.854) of CR-POPF for LPD. Compared with OPD, the soft pancreatic remnant is a more reliable risk factor of PF after LPD. During the reconstruction of the pancreatic-digestive anastomosis, surgeons need to use laparoscopic instruments to achieve the appropriate suture tension without forming the direct sense. Excessive suture tension leads to damage in the soft pancreatic parenchyma. Meanwhile, anastomosis is ineffective and promotes the incidence of POPF. However, the evaluation of pancreatic texture is complex and subjective. With the aid of postoperative histopathology and other objective assessment tools, it may be possible to more accurately and objectively describe the texture of the pancreas.
In this study, determining the pancreatic fatty content as part of routine clinical practice was challenging, as samples were obtained by histological examination, post-surgery. We focused on the relationship between body fat distribution and the consistency of pancreatic remnant. Our study revealed that VFA >82 cm2, TFA >221 cm2, and main pancreatic duct width <3 mm was significantly associated with a soft pancreatic remnant. These findings are consistent with the incidence of abdominal obesity and fatty pancreas infiltration, further softening the pancreas (12,13). Meanwhile, a smaller pancreatic duct was more common in the soft pancreas. Therefore, the pancreatic texture may be evaluated simply by VFA, TFA, and pancreatic duct width, along with CT before surgery, and can serve as preoperative predictive tools of PF.
As previously reported, pancreatic duct diameter has been demonstrated to be an essential risk factor for POPF after PD (7,30,31). These results can be explained by the difficulties in reconstructing the pancreatic-digestive anastomosis in a smaller pancreatic duct. In a study of 233 patients of PD, Pratt et al. revealed that pancreatic duct sizes <3 mm were associated with higher fistula rates, which increased with smaller ducts. The diameter of the pancreatic duct has also been incorporated into predictive scoring systems (32). Recently, a few researchers proposed the pancreatic duct index (pancreas duct width/pancreas width), as measured by CT scan, as an independent risk factor of POPF (33). In our study, the pancreatic duct width of the PF group was smaller than that of the no-PF group (P<0.001). The main pancreatic duct (OR =7.701; P=0.001) was demonstrated to ben an independent risk factor of CR-POPF after LPD.
Recently, the correlation between postoperative blood tests and pancreatic fistula were also extensively analyzed (8,10,34). Kawai et al. (34) analyzed 244 consecutive patients who underwent PD and discovered that serum albumin level ≤3.0 g/dL on POD 4 was an independent predictive factor of clinically relevant pancreatic fistula. The decrease of postoperative albumin level resulted from various factors, including hemodilution, surgical stress, and inflammatory reaction. Therefore, serum albumin levels were an important indicator of nutritional status and prognosis. The tissue edema caused by hypoproteinemia after LPD can affect the healing of pancreatic-digestive anastomosis, which may explain the correlation between the decline of postoperative serum albumin and pancreatic fistula. Similarly, our study found that Alb (POD1–POD3) was strongly associated with CR-POPF after LPD, and Alb (POD1–POD3) <3.1 g/L was a risk factor in univariate analysis. However, it was not an independent risk factor in multivariable logistic regression analysis.
The duration of operative time was closely related to the degree of surgical difficulty and experience of surgeons. After a long period of operation, anesthesia, and hypothermia, the normal physiological function and the internal environment are susceptible to damage. Furthermore, an operation of a longer duration may lead to increased postoperative stress response and severe inflammation or systemic inflammatory response syndrome, which may cause the occurrence of PF. In our study, the operative time was 320 minutes or longer for 71% and 42.6% of patients in the CR-POPF and no-PF groups, respectively (P=0.002) group. Meanwhile, the operative duration of over 320 minutes an independent risk factor of CR-POPF, with an OR of 6.061. These results are consistent with the findings of de Castro (35), who determined that an operative time ≥285 min in 459 consecutive patients who underwent PD was the independent risk factor of PF.
Also, we aimed further to identify the predictive ability of risk factor models. The ROC analysis of risk factors revealed pancreatic texture as the strongest single predictor. The greatest two-predictor model (pancreatic texture and pancreatic duct width) resulted in an AUC of 0.904. VFA combined with pancreatic duct width as a preoperative predictive model had an AUC of 0.886. These models from our study showed excellent predictive abilities of PF and aided in the clinical perioperative management of our patients.
CR-POPF is acknowledged to be one of the most severe postoperative complications and is the subject of extensive international investigation. The ultimate purpose of studying the risk factors of POPF is to achieve early detection in high-risk patients in order to implement preventive measures, modify perioperative management, and reduce the incidence of postoperative complications and mortality. In our study, the VFA, pancreatic texture, main pancreatic duct width, and operative time were the independent predictive factors of CR-POPF after LPD. For obese patients, laparoscopic surgery is complicated due to excessive fat affecting the endoscopic field of vision. We believe that laparotomy for these patients may be safer, particularly for the surgeons in the early stages of the LPD learning curve. For extremely complicated laparoscopic operations with a longer duration, it is beneficial to reduce postoperative complications by turning to open surgery, thereby reducing operation and anesthesia time. Additionally, adopting appropriate pancreaticojejunostomy for different sizes of the pancreatic duct may be beneficial in reducing the incidence of postoperative pancreatic fistula.
Although this study investigated the predictive factors of CR-POPF after LPD by the comprehensive analysis of pre, intra- and postoperative variables, some limitations should be addressed. First, the study did not compare LPD and OPD data across the same period in our pancreatic surgery center. Second, this study was subject to selection bias due to its retrospective nature. The results need to be confirmed by prospective randomized studies.
Conclusions
Main pancreatic duct width <3 mm, VFA >82 cm2, soft pancreatic texture, and operative time >320 min were the independent predictive factors of CR-POPF after LPD. The combination of pancreatic texture and pancreatic duct width was the strongest predictor for CR-POPF (AUC =0.904). Furthermore, VFA and TFA were closely related to pancreatic texture; they may be used as a simple means to assess pancreatic texture and can thus be applied to the prediction of POPF before surgery.
Acknowledgments
The authors acknowledge the contribution of all the investigators at the participating study sites. We would also like to thank E. Tan and J. Chapnick of AME for English language editing.
Funding: This study was supported by grants from the Youth Program of National Natural Science Foundation of China (no. 81902499) and National Natural Science Foundation of China (no. 81772950, 81874205, and 81771801).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-1411.
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-1411
Peer Review File: Available at http://dx.doi.org/10.21037/atm-20-1411
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-1411). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted according to the principles of the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Affiliated Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (approval ID: TJ-IRB20190418). Written informed consent was obtained from patients before enrolment.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang M, Peng B, Liu J, et al. Practice Patterns and Perioperative Outcomes of Laparoscopic Pancreaticoduodenectomy in China: A Retrospective Multicenter Analysis of 1029 Patients. Ann Surg 2021;273:145-53. [PubMed]
- Croome KP, Farnell MB, Que FG, et al. Total laparoscopic pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: oncologic advantages over open approaches? Ann Surg 2014;260:633-8; discussion 638-40. [Crossref] [PubMed]
- Song KB, Kim SC, Hwang DW, et al. Matched Case-Control Analysis Comparing Laparoscopic and Open Pylorus-preserving Pancreaticoduodenectomy in Patients With Periampullary Tumors. Ann Surg 2015;262:146-55. [Crossref] [PubMed]
- Chen K, Pan Y, Liu XL, et al. Minimally invasive pancreaticoduodenectomy for periampullary disease: a comprehensive review of literature and meta-analysis of outcomes compared with open surgery. BMC Gastroenterol 2017;17:120. [Crossref] [PubMed]
- Adam MA, Choudhury K, Dinan MA, et al. Minimally Invasive Versus Open Pancreaticoduodenectomy for Cancer: Practice Patterns and Short-term Outcomes Among 7061 Patients. Ann Surg 2015;262:372-7. [Crossref] [PubMed]
- de Rooij T, Lu MZ, Steen MW, et al. Minimally Invasive Versus Open Pancreatoduodenectomy: Systematic Review and Meta-analysis of Comparative Cohort and Registry Studies. Ann Surg 2016;264:257-67. [Crossref] [PubMed]
- Kirihara Y, Takahashi N, Hashimoto Y, et al. Prediction of pancreatic anastomotic failure after pancreatoduodenectomy: the use of preoperative, quantitative computed tomography to measure remnant pancreatic volume and body composition. Ann Surg 2013;257:512-9. [Crossref] [PubMed]
- Partelli S, Pecorelli N, Muffatti F, et al. Early Postoperative Prediction of Clinically Relevant Pancreatic Fistula after Pancreaticoduodenectomy: usefulness of C-reactive Protein. HPB (Oxford) 2017;19:580-6. [Crossref] [PubMed]
- Roberts KJ, Sutcliffe RP, Marudanayagam R, et al. Scoring System to Predict Pancreatic Fistula After Pancreaticoduodenectomy: A UK Multicenter Study. Ann Surg 2015;261:1191-7. [Crossref] [PubMed]
- Malya FU, Hasbahceci M, Tasci Y, et al. The Role of C-Reactive Protein in the Early Prediction of Serious Pancreatic Fistula Development after Pancreaticoduodenectomy. Gastroenterol Res Pract 2018;2018:9157806. [Crossref] [PubMed]
- Kawai M, Kondo S, Yamaue H, et al. Predictive risk factors for clinically relevant pancreatic fistula analyzed in 1,239 patients with pancreaticoduodenectomy: multicenter data collection as a project study of pancreatic surgery by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci 2011;18:601-8. [Crossref] [PubMed]
- Pecorelli N, Carrara G, De Cobelli F, et al. Effect of sarcopenia and visceral obesity on mortality and pancreatic fistula following pancreatic cancer surgery. Br J Surg 2016;103:434-42. [Crossref] [PubMed]
- Gaujoux S, Cortes A, Couvelard A, et al. Fatty pancreas and increased body mass index are risk factors of pancreatic fistula after pancreaticoduodenectomy. Surgery 2010;148:15-23. [Crossref] [PubMed]
- Wang M, Xu S, Zhang H, et al. Imbedding pancreaticojejunostomy used in pure laparoscopic pancreaticoduodenectomy for nondilated pancreatic duct. Surg Endosc 2017;31:1986-92. [Crossref] [PubMed]
- Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017;161:584-91. [Crossref] [PubMed]
- Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007;142:761-8. [Crossref] [PubMed]
- Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 2011;149:680-8. [Crossref] [PubMed]
- Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007;142:20-5. [Crossref] [PubMed]
- Baker MS, Sherman KL, Stocker SJ, et al. Using a modification of the Clavien-Dindo system accounting for readmissions and multiple interventions: defining quality for pancreaticoduodenectomy. J Surg Oncol 2014;110:400-6. [Crossref] [PubMed]
- Tranchart H, Gaujoux S, Rebours V, et al. Preoperative CT scan helps to predict the occurrence of severe pancreatic fistula after pancreaticoduodenectomy. Ann Surg 2012;256:139-45. [Crossref] [PubMed]
- Gullaksen S, Funck KL, Laugesen E, et al. Volumes of coronary plaque disease in relation to body mass index, waist circumference, truncal fat mass and epicardial adipose tissue in patients with type 2 diabetes mellitus and controls. Diab Vasc Dis Res 2019;16:328-36. [Crossref] [PubMed]
- Elagizi A, Kachur S, Lavie CJ, et al. An Overview and Update on Obesity and the Obesity Paradox in Cardiovascular Diseases. Prog Cardiovasc Dis 2018;61:142-50. [Crossref] [PubMed]
- Deng Y, Zhao B, Yang M, et al. Association Between the Incidence of Pancreatic Fistula After Pancreaticoduodenectomy and the Degree of Pancreatic Fibrosis. J Gastrointest Surg 2018;22:438-43. [Crossref] [PubMed]
- Jang JY, Chang YR, Kim SW, et al. Randomized multicentre trial comparing external and internal pancreatic stenting during pancreaticoduodenectomy. Br J Surg 2016;103:668-75. [Crossref] [PubMed]
- House MG, Fong Y, Arnaoutakis DJ, et al. Preoperative predictors for complications after pancreaticoduodenectomy: impact of BMI and body fat distribution. J Gastrointest Surg 2008;12:270-8. [Crossref] [PubMed]
- Lee SE, Jang JY, Lim CS, et al. Measurement of pancreatic fat by magnetic resonance imaging: predicting the occurrence of pancreatic fistula after pancreatoduodenectomy. Ann Surg 2010;251:932-6. [Crossref] [PubMed]
- Lee JS, Kim SH, Jun DW, et al. Clinical implications of fatty pancreas: correlations between fatty pancreas and metabolic syndrome. World J gastroenterol 2009;15:1869-75. [Crossref] [PubMed]
- Schrover IM, van der Graaf Y, Spiering W, et al. The relation between body fat distribution, plasma concentrations of adipokines and the metabolic syndrome in patients with clinically manifest vascular disease. Eur J Prev Cardiol 2018;25:1548-57. [Crossref] [PubMed]
- Callery MP, Pratt WB, Kent TS, et al. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg 2013;216:1-14. [Crossref] [PubMed]
- Grendar J, Jutric Z, Leal JN, et al. Validation of Fistula Risk Score calculator in diverse North American HPB practices. HPB (Oxford) 2017;19:508-14. [Crossref] [PubMed]
- Hu BY, Wan T, Zhang WZ, et al. Risk factors for postoperative pancreatic fistula: Analysis of 539 successive cases of pancreaticoduodenectomy. World J gastroenterol 2016;22:7797-805. [Crossref] [PubMed]
- Pratt WB, Callery MP, Vollmer CM, et al. Risk prediction for development of pancreatic fistula using the ISGPF classification scheme. World J Surg 2008;32:419-28. [Crossref] [PubMed]
- Roberts KJ, Karkhanis S, Pitchaimuthu M, et al. Comparison of preoperative CT-based imaging parameters to predict postoperative pancreatic fistula. Clin Radiol 2016;71:986-92. [Crossref] [PubMed]
- Kawai M, Tani M, Hirono S, et al. How do we predict the clinically relevant pancreatic fistula after pancreaticoduodenectomy?--an analysis in 244 consecutive patients. World J Surg 2009;33:2670-8. [Crossref] [PubMed]
- de Castro SM, Busch OR, van Gulik TM, et al. Incidence and management of pancreatic leakage after pancreatoduodenectomy. Br J Surg 2005;92:1117-23. [Crossref] [PubMed]
(English Language Editor: J. Gray)


