
The characteristic of cognitive impairments in patients with bipolar II depression and its association with N-acetyl aspartate of the prefrontal white matter
Introduction
Bipolar disorder (BD) is considered one of the most common and most disabling chronic mood disorders, a distinguished type I and type II for a manic or hypomanic episode. Patients with BD experiencing depression episodes result in more severe psychosocial impairment compared to BD type I (1). Meanwhile, bipolar II depression and major depressive disorder may share similar cognitive domain deficits and brain function alterations (2-4), resulting in a similar clinical profile, making the differential diagnosis a challenge.
Cognitive impairment is considered a core feature of BD. Typically, processing speed, executive function, attention, and working memory are characteristic markers for BD (5,6). Cognitive dysfunction was reported as a prodromal marker that existed before mood symptoms and occurred in euthymic episodes and healthy relatives of BD (7-9). However, BD type I and BD type II are pathophysiological different, which is still controversial. Prior studies have noted cognitive deficits in bipolar II depression (4,10) and their first-degree relatives (11), suggesting that cognitive deficits may improve performance as a promising candidate endophenotype of bipolar II depression (12). However, the neural mechanism of cognitive dysfunction in bipolar II depression is still unknown.
Neuroimaging can supply insight into the possible pathogenesis of cognitive deficits. For example, the prefrontal lobe, anterior cingulate cortex (ACC), and thalamus are involved in working memory, attention, and executive function in BD patients (13-18). Proton magnetic resonance spectroscopy (1H-MRS) is a technique that can be performed to measure brain metabolic abnormalities in vivo, including N-acetyl aspartate (NAA), choline-containing compounds (Cho), and creatine (Cr). Although there are a growing number of studies examining the metabolic levels and their alteration after treatment in patients with bipolar II depression (3,8,19-21), limited studies investigated the association of cognitive impairments with brain biochemistry in bipolar II depression. In previous studies about bipolar II depression, the NAA/Cr ratio in the left lenticular nucleus (LN) and thalamus was found to be correlated with executive dysfunction (8,10), and the altered biochemical of ACC has a close relationship with attention and executive function (15,16). However, earlier studies only focused on a specific part of cognitive function with a small sample size of patients. Future comprehensive research of biochemical metabolism and its related-cognitive deficit with a large sample is needed.
We examined four cognitive domains (psychomotor speed, executive function, attention/working memory, and verbal fluency) and biochemical metabolites in prefrontal white matter (PWM), ACC, thalamus, and LN of non-late-life and treatment-naïve patients with bipolar II depression. We are interested in the relationship between cognitive impairments and abnormal biochemical metabolism in bipolar II depression. We hypothesized that the deficits of four cognitive domains and abnormal biochemical metabolism could be found in patients with bipolar II depression, and cognitive deficits may correlate with the abnormal biochemistry. Furthermore, cognitive deficits may correlate with the PWM, ACC, LN, and thalamus’ abnormal biochemistry.
We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/atm-20-7098).
Methods
Participants and clinical assessment
From July 2013 to September 2017, 110 out- or in-patients with bipolar II disorder during a depressive episode were recruited from the psychiatry department, First Affiliated Hospital of Jinan University, Guangzhou, China. We limited the age range of all participants to 18–45 years. In this study, BD type II is diagnosed according to the Diagnostic and Statistical Manual of Mental Disorder IV (DSM-IV). The diagnostic assessment was performed using the Structured Clinical Interview for DSM-IV (SCID) Patient Edition and has never taken medication. Two experienced clinical psychiatrists (Y.J. and S.Z) performed a clinical diagnosis. The current study used 30% of the data reported in our previous studies (4,8,10).
The clinical state of each patient was assessed by using the Young Mania Rating Scale (YMRS) and 24-item Hamilton Depression Rating Scale (24-item HDRS) during the two days before the cognitive assessment and magnetic resonance imaging (MRI) scan. The inclusive criteria were a 24-item HDRS total score >21 and a YMRS total score <7 for BD II depression patients. Exclusion criteria: any other current psychiatric disorder, pregnancy and any contraindication to MRI scanning, alcohol or other substance dependence or abuse (current or past), the presence of a current (or history) organic medical illness, brain injury, or another neurological disease, current (or history) use of any form treatment, including psychotherapy, psychotropic medication or electroconvulsive therapy.
One hundred ten healthy controls matched by age, sex, and education level were recruited from the local community by advertisements. The healthy controls were screened using the SCID non-patient edition. The inclusive criteria were healthy volunteers, and a 24-item HDRS total score <7 and a YMRS total score <7. Exclusion criteria included any active or history of neurological and psychiatric illness, a family history of psychiatric illness in first-degree relatives.
All participants were Chinese Han people and right-handed. All participants were asked to sign a written informed consent following a full written and verbal explanation of the study. The study was approved by the Ethics Committee of the First Affiliated Hospital of Jinan University (No. 2016/030), China, and conducted following the Declaration of Helsinki (as revised in 2013).
Neurocognitive assessment
Each participant was examined individually by the same neuropsychologist, trained to administer and score the battery of tests that span four cognitive domains.
Psychomotor speed
Digit Symbol Substitution Test (DSST) measures psychomotor speed, a subtest of the Wechsler Adult Intelligence Scale-Revised by China (WAIS-RC). DSST was performed to calculate the raw score.
Executive function
The Wisconsin Cart Sorting Test (WCST) was performed to calculate the number of total errors (TE) and the number of categories completed (CC).
Trail Making Test-part B (TMT-B) was performed to calculate the completion time.
Attention/working memory
Digit Span Test (DST) was performed by all participants to assess the attention/working memory, including Digit Span Forwards (DSF) and Digit Span Backwards (DSB). Correct responses on the DSF and DSB were calculated.
TMT-part A (TMT-A) was performed to calculate the completion time.
Verbal fluency
Verbal Fluency Test (VFT) was performed to calculate the valid words. Participants were given 60sec to name as many animals as possible.
MRI data acquisition and preprocessing
All participants received MRI and 1H-MRS scanning within two days of initial contact. All MRI data were obtained on a General Electric (GE) Discovery MR750 3.0T system equipped with an 8-channel phased-array head coil. To confirm the absence of brain signals and structural abnormalities, including brain tumors, vascular diseases, and other brain diseases, routine axial T1-weighted fluid attenuation inversion recovery (T1 FLAIR) and fast spin echo T2-weighted MR images were obtained. The following parameters were used to acquire MRI: T1 FLAIR [repetition time (TR)/echo-time (TE) =1,750/24 ms, numbers of excitation (NEX) = 1,240×240 mm field of view (FOV), 320×256 matrix, 5 mm slice thickness, 1.5 mm gap, acquisition time =1 min and 22 s], fast spin echo T2-weighted MR images (TR/TE =8,400/145 ms, NEX =1,240×240 mm FOV, 256×256 matrix, 5 mm slice thickness, 1.5 mm gap, acquisition time = 2 min and 15 s).
We used the 2D multivoxel technique to obtain the spectrum. The anatomic localization was acquired using axial T2-weighted MR images with the following parameters: TR/TE =3,500/102 ms, NEX = 2,240×240 mm FOV, 256×256 matrices, 5 mm slice thickness with no gap, acquisition time = 1min and 45 s. The volume of interest (VOI), including 49 nominal voxels (7.5×7.5×10 mm3), was positioned in the PWM, ACC, LN, and thalamus (Figure 1A,B,C,D). 1H-MRS with a single section of 2D multi-voxel was performed using a point resolved spectroscopy sequence (PRESS) with chemically selective suppression (CHESS) water suppression. The acquisition parameters were as follows: TR/TE =1,000/144 ms, NEX =1,180×180 mm FOV, 240×240 matrix, 10 mm slice thickness, 7.5×7.5×10 mm3 nominal voxel size. The total acquisition time for 1H-MRS was 10 min and 56 s.
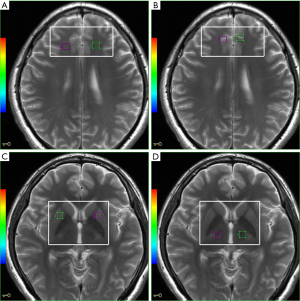
Then, saturation bands were placed outside the VOI to minimize lipid contamination from the scalp. We conduct automatic pre-scanning before each spectroscopic scan for achieving an optimal full width at half-maximum of 10 Hz. As a quality standard, we exclude spectra with a line width better than 10 Hz or water suppression above 98%.
Voxel placements for spectroscopy were performed by an experienced radiologist, who was blinded to each participant’s diagnosis. The GE Advantage Workstation AW4.2_07 FuncTool analyzed the spectral dataset. NAA/Cr and Cho/Cr ratios were used for the analysis of brain biochemical changes.
Statistical analysis
SPSS 21.0 software conducted all data analyses (SPSS, Chicago, IL, USA). All variables were evaluated for normality of distribution by mean of the Kolmogorov-Smirnov goodness-of-fit test. Clinical, demographic data (except gender), cognitive variables, and metabolite ratios between the two groups were performed by independent two-sample t-test (normal variable) and Mann-Whitney U test (skewed variables). Sex distribution in the group was compared using the Chi-squared test. All the statistical tests were two-tailed, and a P value of less than 0.05 was considered statistically significant. Additionally, we applied a Bonferroni correction to adjust α for multiple comparisons; the adjusted critical p value would be the significance level (α=0.05) divided by the total number of comparisons per type of analysis. And P<0.00208 (0.05/24) was considered significant.
Effect sizes (ESs) (Cohen’s D) were computed to weigh the degree of cognitive deficits. The computed formula: (mean value of patient group – mean value of controls)/standard deviation of controls. Cognitive impairment was classified as mild deficit (0.2< ES <0.5), moderate deficit (0.5< ES <0.8), and severe deficit (ES >0.8).
The multiple linear regression was conducted with NAA/Cr, Cho/Cr ratios as the independent variables, and cognitive variables serving as the dependent variables in patients with bipolar II depression. Using the forward likelihood ratio, the probability of F entering ≤ is 0.100, and the probability of F removing ≥ is 0.150. The significance level of the final model maintenance variable was ≤0.05.
Results
Sociodemographic and clinical data
Sociodemographic and clinical data of all participants are summarized in Table 1. No significant differences were observed in age, gender, and education level between the two groups.

Full table
Group differences of cognitive function
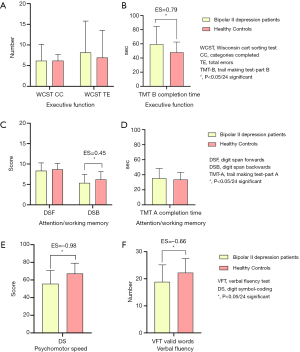
Figure 2 shows the comparison of cognitive function indices between the two groups. However, with the healthy control group, patients with bipolar II depression showed significantly worse on TMT-B (completion time) (Figure 2B), DSB (score) (Figure 2C), DSST (score) (Figure 2E) and VFT (valid word number) (Figure 2F) (z=−6.149, P=0.000; z=−3.930, P=0.000; z=−3.074, P=0.002; t=−4.379, P=0.000). According to the ESs, the DSST (score) (ES =−0.98) exhibited severe deficits, the TMT-B (completion time) (ES =0.79) and VFT (valid words number) (ES =−0.66) exhibited moderate deficits, and the DSB (score) (ES =−0.45) exhibited mild deficit. No significant differences of WCST (CC and TE number) (Figure 2A), DSF (score) (Figure 2C) and TMT-A (completion time) (Figure 2D) were found in the two groups.
Group differences of biochemical metabolite ratios
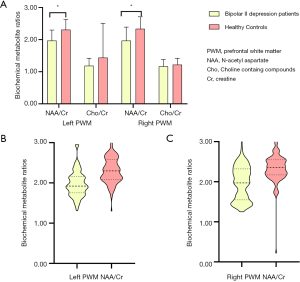
Figures 3 and 4 show the comparisons of biochemical metabolite ratios (NAA/Cr and Cho/Cr) in the four cerebral VOIs between patients with bipolar II depression and healthy controls. Compared to healthy controls, patients with bipolar II depression showed significantly lower NAA/Cr in the bilateral PWM (Figure 3) (t=−7.532, P=0.000; t=−7.132, P=0.000), but did not differ significantly in Cho/Cr ratios of bilateral PWM (Figure 3A). We found there were no significant differences in the other neuro-metabolite ratios of the left and right ACC, LN, and thalamus between the two groups (Figure 4).

Correlations between abnormal 1H-MRI indices and cognitive indices
Table 2 shows the linear regression analyses of abnormal cognitive function indices and biochemical metabolite ratios in bipolar II depression patients. NAA/Crratio of the left PWM was positively correlated with the score of DS (t=3.095, P=0.003) and DSB (t=3.040, P=0.003), and NAA/Crratio of the right PWM was negatively correlated with the completion time of TMT-B (t=−2.968, P=0.004).

Full table
Discussion
The present comprehensive research aims to identify the characteristics of cognitive deficit and neuro-metabolism alterations in a large patient sample with bipolar II depression who have never taken medications. We also explore associations between targeted metabolite levels and cognitive performance. The main findings for bipolar II depression in the current study are the following: (I) worse performance on DSST, TMT-B, DSB, and VFT; (II) decreased NAA/Cr in the bilateral PWM; (III) a positive correlation between abnormal NAA/Cr of the left PWM and the score of DSST and DSB, negative correlation between abnormal NAA/Cr of the right PWM, and the completion time of TMT-B.
Neuro-metabolic abnormalities within the frontal lobe in BD have been implicated in many imaging studies. A lower NAA/Cr ratio was found in the bilateral PWM of bipolar II depression when compared with healthy controls, in agreement with previous studies (3,8). However, normal NAA concentration in the frontal lobe was reported in another study (22). The decreased NAA/Cr ratio is considered to reflect dysfunctional neurons, decreased number, the density of neurons, or mitochondrial function impairment, and increased NAA has been described as improved neuronal function and viability with the response to therapy (23). According to our results, bipolar II depression patients may have a neuronal loss or mitochondrial function impairment in the bilateral PWM. Lower NAA/Cr ratios of bilateral PWM are related to poorer cognitive functions in patients with bipolar II depression, suggesting that PWM's abnormal neuro-metabolism may involve more subtle illness-specific cognitive deficits.
The severe deficit in psychomotor speed was found in bipolar II depression, positively correlated with decreased NAA/Cr of the left PWM, suggesting that the left PWM dysfunction was positively correlated with a deficit in psychomotor speed. Psychomotor speed deficits may be a potential endophenotypic index for BD since it has been found in BD patients and their first-degree relatives (24,25). Moreover, psychomotor speed was an underlying variable that implicitly affected visual memory, performance, working memory, verbal fluency, and cognitive flexibility (26). Slower psychomotor speed was considered related to the smaller gray matter volume in the bilateral prefrontal region. However, better psychomotor speed was generally considered related to larger gray matter volumes in the bilateral prefrontal lobe, bilaterally hippocampus, and left superior temporal gyrus in normal controls (26). Psychomotor speed was associated with degree centrality abnormalities of the temporal lobe in early BD patients (27). Furthermore, a positive relationship between the inferior temporal surface area and psychomotor speed was reported in BD (28).
DSB was used to measure working memory, and DSF and TMT-A were used to measure attention. A mild deficit of working memory was found in bipolar II depression, consistent with the previous study (29). Working memory’s efficient functioning depends on inhibitory processes, limiting the access of information into working memory, and updating the contents of working memory by removing irrelevant information. Healthy relatives of BD performed less accurately and required additional time in working memory tasks (30). Additionally, decreased working memory also existed in BD patients at the remission phase, suggesting that working memory deficit was not only a phenomenon of mood but a consequence of enduring brain dysfunction (31). In this study, we found evidence that working memory deficit in bipolar II depression could be accounted for by the generalized NAA/Cr ratio decrease of the left PWM. Working memory is one central function of the prefrontal cortex. Previous studies have identified aberrant activity in the prefrontal cortex during working memory performance (30,32,33). Specifically, one study reported that working memory-related dorsolateral prefrontal cortex (DLPFC) activity in remitted patients with BD was modulated by the catechol-O-methyltransferase (COMT) Val158Met genotype (34). Greater ACC activation during working memory tasks is found in bipolar II depression, which may distinguish between unipolar and bipolar depression (35). However, no evidence of a relationship between working memory and ACC biochemical metabolism was detected in our study. An explanation for this might be that we investigated the biochemical metabolism at a resting state rather than during a working memory task.
A moderate deficit of executive function was found in bipolar II depression in the current study, which also found in their unaffected first-degree relatives (6,36,37). Executive dysfunction may occur in the early stages of bipolar II depression and may remain during remission (8). Positive relationships between executive function and NAA levels of the LN and ACC were reported in bipolar II depression (8,38), and a negative relationship between increased volume in the right caudate and poorer performance of executive function was reported in BD type I patients at remission phase (37). In this study, a negative relationship between executive dysfunction and abnormal NAA/Crratio in the right PWM was found in bipolar II depression. According to our results, abnormal NAA/Crratio in the right PWM may be a crucial factor underlying executive function impairments in bipolar II depression.
Verbal fluency is a higher executive function, a series of cognitive processes necessary to control and regulate lower-level processing and goal-directed behavior. In this study, a moderate deficit of verbal fluency was found in bipolar II depression. Significant improvements in verbal fluency were found in patients with BD after cognitive therapy (39). Increased activation in the ACC during the verbal fluency task has been reported in patients with BD (40). Hypoactivation of the prefrontal cortex and hyperactivation of the bilateral precuneus were also found during verbal fluency tasks in euthymic patients with BD (41,42). The pattern of response to verbal fluency is highly diagnostic for schizophrenia and distinct from BD (40). Another important finding was that verbal fluency deficits were associated with the reduced white matter integrity in the left inferior frontal-occipital fasciculus and the forceps minor in BD (43). Verbal fluency may be related to the prefrontal lobe function (44), presumed to be correlated with the PWM's abnormal biochemistry. However, no relationship between verbal fluency and neuro-metabolism of the PWM was found in bipolar II depression in this study.
The association between cognitive deficits and brain biochemistry in bipolar II depression was investigated in this study. The findings of this study are promising, but some limitations must be noted. Firstly, only acute episode patients with bipolar II depression were recruited in this current study, with no euthymic episode or manic/hypomanic episode patients and their first-degree relatives, it is still not clear whether the association between cognitive dysfunction and neuro-metabolism was specific to the depression episode or shared by all episodes of BD. Secondly, all patients carried on with follow-up for two years after inclusion in this study. If patients had presented a manic episode during the observation period, he would exclude from the analysis, but some patients still do not show manic or hypomanic episodes. Thirdly, we investigated the biochemical metabolism in resting state rather than cognitive functional tasks, including working memory tasks and verbal fluency tasks. However, the neuro-biochemical changes during cognitive tasks associated with bipolar II depression have yet to be explored. Fourthly, a semi-quantitative analysis was performed in this study, using Cr as an internal reference. However, the accuracy of Cr as a standard is controversial. Subsequent studies with absolute measures could revalidate this finding. Finally, previous studies have reported the association between cognitive function and gene polymorphism (45) or gene expression in BD (46,47). Further studies are needed to investigate whether the dysfunction is related to bipolar II depression's genetic risk.
Conclusions
Our study’s main findings are partially consistent with earlier studies showing cognitive impairments in bipolar II depression. There are positive correlations between abnormal NAA/Cr of the left PWM and the score of DSST and DSB and a negative correlation between abnormal NAA/Crof the right PWM and the completion time of TMT-B. Our findings suggested that psychomotor speed, executive function, working memory, and verbal fluency are impaired in BD II depression patients. Also, hypoactivity NAA/Cr in bilateral PWM may be associated with the pathophysiology of bipolar II depression and results in cognitive dysfunction. These findings may improve our understanding of cognitive impairments, neural pathogenesis, and supply a marker for the early identification of bipolar II depression.
Acknowledgments
Funding: Funding for this work was provided by the National Natural Science Foundation of China (No: 81671351; 81801347), Planned Science and Technology Project of Guangdong Province, China (No: 2017B020227011), Medical Science and Technology Research Project of Guangdong Province, China (No: A2018115). The founders have not played any study design roles, data collection, analysis, manuscript writing, and decision to publish.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-7098
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-7098
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-7098). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All participants were Chinese Han people and right-handed. All participants were asked to sign a written informed consent following a full written and verbal explanation of the study. The study was approved by the Ethics Committee of the First Affiliated Hospital of Jinan University (No. 2016/030), China, and conducted following the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Judd LL, Akiskal HS, Schettler PJ, et al. Psychosocial disability in the course of bipolar I and II disorders: a prospective, comparative, longitudinal study. Arch Gen Psychiatry 2005;62:1322-30. [Crossref] [PubMed]
- Wang Y, Wang J, Jia Y, Zhong S, Niu M, Sun Y, et al. Shared and Specific Intrinsic Functional Connectivity Patterns in Unmedicated Bipolar Disorder and Major Depressive Disorder. Sci Rep 2017;7:3570. [Crossref] [PubMed]
- Zhong S, Wang Y, Zhao G, et al. Similarities of biochemical abnormalities between major depressive disorder and bipolar depression: a proton magnetic resonance spectroscopy study. J Affect Disord 2014;168:380-6. [Crossref] [PubMed]
- Liu T, Zhong S, Wang B, et al. Similar profiles of cognitive domain deficits between medication-naive patients with bipolar II depression and those with major depressive disorder. J Affect Disord 2019;243:55-61. [Crossref] [PubMed]
- Bora E, Yucel M, Pantelis C, et al. Meta-analytic review of neurocognition in bipolar II disorder. Acta Psychiatr Scand 2011;123:165-74. [Crossref] [PubMed]
- Solé B, Bonnin CM, Torrent C, et al. Neurocognitive impairment and psychosocial functioning in bipolar II disorder. Acta Psychiatr Scand 2012;125:309-17. [Crossref] [PubMed]
- Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J Affect Disord 2009;113:1-20. [Crossref] [PubMed]
- Lai S, Zhong S, Liao X, et al. Biochemical abnormalities in basal ganglia and executive dysfunction in acute- and euthymic-episode patients with bipolar disorder: A proton magnetic resonance spectroscopy study. J Affect Disord 2018;225:108-16. [Crossref] [PubMed]
- Calafiore D, Rossell SL, Van Rheenen TE. Cognitive abilities in first-degree relatives of individuals with bipolar disorder. J Affect Disord. 2018;225:147-52. [Crossref] [PubMed]
- Zhong S, Wang Y, Lai S, et al. Associations between executive function impairment and biochemical abnormalities in bipolar disorder with suicidal ideation. J Affect Disord 2018;241:282-90. [Crossref] [PubMed]
- Lin K, Shao R, Lu R, et al. Resting-state fMRI signals in offspring of parents with bipolar disorder at the high-risk and ultra-high-risk stages and their relations with cognitive function. J Psychiatr Res 2018;98:99-106. [Crossref] [PubMed]
- Merikangas AK, Cui L, Calkins ME, et al. Neurocognitive performance as an endophenotype for mood disorder subgroups. J Affect Disord 2017;215:163-71. [Crossref] [PubMed]
- Takeuchi H, Taki Y, Sassa Y, et al. Verbal working memory performance correlates with regional white matter structures in the frontoparietal regions. Neuropsychologia 2011;49:3466-73. [Crossref] [PubMed]
- Oertel-Knöchel V, Reinke B, Alves G, et al. Frontal white matter alterations are associated with executive cognitive function in euthymic bipolar patients. J Affect Disord 2014;155:223-33. [Crossref] [PubMed]
- Stevens FL, Hurley RA, Taber KH. Anterior cingulate cortex: unique role in cognition and emotion. J Neuropsychiatry Clin Neurosci 2011;23:121-5. [Crossref] [PubMed]
- Zimmerman ME, DelBello MP, Getz GE, et al. Anterior cingulate subregion volumes and executive function in bipolar disorder. Bipolar Disord 2006;8:281-8. [Crossref] [PubMed]
- Piras F, Caltagirone C, Spalletta G. Working memory performance and thalamus microstructure in healthy subjects. Neuroscience 2010;171:496-505. [PubMed]
- van Dam WO, Decker SL, Durbin JS, et al. Resting state signatures of domain and demand-specific working memory performance. NeuroImage 2015;118:174-82. [Crossref] [PubMed]
- Li H, Xu H, Zhang Y, et al. Differential neurometabolite alterations in brains of medication-free individuals with bipolar disorder and those with unipolar depression: a two-dimensional proton magnetic resonance spectroscopy study. Bipolar Disord 2016;18:583-90. [Crossref] [PubMed]
- Machado-Vieira R, Gattaz WF, Zanetti MV, et al. A Longitudinal (6-week) 3T (1)H-MRS Study on the Effects of Lithium Treatment on Anterior Cingulate Cortex Metabolites in Bipolar Depression. Eur Neuropsychopharmacol 2015;25:2311-7. [Crossref] [PubMed]
- Croarkin PE, Thomas MA, Port JD, et al. N-acetylaspartate normalization in bipolar depression after lamotrigine treatment. Bipolar Disord 2015;17:450-7. [Crossref] [PubMed]
- Bertolino A, Frye M, Callicott JH, et al. Neuronal pathology in the hippocampal area of patients with bipolar disorder: a study with proton magnetic resonance spectroscopic imaging. Biol Psychiatry 2003;53:906-13. [Crossref] [PubMed]
- Ariyannur PS, Madhavarao CN, Namboodiri AM. N-acetylaspartate synthesis in the brain: mitochondria vs. microsomes. Brain Res 2008;1227:34-41. [Crossref] [PubMed]
- Hellvin T, Sundet K, Simonsen C, et al. Neurocognitive functioning in patients recently diagnosed with bipolar disorder. Bipolar Disord 2012;14:227-38. [Crossref] [PubMed]
- Ferrier IN, Chowdhury R, Thompson JM, et al. Neurocognitive function in unaffected first-degree relatives of patients with bipolar disorder: a preliminary report. Bipolar Disord 2004;6:319-22. [Crossref] [PubMed]
- Sanfilipo M, Lafargue T, Rusinek H, et al. Cognitive performance in schizophrenia: relationship to regional brain volumes and psychiatric symptoms. Psychiatry Res 2002;116:1-23. [Crossref] [PubMed]
- Deng W, Zhang B, Zou W, et al. Abnormal Degree Centrality Associated With Cognitive Dysfunctions in Early Bipolar Disorder. Front Psychiatry 2019;10:140. [Crossref] [PubMed]
- Hartberg CB, Sundet K, Rimol LM, et al. Brain cortical thickness and surface area correlates of neurocognitive performance in patients with schizophrenia, bipolar disorder, and healthy adults. J Int Neuropsychol Soc 2011;17:1080-93. [Crossref] [PubMed]
- Van Rheenen TE, Rossell SL. An empirical evaluation of the MATRICS Consensus Cognitive Battery in bipolar disorder. Bipolar Disord 2014;16:318-25. [Crossref] [PubMed]
- Thermenos HW, Goldstein JM, Milanovic SM, et al. An fMRI study of working memory in persons with bipolar disorder or at genetic risk for bipolar disorder. Am J Med Genet B Neuropsychiatr Genet 2010;153B:120-31. [PubMed]
- Thompson JM, Gray JM, Hughes JH, et al. Impaired working memory monitoring in euthymic bipolar patients. Bipolar Disord 2007;9:478-89. [Crossref] [PubMed]
- Drapier D, Surguladze S, Marshall N, et al. Genetic liability for bipolar disorder is characterized by excess frontal activation in response to a working memory task. Biol Psychiatry 2008;64:513-20. [Crossref] [PubMed]
- Brooks JO 3rd, Vizueta N, Penfold C, et al. Prefrontal hypoactivation during working memory in bipolar II depression. Psychol Med 2015;45:1731-40. [Crossref] [PubMed]
- Miskowiak KW, Kjaerstad HL, Stottrup MM, et al. The catechol-O-methyltransferase (COMT) Val158Met genotype modulates working memory-related dorsolateral prefrontal response and performance in bipolar disorder. Bipolar Disord 2017;19:214-24. [Crossref] [PubMed]
- Bertocci MA, Bebko GM, Mullin BC, et al. Abnormal anterior cingulate cortical activity during emotional n-back task performance distinguishes bipolar from unipolar depressed females. Psychol Med 2012;42:1417-28. [Crossref] [PubMed]
- Schulze KK, Walshe M, Stahl D, et al. Executive functioning in familial bipolar I disorder patients and their unaffected relatives. Bipolar Disord 2011;13:208-16. [Crossref] [PubMed]
- Kozicky JM, Ha TH, Torres IJ, et al. Relationship between frontostriatal morphology and executive function deficits in bipolar I disorder following a first manic episode: data from the Systematic Treatment Optimization Program for Early Mania (STOP-EM). Bipolar Disord 2013;15:657-68. [Crossref] [PubMed]
- Huber RS, Kondo DG, Shi XF, et al. Relationship of executive functioning deficits to N-acetyl aspartate (NAA) and gamma-aminobutyric acid (GABA) in youth with bipolar disorder. J Affect Disord 2018;225:71-8. [Crossref] [PubMed]
- Ives-Deliperi VL, Howells F, Stein DJ, et al. The effects of mindfulness-based cognitive therapy in patients with bipolar disorder: a controlled functional MRI investigation. J Affect Disord 2013;150:1152-7. [Crossref] [PubMed]
- Costafreda SG, Fu CH, Picchioni M, et al. Pattern of neural responses to verbal fluency shows diagnostic specificity for schizophrenia and bipolar disorder. BMC Psychiatry 2011;11:18. [Crossref] [PubMed]
- Matsuo K, Kouno T, Hatch JP, et al. A near-infrared spectroscopy study of prefrontal cortex activation during a verbal fluency task and carbon dioxide inhalation in individuals with bipolar disorder. Bipolar Disord 2007;9:876-83. [Crossref] [PubMed]
- Yoshimura Y, Okamoto Y, Onoda K, et al. Psychosocial functioning is correlated with activation in the anterior cingulate cortex and left lateral prefrontal cortex during a verbal fluency task in euthymic bipolar disorder: a preliminary fMRI study. Psychiatry Clin Neurosci 2014;68:188-96. [Crossref] [PubMed]
- Bauer IE, Ouyang A, Mwangi B, et al. Reduced white matter integrity and verbal fluency impairment in young adults with bipolar disorder: a diffusion tensor imaging study. J Psychiatr Res 2015;62:115-22. [Crossref] [PubMed]
- Curtis VA, Bullmore ET, Brammer MJ, et al. Attenuated frontal activation during a verbal fluency task in patients with schizophrenia. Am J Psychiatry 1998;155:1056-63. [Crossref] [PubMed]
- Mitchell ES, Conus N, Kaput J. B vitamin polymorphisms and behavior: evidence of associations with neurodevelopment, depression, schizophrenia, bipolar disorder and cognitive decline. Neurosci Biobehav Rev 2014;47:307-20. [Crossref] [PubMed]
- Kunii Y, Hyde TM, Ye T, et al. Revisiting DARPP-32 in postmortem human brain: changes in schizophrenia and bipolar disorder and genetic associations with t-DARPP-32 expression. Mol Psychiatry 2014;19:192-9. [Crossref] [PubMed]
- Li M, Luo XJ, Rietschel M, et al. Allelic differences between Europeans and Chinese for CREB1 SNPs and their implications in gene expression regulation, hippocampal structure and function, and bipolar disorder susceptibility. Mol Psychiatry 2014;19:452-61. [Crossref] [PubMed]


