
Poncirin suppresses lipopolysaccharide (LPS)-induced microglial inflammation and ameliorates brain ischemic injury in experimental stroke in mice
Introduction
Stroke, a type of cerebrovascular disease that manifests as cerebral ischemia or cerebral hemorrhage, has very high mortality and disability rates and has become the leading cause of death in China. Ischemic stroke accounts for approximately 80% of all strokes and is caused by various factors, such as hypertension, diabetes and atherosclerosis (1,2). The obstruction of blood flow caused by various factors deprives the brain tissue of oxygen and glucose, which leads to irreversible neuronal apoptosis and hypoxic brain injury (3). Currently, thrombolysis and mechanical thrombectomy are the main acute treatments for ischemic stroke (2). However, less than 10% of patients with acute ischemic stroke are able to receive these therapies due to their limited treatment window and side effects. Therefore, new and effective prevention measures and safe treatments for stroke are urgently needed.
Although multiple pathological mechanisms are involved in ischemic stroke, accumulating evidence has revealed that inflammation is the main contributor to stroke progression (4,5). Microglia are the resident immune cells in the central nervous system (CNS) and function as key mediators of inflammation in the brain (6). They are derived from myeloid progenitor cells and migrate into the cerebral rudiment by embryonic day 9.5 (6). In the resting state, similar to a housekeeper, microglia continue to surveil the brain parenchyma via their long processes and protrusions (7). When the environment changes drastically, microglia promptly adopt an activated state, migrate to the injury site, engulf necrotic cells and release numerous messenger molecules of the immune response that enhance the inflammatory responses (8,9). Microglial activation is necessary for host defenses, clearing debris and tissue repair, but microglial overactivation is neurotoxic. Overactivated microglia-mediated inflammatory responses contribute to neuronal injury through the secretion of various destructive mediators, such as interleukin (IL)-1β, IL-6, nitric oxide (NO) and tumor necrosis factor (TNF)-α (10). Excess NO and TNF-α kill oligodendrocytes, which further aggravates white matter damage under hypoxic conditions (11). Furthermore, excess cytokines exacerbate the disintegration of blood vessels (12). Thus, inhibitors that target excessive microglia activation and the inflammatory response in which it participates have become a treatment strategy for ischemic stroke.
Poncirin (Pon) is a flavanone glycoside extracted from the fruits of Poncirus trifoliata and is used as a component of traditional Chinese medicines for the treatment of asthma and inflammation (Figure 1A) (13). According to numerous studies, Pon exerts a variety of pharmacological effects on many disease models. For example, Pon relieves acetic acid-induced and formalin-induced tonic pain and prevents the infiltration of inflammatory cells (14). It promotes apoptosis in AGS cells (human gastric cancer cells) via the FasL-dependent extrinsic apoptotic pathway (15). Furthermore, Pon downregulates the expression of nuclear factor of activated T cells c1 (NFATc1) to ameliorate osteoclast differentiation and bone loss (16). In addition, Pon has also been shown to possess anti-inflammatory properties. Pon and its metabolites attenuate inflammation of the lining of the colon (colitis) by preventing lipopolysaccharide (LPS) from binding to Toll-like receptors (TLRs) and inhibiting nuclear factor kappa B (NF-κB) activation (13). Nevertheless, the effects of Pon on the treatment of ischemic stroke related to the inhibition of microglia-induced inflammation remain unknown.

We conducted a series of in vitro and in vivo experiments to investigate the anti-inflammatory effects of Pon on microglia. BV2 cells and primary mouse microglia were used to analyze whether Pon inhibits LPS-induced inflammation and to elucidate the underlying molecular mechanism by which Pon inhibits the inflammatory response. Subsequently, we elucidated the contribution of Pon to controlling inflammation and promoting stroke recovery after ischemic injury in the brains of male C57BL/6J mice. The middle cerebral artery occlusion (MCAO) model was used as a stroke model.
We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-3470).
Methods
Regents and antibodies
Pon (CAS: 14941-08-3, purity: ≥95%) was purchased from ALADDIN Ltd. (Shanghai, China) and dissolved in dimethyl sulfoxide (DMSO, Amresco, Solon, OH, USA) for use in subsequent experiments. The concentration of DMSO was less than 0.1%. LPS (from Escherichia coli 0111: B4) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Antibodies against iNOS, ERK1/2, phospho-ERK1/2, JNK, phospho-JNK, p38, phospho-p38, IκBα, phospho-IκBα, NF-κB p65 and phospho-p65 were purchased from Cell Signaling Biotechnology (Hertfordshire, UK). Antibodies against GAPDH and COX2 were obtained from Bioworld Biotechnology (Minneapolis, MN, USA). The polyclonal anti-Iba1 antibody was purchased from Abcam (Cambridge, UK).
Cell culture
The murine microglia cell line BV2 was obtained from the China Infrastructure of Cell Line Resources (Beijing, China) and cultured in medium comprising 90% DMEM (Invitrogen, Frederick, MD, USA), 10% fetal bovine serum (FBS, Hyclone, Logan, UT, USA) and 1% antibiotics (100 U/mL penicillin and 100 µg/mL streptomycin) at 37 °C in a humidified atmosphere of 5% CO2. All experiments with BV2 cells were performed at less than passage 10. Primary microglia were prepared from neonatal B6 mice, maintained in medium for 10–12 days, harvested by shaking for 10 min and reseeded in new plates. The medium used for primary microglia was the same as the medium used for BV2 cells. The purity of the primary microglia was greater than 95%, as determined using Iba1 staining.
Animals and a mouse model of MCAO
Approximately 8-week-old healthy male C57BL/6J mice weighing 20–25 g were obtained from the Animal Model Center of Nanjing Medical University (Nanjing, Jiangsu, China). All animal experiments were approved by the Animal Care and Use Committee at Nanjing University (reference number: 2019AE01073) and performed according to institutional guidelines. The mice were housed in standard cages and provided with adequate food and water in a room with suitable light and temperature conditions.
All mice were randomly divided into three groups with each group consisting of 16 animals (10 mice were used to measure infarct volume and neurobehavioral deficits; 6 mice were used to detect the expression of pro-inflammatory factors and Iba-1) as follows: the sham-operated (sham) group; the saline-treated MCAO (MCAO + saline) group; and the 30 mg/kg Pon-treated MCAO (MCAO + Pon) group. The animals were anaesthetized with pentobarbital sodium. The MCAO mouse model was established as previously described (17). Briefly, a 6/0 monofilament nylon suture (Doccol Corporation, MA, USA) with a heat-rounded tip was inserted into the beginning of the MCA through the internal carotid artery until the ipsilateral blood flow decreased to less than 30% of the baseline value, as monitored using laser Doppler flowmetry (Perimed Corporation, Stockholm, Sweden). After 60 min of occlusion, the filament was withdrawn to allow blood reperfusion. In the sham-operated group, the aforementioned procedure was performed, but a filament was not inserted into the MCA. After the operation, we fed the mice jelly or performed an intraperitoneal (i.p.) injection of normal saline to rehydrate. Pre-dissolved Pon or the same volume of saline was administered to each animal by an i.p. injection at 30 min, 24 h and 48 h after MCAO in a double-blind manner.
Cell viability assays
BV2 and primary microglia were planted in 96-well plates and treated with different concentrations of Pon for 24 h. Then, the medium was removed, and cell viability was assessed using the Cell Counting Kit-8 (CCK-8; Dojindo Laboratories, Tokyo, Japan) according to the manufacturer’s instructions. The optical density (OD) was measured at 450 nm with a microplate reader (Bio-Rad, Hercules, CA, USA).
Nitrite analysis
BV2 and primary microglia seeded in 24-well plates were pre-treated with Pon for 2 h and stimulated with LPS (0.5 µg/mL for BV2 cells and 0.1 µg/mL for primary microglia) for 24 h. The concentrations of NO in the supernatants were detected using the Griess reagent (Beyotime Biotech, Nantong, China). The OD value was measured at 540 nm with a microplate reader.
Cytokine measurements
BV2 and primary microglia were pre-treated with the indicated doses of Pon for 2 h and then stimulated with LPS (0.5 µg/mL for BV2 cells and 0.1 µg/mL for primary microglia) for 24 h. The supernatants were collected, and the concentrations of the cytokines PGE2, IL-1β, IL-6 and TNF-α were measured using enzyme-linked immunosorbent assays (ELISAs) according to the manufacturer’s instructions (Cusabio Biotech, Wuhan, China).
Real-time PCR
Primary microglia and BV2 cells were pre-treated with Pon for 2 h and stimulated with LPS (0.5 µg/mL for BV2 cells and 0.1 µg/mL for primary microglia) for 3 h. The mice were sacrificed by cervical dislocation under anesthesia with pentobarbital sodium, and brain tissues from the penumbra were isolated. Total RNA was extracted from these cells and tissue mentioned above using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Then, the RNA was transcribed into cDNA using the PrimeScript RT Reagent Kit (Vazyme, Nanjing, China). A 20 µL reaction mixture was prepared, and real-time PCR was performed using a Step One Plus PCR system (Applied Biosystems, Foster City, CA, USA) with a SYBR Green Kit (Applied Biosystems). The corresponding primers were as follows: IL-1β, F: AAGCCTCGTGCTGTCGGACC, R: TGAGGCCCAAGGCCACAGGT; IL-6, F: GCTGGTGACAACCACGGCCT, R: AGCCTCCGACTTGTGAAGTGGT; TNF-α, F: CAAGGGACAAGGCTGCCCCG, R: GCAGGGGCTCTTGACGGCAG; GAPDH, F: GCCAAGGCTGTGGGCAAGGT, R: TCTCCAGGCGGCACGTCAGA.
Western blot
Brain tissue from the ischemic penumbra and all cells were homogenized with lysis buffer (Thermo Fisher Scientific, Rockford, IL, USA) containing 1% protease inhibitor cocktail for 30 min. Afterwards, the mixtures were centrifuged (15,000 ×g) at 4 °C for 15 min. The protein concentrations in the supernatants were quantified using a BCA protein assay kit (Beyotime Biotech). Equal quantities of the proteins were denatured in SDS, electrophoresed on 8–12% SDS-PAGE gels and then transferred to PVDF membranes. The membranes were blocked with 5% skim milk for 2 h at room temperature, and then incubated with the appropriate primary antibodies overnight at 4 °C. Subsequently, the membranes were incubated with secondary antibodies for another 2 h, and the proteins on the membranes were detected with the ECL Detection Kit (Bioworld Biotechnology). Images were acquired using the Gel-Pro system (Tanon Technologies, Shanghai, China), and the intensity of each band was analyzed using ImageJ software (ImageJ 1.5, NIH, USA).
Immunofluorescence staining
The mice were anaesthetized and executed via cardiac perfusion with 0.9% saline followed by 4% paraformaldehyde. Their brain tissues were removed, dehydrated and sectioned into 20 µm slices. The brain sections and BV2 cells were permeabilized with 0.1% Triton X-100 for 10 min, and blocked with 2% BSA for 90 min. After washing 3 times with PBS, the slices and cells were incubated with primary antibodies against Iba1 (1:500) and NF-κB p65 (1:500) overnight at 4 °C. The brain sections and BV2 cells were incubated with the indicated secondary antibodies for 2 h in the dark on the next day. DAPI (5 g/mL) was added for 15 min to stain nuclei. Images were captured with a fluorescence microscope (Olympus BX51, Japan).
TTC staining
The mice were sacrificed by cervical dislocation at 72 h after the operation. Their brains were removed carefully, cut into five slices and immersed in 0.2% (w/v) 2,3,5-triphenyltetrazolium chloride (TTC, Sigma-Aldrich) for 15 min for staining. The pale grey region was the infarct area, while the dark red region represented the intact brain area. Images were captured with a digital camera and analyzed using ImageJ software. The percentage of the infarct volume was calculated using the following formula: percentage of the infarct size = (contralateral area − ipsilateral non-infarct area)/(2 × contralateral area) ×100% (18).
Neurobehavioral tests
The modified neurological severity score (mNSS) test was conducted on mice 72 h after the operation. The mNSS test comprised motor (muscle status and abnormal movement), sensory (visual, tactile, and proprioceptive), and reflex tests. Scores ranged from 0 to 18 points. Larger numbers indicated a more severe impairment in brain function.
The mice were trained on a rotarod device (RWD Life Science, Shenzhen, China) for 3 days before MCAO. All mice were trained twice a day, and each training trial lasted for 5 min, during which the rotating rod accelerated from 4 to 40 rpm. Seventy-two hours after MCAO, the mice were placed on the device again, and the time until they fell from the rod was recorded. This test was used to quantify the motor function of the mice.
The forelimb muscle strength of the mice was assessed using the grip strength test 72 h after the operation (17). Each mouse was suspended by the tail, allowed to grasp the T-bar of a grip strength metre (GS3, Bioseb, France), and pulled backward in a straight line until its grip was broken. The maximum grip strength value was recorded to measure the maximum forelimb muscle strength.
Statistical analysis
SPSS 18.0 software was used to conduct statistical analysis. The normality of data distribution was analyzed by the Shapiro-Wilk test. Normally distributed data are presented as the mean ± SEM and otherwise by the median with inter-quartile range. The normally distributed continuous variables were compared by Student’s t-test, while non-normally distributed variables were tested by the Mann-Whitney test. For multiple comparison among the groups, the data were tested using the one-way analysis variance (ANOVA) followed by Bonferroni’s post-hoc test. If P<0.05, the experimental results were considered significantly different.
Results
Effects of Pon on the viability of BV2 cells and primary microglia
Prior to evaluating the anti-inflammatory and neuroprotective activities of Pon, we examined the cytotoxicity of Pon in microglia. BV2 cells and primary microglia were treated with Pon at concentrations ranging from 0 to 100 µM for 24 h. As shown in Figure 1B,C, Pon did not alter cell viability at concentrations less than 50 µM. However, cell viability was slightly decreased when the concentration of Pon in the medium was 100 µM. Based on these findings, Pon was used at concentration less than 50 µM in subsequent experiments.
Pon attenuated the production of NO and PGE2 and the expression of iNOS and COX-2 in LPS-induced microglia
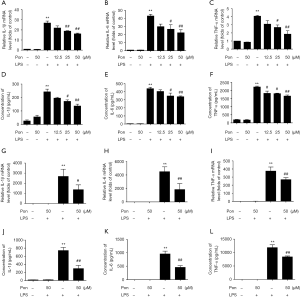
ELISAs and the Griess assay were performed to evaluate the effect of Pon on NO and PGE2 levels. LPS treatment significantly increased the levels of NO and PGE2 in BV2 cells compared with the controls. Pre-treatment with Pon (12.5, 25, or 50 µM) inhibited the increase in NO and PGE2 levels in a dose-dependent manner (Figure 2A,B). Similar results were obtained from primary microglia (Figure 2C,D). Considering that NO and PGE2 are produced by iNOS and COX-2, respectively, we subsequently evaluated the changes in the levels of the iNOS and COX-2 proteins in BV2 cells treated with Pon and LPS. Compared with LPS treatment alone, pre-treatment with Pon significantly suppressed the increased in iNOS and COX-2 protein levels in microglial cells (Figure 2E,F).
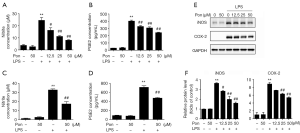
Pon reduced the LPS-induced production of pro-inflammatory cytokines in BV2 cells and primary microglia
Because microglia release various cytokines under pathological conditions and because some of these cytokines (such as IL-1β, IL-6 and TNF-α) induce inflammatory responses that aggravate tissue injury, we evaluated whether Pon exerted an effect on the LPS-induced release of pro-inflammatory factors. We collected RNA and culture medium from BV2 cells and primary microglia stimulated with LPS for 3 or 24 h, as described above. Then, we evaluated the levels of the IL-1β, IL-6 and TNF-α mRNAs and proteins using real-time PCR and ELISAs, respectively. Consistent with our hypothesis, Pon attenuated the LPS-induced increase in the expression of IL-1β, IL-6 and TNF-α in BV2 cells in a dose-dependent manner (Figure 3A,B,C,D,E,F). Additionally, 50 µM Pon inhibited the mRNA expression and secretion of the three pro-inflammatory cytokines in LPS-treated primary microglia (Figure 3G,H,I,J,K,L).
Pon suppressed the phosphorylation of ERK1/2, JNK and NF-κB in LPS-activated BV2 microglial cells
The MAPK signaling pathway is a well-known pathway involved in the inflammatory response that mainly consists of three MAPK subfamilies, including the ERK1/2, JNK and p38 families (19). Therefore, we aimed to investigate the molecular mechanism by which Pon inhibits the LPS-stimulated inflammatory response. As shown in Figure 4A, exposure of BV2 cells to LPS for 1 h increased the levels of the phosphorylated ERK1/2, JNK, and p38 proteins, consistent with the results of our previous study (20). When BV2 cells were pretreated with Pon, the phosphorylation of ERK1/2 and JNK, but not p38, was suppressed (Figure 4A,B). According to these results, we postulated that Pon inhibits inflammation by suppressing the ERK1/2 and JNK MAPK signaling pathways without altering the activation of p38 MAPK.
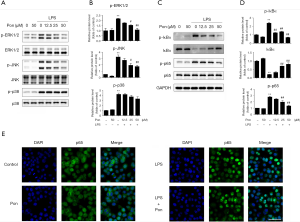
The NF-κB family is a family of transcription factors that responds quickly to inflammation and tissue injury (21). Proteins of the inhibitory κB (IκB) family inhibit NF-κB, particularly dimers containing the p65 or c-Rel subunit (22). Levels of the NF-κB p65 and IκBα proteins were analysed in BV2 cells treated with LPS for 1 h using Western blotting to investigate the effect of Pon on the NF-κB signalling pathway. LPS treatment significantly increased the level of phosphorylated NF-κB p65, while Pon suppressed this phosphorylation in a dose-dependent manner. IκBα was markedly phosphorylated and degraded in BV2 cells after LPS stimulation. However, pre-treatment with Pon significantly decreased the degradation and phosphorylation of IκBα (Figure 4C,D). The inhibitory effect of Pon on the activation of p65 was confirmed by the results of immunofluorescence staining. NF-κB p65 was mainly located in the cytoplasm in the absence of LPS and in the presence of Pon alone, whereas the LPS treatment markedly increased the fluorescence intensity of NF-κB p65 in the nucleus. Pre-treatment with Pon significantly blocked the translocation of NF-κB p65 from the cytoplasm to the nucleus (Figure 4E). Thus, we propose that Pon inhibits inflammatory responses through the NF-κB signaling pathway.
Pon decreased the infarct volume and improved neurological deficits after ischemic stroke
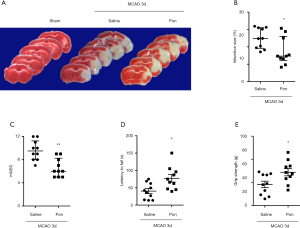
To investigate whether the in vitro anti-inflammatory effects of Pon are associated with therapeutic activities in vivo, an experimental mouse model of ischemic stroke in which neuroinflammation is the main pathogenic event was used. We did not find any studies that mentioned the use of Pon in stroke research. However, Pon (30 mg/kg) markedly reduces pain behaviors in experimental models of inflammatory pain (14). Thus, we chose 30 mg/kg Pon for the in vivo study. We excluded two mice that died within 72 h after MCAO and replaced them with another two mice. TTC staining was performed to analyze infarct volume, and no infarction was detected in the sham group. In addition, the infarct volume was significantly decreased in mice that received Pon via an i.p. injection at 30 min, 24 h and 48 h after MCAO compared with the vehicle-treated MCAO group (17.41%±2.50% in the group treated with MCAO-Pon vs. 24.95%±1.85% in the group treated with MCAO-saline at 72 h, P<0.05, n=10, Figure 5A,B). Subsequently, we evaluated changes in brain function deficits in each group. Consistent with a smaller infarct size, Pon led to a lower mNSS score, increased motor function and stronger grip strength (mNSS: 6.60±0.50 points in the group treated with MCAO-Pon vs. 9.60±0.54 points in the group treated with MCAO-saline at 72 h, P<0.01; rotarod test: 154.50±21.36 for the group treated with MCAO-Pon vs. 80.86±14.65 for the group treated with MCAO-saline at 72 h, P<0.05; grip strength test: 72.11±7.83 for the group treated with MCAO-Pon vs. 45.75±7.09 for the group treated with MCAO-saline at 72 h, P<0.05, n=10, Figure 5C,D,E). Based on these results, Pon attenuates ischemic brain injury and behavioral deficits in a mouse model of experimental stroke.
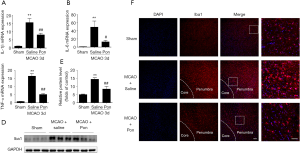
Pon attenuated the production of inflammatory cytokines by restraining microglial activation after ischemic stroke
Microglia are usually activated after tissue injury and activated microglia aggravate the inflammatory response by simultaneously releasing many pro-inflammatory factors (12). To investigate whether the neuroprotective effects of Pon are related to the inhibition of microglial activation and pro-inflammatory factor production, we further assessed the levels of the IL-1β, IL-6 and TNF-α mRNA in tissues from the ischemic hemisphere at 3 days after MCAO. The mRNA levels of these three pro-inflammatory cytokines were increased in the MCAO-saline group compared with the sham group, but this elevation was reversed by the Pon injection (Figure 6A,B,C). Finally, we detected the expression of Iba1, an indicator of microglial activation, in the ischemic hemisphere 3 days after MCAO by performing a Western blot analysis. Compared with saline treatment, Pon significantly suppressed the expression of Iba1 after MCAO (Figure 6D,E). Afterwards, we evaluated the degree of microglial activation in the ischemic penumbra 3 days after MCAO using immunofluorescence staining (Figure 6F). The microglia activation caused by cerebral ischemic injury was attenuated by Pon as well. Thus, Pon decreases the infarct volume and improves neurological deficits after ischemic stroke, which might be the result of the modulation of microglial activation and subsequent inflammatory responses.
Discussion
Ischemic stroke is a global public health issue, and its high morbidity and mortality rates are closely related to the severity of ischemic brain injury (1). The pathological mechanisms of ischemic brain injury are very complicated and involve many factors. Among them, the microglia-mediated inflammatory response participates in the entire process of this disease and is one of the most important factors affecting the prognosis of patients with ischemic stroke (4). In the present study, pre-treatment with Pon effectively inhibited the LPS-induced production of pro-inflammatory mediators in BV2 microglial cells and primary microglia by regulating the activation of the MAPK and NF-κB signaling pathways. According to our in vivo data, the i.p. injection of Pon attenuated the brain infarct volume and improved neurological deficits induced by transient focal ischemia. Furthermore, Pon significantly suppressed microglial activation in the cerebral cortical penumbra and the release of inflammatory cytokines, suggesting that the protective effects of Pon on experimentally induced stroke were at least partially mediated by the attenuation of microglia-mediated neuroinflammation. Taken together, Pon potentially represents a neuroprotective agent to treat ischemic brain injury.
NO and PGE2 produced by iNOS and COX-2 are two of the main proteins involved in post-ischemic inflammation (23). Importantly, iNOS is expressed in the human brain after ischemic infarction (24). Inhibition of iNOS activity by its inhibitor aminoguanidine results in large decreases in infarct volume in MCAO models (25). Likewise, COX-2 inhibition by specific inhibitors and COX-2 knockout in mice result in a significant reduction in brain injury produced by focal ischemia (26,27). In the present study, pre-treatment with Pon dose-dependently decreased NO and PGE2 production and inhibited iNOS and COX-2 expression at the protein level in LPS-induced microglia (Figure 2). These observations are consistent with those of previous studies describing the anti-inflammatory effects of Pon on ethanol-induced gastric damage in mice (28).
IL-1β, IL-6 and TNF-α are likely the most extensively studied pro-inflammatory cytokines involved in stroke. The levels of IL-1β, IL-6 and TNF-α are significantly increased by many folds (up to 40-fold) in the brain within the first 24 h after the induction of focal cerebral ischemia in mice (29). These three cytokines are mainly synthesized by microglia in the stroke-lesioned rodent brain (30). In a mouse model of experimental stroke, the administration of neutralizing antibodies against IL-1β and TNF-α reduce the infarct volume after MCAO, while a striatal injection of IL-1β exacerbates ischemic damage (30,31). IL-6 is a pleiotropic pro-inflammatory cytokine, and the role of IL-6 in ischemic stroke is controversial. Several studies have reported strong neuroprotective effects of IL-6 inhibition, while Loddick et al. found that intracerebroventricular injection of recombinant IL-6 significantly reduced ischemic brain damage after MCAO (32-34). In the present study, we confirmed that Pon not only suppresses the LPS-induced expression of the IL-1β, IL-6 and TNF-α mRNA and proteins in microglia, but also inhibits the production of these pro-inflammatory cytokines in the mouse brain after MCAO (Figures 3,6).
Several studies have reported that the MAPK signaling pathways, including the ERK1/2, JNK and p38 pathways, play vital roles in microglial activation and inflammatory reactions (19). Inhibition of phospho-ERK1/2 and p38 in activated microglia by two specific kinase inhibitors (PD98059 and SB203580) almost completely reduces the production of NO and TNF-α (35). JNKs are essential mediators of relevant pro-inflammatory functions in microglia (36). Luteolin, a flavonoid, has been shown to inhibit LPS-induced IL-6 production in the brain by inhibiting the JNK pathway in microglia (32). Thus, treatments that inhibit MAPKs may be a promising intervention for inflammatory responses induced by overactivated microglia. In our current study, we confirmed that these three MAPK signaling pathways were involved in microglial activation and that LPS-stimulated phosphorylation of ERK1/2 and JNK was markedly diminished in BV2 microglia after pre-treatment with Pon, suggesting that ERK1/2 and JNK are two important molecular targets of Pon (Figure 4). Furthermore, Pon tended to decrease the levels of phospho-p38. However, a study performed by Chun et al. suggested that Pon inhibits receptor activator of NF-κB ligand (RANKL)-induced JNK activation without substantially altering the levels of phosphorylated p38 and ERK in RAW 264.7 cells, which are murine pre-osteoclasts/macrophages. The discrepancy in phospho-MAPK expression may result from the use of different cell types and experimental conditions.
In addition to MAPK pathways, NF-κB activation is also critically required for microglia-mediated CNS inflammation (21). In the resting state, NF-κB is sequestered in the cytoplasm by binding with the inhibitory protein IκB. Upon activation by many stimuli, IκBs are phosphorylated and degraded by ubiquitination. IκB degradation leads to the phosphorylation of the NF-κB p65 subunit and its translocation into the nucleus to induce the transcription of several pro-inflammatory mediators. As shown in a recent study by Ganbold et al., NF-κB p65 silencing decreases the expression of pro-inflammatory cytokines and facilitates the anti-inflammatory polarization of microglia (37). Overexpression of heat shock protein 70 (HSP70) in microglia has been reported to protect against focal and global cerebral ischemia by preventing IκB phosphorylation (38). Pon has been shown to inhibit NF-κB in RAW 264.7 macrophages exposed to LPS (39). Consistent with these results, we unequivocally demonstrated that pre-treatment with Pon significantly reduced LPS-induced IκBα phosphorylation and degradation in microglia. Subsequently, Pon suppressed NF-κB p65 activation and nuclear translocation, suggesting that the IκB/NF-κB p65 signaling pathway is involved in the inhibitory effects of Pon on the overexpression of pro-inflammatory mediators in LPS-treated microglia (Figure 4).
The MCAO model is the most commonly used animal model in studies of ischemic brain injury, and one of the most prominent features of ischemic stroke is neuroinflammation mediated by activated microglia. In follow-up experiments, we observed the neuroprotective effect of Pon on the MCAO model. Our results showed that Pon administration at 30 min, 24 h and 48 h after MCAO obviously attenuated the brain infarct size and neurological deficits on day 3 after MCAO (Figure 5). Moreover, Pon reduced the expression of Iba1, an indicator of microglial activation, suggesting that Pon exerts its potential neuroprotective effect by suppressing inflammation caused by microglia activation (Figure 6). Nevertheless, to reduce the use of experimental animals, the number of mice in each group was not large which may be a potential source of bias. Increasing evidence in recent years has shown that activated microglia can be divided into pro-inflammatory (M1) and anti-inflammatory (M2) phenotypes. As shown in our previous study, M1 and M2 microglia can switch phenotypes during the process of stroke (40). Malibatol A, a natural resveratrol oligomer, protects against focal cerebral ischemia-reperfusion injury by modulating microglial polarization from the M1 to the M2 phenotype (41). Thus, treatments that inhibit overactivated pro-inflammatory M1 microglia by switching them to the protective M2 phenotype has been suggested as a potential therapeutic strategy for ischemic stroke. Further experiments will study the effect of Pon on microglial polarization in in vivo and in vitro mouse models of ischemic stroke to make our research more meaningful and interesting.
Conclusions
In summary, Pon exerts anti-inflammatory and neuroprotective effects on LPS-induced inflammatory responses in microglia and ischemic injury in the brain of a mouse model experimentally induced stroke. These findings suggest that Pon may represent a potential treatment for ischemic stroke.
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (No. 81701170, 81630028, 81920108017 and 81571134), the Natural Science Foundation of Jiangsu Province of China (No. BK20170122), the Young Talent Support Program from Jiangsu Association for Science and Technology and Jiangsu Province Key Medical Discipline (No. ZDXKA2016020).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-3470
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-3470
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-3470). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal experiments were approved by the Animal Care and Use Committee at Nanjing University (reference number: 2019AE01073) and performed according to institutional guidelines.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Phipps MS, Cronin CA. Management of acute ischemic stroke. BMJ 2020;368:l6983. [Crossref] [PubMed]
- Stoll G, Nieswandt B. Thrombo-inflammation in acute ischaemic stroke - implications for treatment. Nat Rev Neurol 2019;15:473-81. [Crossref] [PubMed]
- Radak D, Katsiki N, Resanovic I, et al. Apoptosis and Acute Brain Ischemia in Ischemic Stroke. Curr Vasc Pharmacol 2017;15:115-22. [Crossref] [PubMed]
- Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med 2017;23:1018-27. [Crossref] [PubMed]
- Hu X, Leak RK, Shi Y, et al. Microglial and macrophage polarization-new prospects for brain repair. Nat Rev Neurol 2015;11:56-64. [Crossref] [PubMed]
- Tay TL, Savage JC, Hui CW, et al. Microglia across the lifespan: from origin to function in brain development, plasticity and cognition. J Physiol 2017;595:1929-45. [Crossref] [PubMed]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005;308:1314-8. [Crossref] [PubMed]
- Neher JJ, Emmrich JV, Fricker M, et al. Phagocytosis executes delayed neuronal death after focal brain ischemia. Proc Natl Acad Sci U S A 2013;110:E4098-107. [Crossref] [PubMed]
- Michelucci A, Heurtaux T, Grandbarbe L, et al. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: Effects of oligomeric and fibrillar amyloid-beta. J Neuroimmunol 2009;210:3-12. [Crossref] [PubMed]
- Xu L, He D, Bai Y. Microglia-Mediated Inflammation and Neurodegenerative Disease. Mol Neurobiol 2016;53:6709-15. [Crossref] [PubMed]
- Merrill JE, Ignarro LJ, Sherman MP, et al. Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J Immunol 1993;151:2132-41. [PubMed]
- Qin C, Zhou LQ, Ma XT, et al. Dual Functions of Microglia in Ischemic Stroke. Neurosci Bull 2019;35:921-33. [Crossref] [PubMed]
- Kang GD, Kim DH. Poncirin and its metabolite ponciretin attenuate colitis in mice by inhibiting LPS binding on TLR4 of macrophages and correcting Th17/Treg imbalance. J Ethnopharmacol 2016;189:175-85. [Crossref] [PubMed]
- Afridi R, Khan AU, Khalid S, et al. Anti-hyperalgesic properties of a flavanone derivative Poncirin in acute and chronic inflammatory pain models in mice. BMC Pharmacol Toxicol 2019;20:57. [Crossref] [PubMed]
- Saralamma VV, Nagappan A, Hong GE, et al. Poncirin Induces Apoptosis in AGS Human Gastric Cancer Cells through Extrinsic Apoptotic Pathway by up-Regulation of Fas Ligand. Int J Mol Sci 2015;16:22676-91. [Crossref] [PubMed]
- Chun KH, Jin HC, Kang KS, et al. Poncirin Inhibits Osteoclast Differentiation and Bone Loss Through Down-Regulation of NFATc1 In Vitro and In Vivo. Biomol Ther (Seoul) 2020;28:337-43. [Crossref] [PubMed]
- Liu PY, Zhang Z, Liu Y, et al. TMEM16A Inhibition Preserves Blood-Brain Barrier Integrity After Ischemic Stroke. Front Cell Neurosci 2019;13:360. [Crossref] [PubMed]
- Zhang Z, Guo MD, Liu Y, et al. RNPS1 inhibition aggravates ischemic brain injury and promotes neuronal death. Biochem Biophys Res Commun 2020;523:39-45. [Crossref] [PubMed]
- Ji RR, Gereau RW, Malcangio M, et al. MAP kinase and pain. Brain Res Rev 2009;60:135-48. [Crossref] [PubMed]
- Weng L, Zhang H, Li XX, et al. Ampelopsin attenuates lipopolysaccharide-induced inflammatory response through the inhibition of the NF-κB and JAK2/STAT3 signaling pathways in microglia. Int Immunopharmacol 2017;44:1-8. [Crossref] [PubMed]
- Sochocka M, Diniz BS, Leszek J. Inflammatory Response in the CNS: Friend or Foe? Mol Neurobiol 2017;54:8071-89. [Crossref] [PubMed]
- Wang Q, Zhou X, Yang L, et al. Gentiopicroside (GENT) protects against sepsis induced by lipopolysaccharide (LPS) through the NF-kappaB signaling pathway. Ann Transl Med 2019;7:731. [Crossref] [PubMed]
- Caso JR, Pradillo JM, Hurtado O, et al. Toll-like receptor 4 is involved in subacute stress-induced neuroinflammation and in the worsening of experimental stroke. Stroke 2008;39:1314-20. [Crossref] [PubMed]
- Forster C, Clark HB, Ross ME, et al. Inducible nitric oxide synthase expression in human cerebral infarcts. Acta Neuropathol 1999;97:215-20. [Crossref] [PubMed]
- Iadecola C, Zhang F, Xu X. Inhibition of inducible nitric oxide synthase ameliorates cerebral ischemic damage. Am J Physiol 1995;268:R286-92. [PubMed]
- Iadecola C, Niwa K, Nogawa S, et al. Reduced susceptibility to ischemic brain injury and N-methyl-D-aspartate-mediated neurotoxicity in cyclooxygenase-2-deficient mice. Proc Natl Acad Sci U S A 2001;98:1294-9. [Crossref] [PubMed]
- Carlson NG. Neuroprotection of cultured cortical neurons mediated by the cyclooxygenase-2 inhibitor APHS can be reversed by a prostanoid. J Neurosci Res 2003;71:79-88. [Crossref] [PubMed]
- Kang GD, Kim DH. Ponciretin attenuates ethanol-induced gastric damage in mice by inhibiting inflammatory responses. Int Immunopharmacol 2017;43:179-86. [Crossref] [PubMed]
- Hill JK, Gunion-Rinker L, Kulhanek D, et al. Temporal modulation of cytokine expression following focal cerebral ischemia in mice. Brain Res 1999;820:45-54. [Crossref] [PubMed]
- Lambertsen KL, Biber K, Finsen B. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab 2012;32:1677-98. [Crossref] [PubMed]
- Stroemer RP, Rothwell NJ. Exacerbation of ischemic brain damage by localized striatal injection of interleukin-1beta in the rat. J Cereb Blood Flow Metab 1998;18:833-9. [Crossref] [PubMed]
- Jang S, Kelley KW, Johnson RW. Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc Natl Acad Sci U S A 2008;105:7534-9. [Crossref] [PubMed]
- Chen X, Wu S, Chen C, et al. Omega-3 polyunsaturated fatty acid supplementation attenuates microglial-induced inflammation by inhibiting the HMGB1/TLR4/NF-κB pathway following experimental traumatic brain injury. J Neuroinflammation 2017;14:143. [Crossref] [PubMed]
- Loddick SA, Turnbull AV, Rothwell NJ. Cerebral interleukin-6 is neuroprotective during permanent focal cerebral ischemia in the rat. J Cereb Blood Flow Metab 1998;18:176-9. [Crossref] [PubMed]
- Bhat NR, Zhang P, Lee JC, et al. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci 1998;18:1633-41. [Crossref] [PubMed]
- Waetzig V, Czeloth K, Hidding U, et al. c-Jun N-terminal kinases (JNKs) mediate pro-inflammatory actions of microglia. Glia 2005;50:235-46. [Crossref] [PubMed]
- Ganbold T, Bao Q, Zandan J, et al. Modulation of Microglia Polarization through Silencing of NF-κB p65 by Functionalized Curdlan Nanoparticle-Mediated RNAi. ACS Appl Mater Interfaces 2020;12:11363-74. [Crossref] [PubMed]
- Zheng Z, Kim JY, Ma H, et al. Anti-inflammatory effects of the 70 kDa heat shock protein in experimental stroke. J Cereb Blood Flow Metab 2008;28:53-63. [Crossref] [PubMed]
- Kim JB, Han AR, Park EY, et al. Inhibition of LPS-induced iNOS, COX-2 and cytokines expression by poncirin through the NF-kappaB inactivation in RAW 264.7 macrophage cells. Biol Pharm Bull 2007;30:2345-51. [Crossref] [PubMed]
- Meng H, Zhao HR, Cao X, et al. Double-negative T cells remarkably promote neuroinflammation after ischemic stroke. Proc Natl Acad Sci U S A 2019;116:5558-63. [Crossref] [PubMed]
- Pan J, Jin JL, Ge HM, et al. Malibatol A regulates microglia M1/M2 polarization in experimental stroke in a PPARγ-dependent manner. J Neuroinflammation 2015;12:51. [Crossref] [PubMed]


