Will the 40-gene expression classifier predict responders to EGFR targeted chemotherapy for the invasive bladder cancer patients?
Bladder cancer (BC) is the second most common genitourinary malignancy and the fourth most common cancer in the US, and costs over an estimated $4 billion annually in the US alone. BC ranks amongst the most expensive cancer treatment to date. Most patients have curable transitional cell carcinoma and non-muscle invasive BC (NMIBC). Only approximately 30% of NMIBC patients progress to muscle-invasive BCs (MIBC), however, about 50% of these patients develop metastases within 2 years after cystectomy, and about half of patients die within 5 years. MIBC, which are notorious as heterogeneous and hard-to-treat cancers, do not effectively respond to adjuvant chemotherapy generally associated with a poor prognosis, causing most BC deaths (1).
More recently, neo-adjuvant chemotherapy has been reported to improve overall survival of MIBC patients, however, it remains an important and urgent task to identify prognostic marker(s) of an acquired resistance after cisplatin therapy and to understand the functional contribution of potential predictive markers in chemoresistant MIBC. Current staging systems are inadequate for identifying those patients who would benefit most from chemotherapy. No new systemic therapies have been approved over the past 20 years, mainly due to the heterogeneity of MIBC, which has posed an obstacle for physicians and researchers (2).
Targeted therapy with new agents directed at specific molecular pathways is a promising avenue to achieve such progress. Improvements in understanding the molecular mechanisms involved in bladder carcinoma have highlighted several potential therapeutic targets (3). However, no targeted treatment for MIBC is currently used in clinical practice, and the few clinical trials that attempted using targeted drugs without molecular-based patient selection have been disappointing. The development of successful molecular-based therapies in BC remains challenging because of the high degree of biological diversity of bladder carcinomas. A number of large-scale molecular studies have been conducted in BC (4,5), but not with the focus of identifying a therapeutic target for a particular subgroup. Thus, classification of targetable population and targeted clinical trials on a particularly aggressive subgroup of MIBC are greatly needed (6-8).
A promising finding by a research team led by François Radvanyi (the Institut Curie in Paris, France), along with previous studies from this group and from other laboratories, provided persuasive evidence that a subtype of the cancer is vulnerable to EGFR inhibitors in the Sci Transl Med (9). In this paper, authors showed that a specific subgroup of MIBC was aggressive, with most deaths occurring within 1 year of diagnosis by computational approaches combined with biological validation. This subgroup expressing basal markers was dependent on the activity of a signaling pathway called epidermal growth factor receptor (EGFR). Furthermore, the basal type of MIBC was significantly sensitive to treatment with drugs that inhibit the EGFR pathway, which the authors confirmed in vitro as well as in vivo preclinical models (Figure 1).
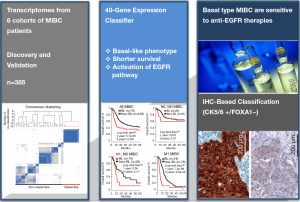
In order to determine the diversity of MIBC, the expression profiles of 85 human MIBC in the discovery cohort were clustered, leading to a homogeneous subgroup of approximately 25% (21 from 85 tumors). Integrative analysis of their own transcriptome data and six publicly available transcriptome data sets with enriched clinical information (n=383 tumors) showed that a MIBC subgroup was associated with shorter survival and exhibited similar a “basal-like” phenotype, which was observed in several other cancers (e.g., breast, lung, head and neck, ovarian, and salivary cancers). Basal-like MIBC subgroup was identified to express epithelial basal cell markers, and approximately 50% of basal-like tumors associated with particular histological parameter, squamous cell differentiation phenotype. Basal-type bladder tumors were found more aggressive, more sensitive to chemotherapy and increased immune cell infiltration, compared to non-basal tumors.
For searching the targetable pathways specifically deregulated in the basal-like tumors, molecular pathway analysis based on four algorithms and knowledge-based databases including Biocarta and KEGG was performed. The EGFR signaling pathway was identified as one of the most significantly enriched pathways. EGFR is overexpressed in BCs and associated with survival rate of patients. EGFR activation stimulates several important downstream signaling pathways, and leads to the increased cell proliferation, invasion and survival. In the basal-like tumors, the expression of EGFR, EGFR ligands (amphiregulin, amphiregulin B, epiregulin, heparin-binding EGF-like growth factor, and transforming growth factor alpha), and EGFR downstream targets (MYC, Interleukin-8 and SOX9) were significantly increased in the basal-like MIBC. Given the findings showing the overexpression of genes in the EGFR pathway, including EGFR itself, and phosphorylation/activation of EGFR and downstream pathways in basal-like tumors, EGFR pathway could be considered as a key therapeutic target candidate. The expression of genes associated with wound healing was also increased in the basal-like MIBC, suggesting that basal-like tumors exploited the bladder repair/reprogramming program.
The 40-gene expression classifier derived from human tumors was further applied to test whether the classifier can subgroup human BC cell lines and tumors from a chemically induced BC mouse model. Among 22 BC-derived cell lines, 11 cells were classified as basal-like based on gene expression. Basal-like MIBC cell lines were responsive to EGFR inhibitors such as erlotinib, (also called as Tarceva, a small-molecule inhibitor of EGFR) or cetuximab (an antibody targeting EGFR) in vitro and in vivo settings. Both erlotinib and cetuximab inhibited the EGFR phosphorylation and the activation of the downstream signaling molecules of EGFR pathway, such as AKT and ERK1/2, only in basal-like BC cell lines. The EGFR inhibitor erlotinib effectively suppressed growth of xenografts of human basal-like cells. In MIBC mouse model induced by treatment with BBN [N-butyl-N-(4-hydroxybutyl)nitrosamine], treatment with erlotinib delayed the appearance of the tumors and increased the animals’ survival.
The data shown by Rebouissou et al. (9) also implicated that basal-like patient tumor-derived xenografts and/or the BBN-induced mouse model could be used for as preclinical models to test the efficacy of combination therapies. Tumors from the chemically induced mouse model resemble the human basal-like MIBC based on the following observations; they were (I) dependent on an EGFR signal pathway; and (II) exhibited frequent mutations of a transcription factor, p53, consistent to those in human basal-like tumors; (III) gene expression analysis using the 37 orthologous mouse genes based on human 40-gene classifier could identify suggested that the BBN-induced bladder tumors resembled human basal-like MIBC; and (IV) they have strong phosphorylation of EGFR, compared to normal mouse urothelium, just like in human basal-like tumors.
Previous clinical trials in MIBC patients in neoadjuvant or adjuvant settings have concluded that EGFR inhibitors have limited effects in patients with MIBC. Given that all the clinical trials of EGFR inhibitors have been performed in unselected MIBC patients, these negative results could be explained by present findings from this study showing that patients with non-basal-like tumors would not respond to EGFR inhibitors. Although further clinical validation in prospective studies will be required, the findings from Radvanyi’s work have provided evidence suggesting the potential existence of the basal-like subtype tumor and the possibility of stratification based on transcriptome and/or IHC signature. The in vivo experimental results using preclinical models strongly suggested that stratifying patients according to the basal-like phenotype should improve treatment efficacy, which might be integrated to the physician’s decision-making process for high risk MIBC as important prognostic biomarkers, and to identification of subgroup-specific therapeutic strategies.
Acknowledgements
Funding: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. NRF-2014R1A2A1A09006983), the Ministry of Education, Science and Technology (2012R1A1A4A01008753 and 2013R1A1A2004740) (to Wun-Jae Kim), the NIH grants 1R01DK100974-01, U24 DK097154, NIH NCATS UCLA CTSI UL1TR000124, the Steven Spielberg Discovery Fund in Prostate Cancer Research Career Development Award, IMAGINE NO IC Research Program Award, Interstitial Cystitis Association (ICA) Pilot Grant, and a Fishbein Family IC Research Grant by ICA; New York Academy of Medicine; Boston Children’s Hospital Faculty Development (to Jayoung Kim).
Disclosure: The authors declare no conflict of interest.
References
- Noon AP, Catto JW. Bladder cancer in 2012: Challenging current paradigms. Nat Rev Urol 2013;10:67-8. [PubMed]
- Resnick MJ, Bassett JC, Clark PE. Management of superficial and muscle-invasive urothelial cancers of the bladder. Curr Opin Oncol 2013;25:281-8. [PubMed]
- Richter S, Sridhar SS. New directions for biologic targets in urothelial carcinoma. Mol Cancer Ther 2012;11:1226-35. [PubMed]
- Kim WJ, Kim SK, Jeong P, et al. A four-gene signature predicts disease progression in muscle invasive bladder cancer. Mol Med 2011;17:478-85. [PubMed]
- Kim WJ, Kim EJ, Kim SK, et al. Predictive value of progression-related gene classifier in primary non-muscle invasive bladder cancer. Mol Cancer 2010;9:3. [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014;507:315-22. [PubMed]
- Hurst CD, Knowles MA. Molecular subtyping of invasive bladder cancer: time to divide and rule? Cancer Cell 2014;25:135-6. [PubMed]
- Choi W, Porten S, Kim S, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014;25:152-65. [PubMed]
- Rebouissou S, Bernard-Pierrot I, de Reyniès A, et al. EGFR as a potential therapeutic target for a subset of muscle-invasive bladder cancers presenting a basal-like phenotype. Sci Transl Med 2014;6:244ra91.


