
Profiling circulating T follicular helper cells and their effects on B cells in post-cardiac transplant recipients
Introduction
Heart transplantation is a life-saving treatment used for patients with end-stage heart failure. Due to advancements in medical technologies in the fields of cardiac protection, cardiac surgery, and immunology, the post-transplantation 1-year survival rate is 82%, and the 5-year survival rate is 70%, as reported by the International Society of Heart and Lung Transplantation (ISHLT) (1). As of 2017, more than 144,230 heart transplants had been conducted worldwide. With the development and clinical application of multiple inhibitors of immune rejection, such as adrenal glucocorticoids (2,3), new polyclonal and monoclonal lymphocyte antibodies, and other immunosuppressive drugs (4-6), acute immune rejection of a transplanted heart can be controlled more effectively. Chronic rejection, which causes functional loss of the transplanted heart, has become the primary factor that significantly affects the long-term survival of cardiac transplant patients.
The underlying mechanisms of chronic rejection are multifactorial, although the activation of the innate and adaptive immune systems is the main mediating factor in chronic rejection-linked injury of transplanted hearts. Chronic immune damage can involve multiple T cell subsets (7). T follicular helper (Tfh) cells, which are CXCR5-positive CD4+ T cells, are a recently defined subset of T cells (8-10). These cells are activated in the germinal centers (GCs) of secondary lymphoid organs and have a stronger ability to promote immunoglobulin formation and B cell differentiation compared with CXCR5-negative CD4+ T cells. In 2000, circulating Tfh (cTfh) cells were first identified as a CD4+CXCR5+ T cell subset in peripheral circulation that play a similar role to GC Tfh cells (11). Recently, the de Graav (12) and Forcade (13) groups revealed the pivotal role of cTfh cells in patients who undergo kidney transplants or who have chronic graft-versus-host disease. Combined with the fact that CXCR5 can be induced during lymphocytic choriomeningitis virus infection (14), exactly how the cTfh subtypes are distributed in the peripheral blood of chronic-phase patients after heart transplant is not well understood.
In this study, we profiled cTfh subtypes and examined the levels of their related cytokines, CXCL13 and interleukin-21 (IL-21), in the peripheral blood of patients after heart transplantation. Our findings offer novel insights that help elucidate the pathological connection between cTfh cells and B cells in chronic-phase recipients after heart transplantation, providing potential therapeutic targets for ameliorating chronic rejection.
Methods
Study population
This study complied with the Declaration of Helsinki (as revised in 2013) and was approved by the review boards of Union Hospital and Tongji Medical College (IORG number: IORG0003571). Informed consent was obtained from all patients and healthy control (HC) subjects. The study participants were divided into the following three groups: the preoperative group (Pre group, n=40), the group of chronic-phase recipients at 1-year post-heart transplantation (1-year group, n=40), and an age and gender-matched HC group (n=40) comprising volunteers. The demographic information of patients and HC subjects is summarized in Table 1, and the detailed perioperative and follow-up data of the 1-year group is shown in Table 2.

Full table
Full table
After being discharged from our hospital, all patients were admitted to the outpatient department weekly for the 1st month, biweekly until the 3rd month, monthly from the 4th to 12th month, and twice for one year thereafter. Outpatients who had undergone orthotopic heart transplantation over 1 year before at our institute were enrolled in this study. The maintenance immunotherapies used for all patients included tacrolimus (0–90 days, 10 ng/mL; 90 days–1 year, 8–10 ng/mL; >1 year, 5–8 ng/mL), mycophenolate (maintenance dose 1.5–2 g/day), and prednisone. The exclusion criteria for patients in the Pre and 1-year groups were: (I) a history of autoimmune disease (such as rheumatoid arthritis, systemic lupus erythematosus, or rheumatic heart disease); (II) a medical history of malignant tumors, hepatitis virus infection, or human immunodeficiency virus (HIV) infection; (III) fever or cough symptoms, with lung computed tomography (CT) scans showing infectious lesions; (IV) acute rejection symptoms with a biopsy diagnosed with a grade exceeding 2R according to the ISHLT criteria (15); (V) a blood test showing a white blood cell (WBC) count of >9.5 g/L, erythrocyte sedimentation rate (ESR) of >20 mm/h, and C-reactive protein (CRP) level of >8 mg/L. The exclusion criteria for the HC group included: (I) fever, cough, or phlegm in the week before the start of the trial, which were treated with antibiotics; or (II) a previous history of autoimmune diseases, hepatitis virus infection, or HIV infection.
For the 1-year group patient, they would have coronary angiography once per year. For patients who underwent a coronary angiography to detect cardiac allograft vasculopathy (CAV), the diagnosis of CAV included a detectable angiographic lesion including any branch stenosis (including diffuse narrowing), with or without allograft dysfunction (16).
Blood sample collection
Overnight-fasted blood samples (6 mL) from each participant were collected into heparin-treated anticoagulation tubes. Each blood sample (3 mL) was then carefully added to human lymphocyte-separation solution (3 mL) in a plastic centrifuge tube, followed by centrifugation at 500 ×g for 20 min at 4 °C to separate the plasma, mononuclear cells, and erythrocytes.
Human tissue collection and H&E staining
A post-cardiac transplant patient diagnosed with CAV by coronary angiography was admitted to the cardiovascular department of our institute for repeat heart transplantation, because of progressive deterioration in cardiac function. Myocardial tissue was collected during the operation. Tissue samples were either frozen in liquid nitrogen until use, or fixed with formalin and embedded in paraffin. Hematoxylin and eosin (H&E) staining was performed on 4-µm-thick cardiac tissue sections according to a standard protocol.
Cell staining and flow cytometry
Single-cell suspensions were stained for 30 min using the following fluorophore-conjugated antibodies: FITC anti-CD3 (BD Biosciences Cat# 555332, RRID:AB_395739), APC/Cy7 anti-CD4 (BD Biosciences Cat# 341095, RRID:AB_400218), Percp-Cy5.5 anti-CXCR5 (BD Biosciences Cat# 562781, RRID:AB_2313576), PE anti-PD-1 (BD Biosciences Cat# 560795, RRID:AB_2033989), PE/Cy7 anti-CCR6 (BD Biosciences Cat# 560620, RRID:AB_1727440), APC anti-CCR6 (BD Biosciences Cat# 560619, RRID:AB_1727439), PECy7 anti-CXCR3 (BD Biosciences Cat# 560831, RRID:AB_2033944), APC anti-CXCR3 (BD Biosciences Cat# 565223, RRID:AB_2687488), FITC anti-CD19 (BD Biosciences Cat# 555412, RRID:AB_395812), BV421 anti-CD27 (BioLegend Cat# 302823, RRID:AB_10900425), APC anti-CD38 (BD Biosciences Cat# 555462, RRID:AB_398599), PECy7 anti-IgD (BD Biosciences Cat# 555776, RRID:AB_396111), and PE anti-CD138 (BD Biosciences Cat# 552026, RRID:AB_394323). For intracellular staining, cells were permeabilized using the Foxp3 Staining Buffer Set (eBioscience), then stained with PECy7 anti-Ki67 (BD Biosciences Cat# 561283, Clone B56), PE anti-Foxp3 (BD Biosciences Cat# 560082, RRID:AB_1645509), and PE anti-Bcl2 (BD Biosciences Cat# 340576, RRID:AB_400061). All samples were analyzed with an LSRFortessa flow cytometer (Beckton Dickinson), and data were processed with FlowJo software, version 10 (TreeStar, Inc.).
Enzyme-linked immunosorbent assay (ELISA)
ELISAs were performed to determine the levels of human plasma IL-21, CXCL13, immunoglobulin (IgG), immunoglobulin G1 (IgG1), and immunoglobulin G3 (IgG3) according to the protocols provided by the manufacturers. The following ELISA kits were used: human IL-21 ELISA kit (BioLegend Cat# 433808, RRID:AB_10663410), human CXCL13 ELISA kit (R&D Systems, USA), human IgG1 ELISA kit, and human IgG3 ELISA kit (eBioscience, USA).
Co-culture of cTfh cells and B cells
WBCs were isolated as described above and analyzed with a FACS Aria flow cytometer (Beckton Dickinson) to obtain CD4+CXCR5− T cells, CD4+CXCR5+ cells, and CD19+CD27+ memory B cells (mB) (4×105/mL of each cell type). The following fluorescently labeled antibodies were used for fluorescence-activated cell sorting (FACS) analysis: APC anti-CXCR5, APCCy7 anti-CD4, PE anti-CD27, PECy7 anti-CD19, and BV421 anti-CD38 (all from BD Bioscience).
Cells were maintained in RPMI 1640 medium supplemented with 50% fetal bovine serum (FBS). For co-culture experiments, 1×105 T and B cells were added to wells of a 96-well plate and co-cultured in 200 µL complete medium containing 10% FBS and 0.2 µg/mL staphylococcal enterotoxin B (SEB). Half of the medium was changed every 2 days to keep the SEB concentration constant. After 6 days of culture, co-cultured cells were subjected to flow cytometry, based on positive staining against the markers fixable viability dye (FVD), CD4, CXCR5, CD19, CD27, and CD38 to reveal changes in the B cell subtype.
Statistical analysis
Unless otherwise stated, continuous variables conforming to a normal distribution were articulated as a mean ± standard deviation and analyze by ANOVA with post-hoc Bonferroni or Tamhane T2 correction. Two-group data were analyzed by a 2-sample t-test. Variables fitting a skewed distribution, which were reported as the median [inter-quartile range (IQR)], were analyzed by the Mann-Whitney test. Categorical variables were presented as counts followed by percentages in parentheses and analyzed by the Chi-square test. Correlations between two variables were evaluated using Pearson’s correlation coefficient, except in cases of non-normal data distribution, where Spearman’s test was used. Data were analyzed with GraphPad Prism software, version 6.04 for Windows (GraphPad Software, Inc.), differences with P<0.05 were statistically significant.
Results
The percentage of cTfh cells was elevated 1-year post-heart transplantation
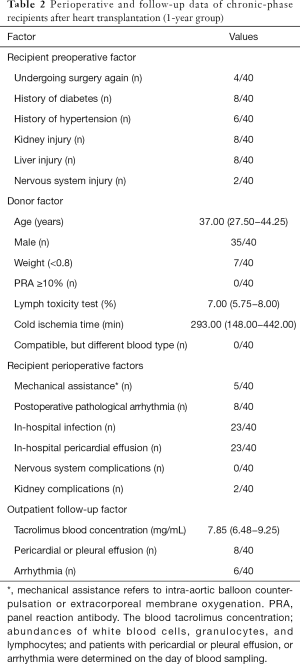
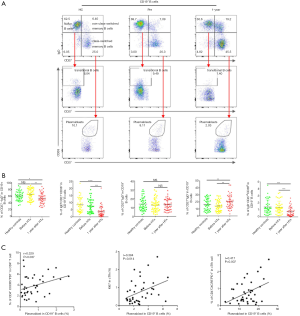
A total of 120 subjects (each group, n=40) were included in this study. After gender and age matching, the baseline characteristics of the three groups were comparable (Table 1). We first evaluated CXCR5 expression in CD4+ T cells. As shown in Figure 1A and B, the 1-year group had significantly higher CXCR5 expression in their CD4+ T cells compared with the HC and Pre groups (22.95%±1.17% vs. 17.20%±0.80% and 18.03%±0.87%, P<0.001 and P=0.0012, respectively), but no significant difference was found between the HC and Pre group.
Programmed cell death protein 1 (PD-1) and inducible T cell costimulator (ICOS), which are expressed on cTfh cells, show better function of promoting B cell differentiation and maturation in vitro (13,17), defined by the CD4+CXCR5+PD-1+ T cells (polarized cTfh). As shown in Figure 1A and C, the percentage of CD4+CXCR5+PD-1+ T cells among CD4+ T cells was also elevated in the 1-year group (4.08%±0.33% vs. 2.52%±0.19% and 2.52%±0.19%, P<0.001, respectively). These results demonstrated that the percentage of cTfh cells increased significantly during the chronic phase in cardiac transplant recipients.
Comparison of the proliferative and anti-apoptotic capabilities of cTfh cell subtypes in the HC, Pre, and 1-year groups
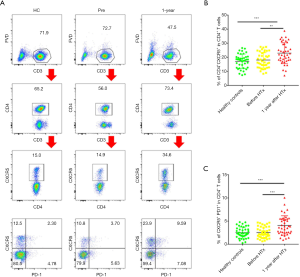
Peripheral blood cTfh cells can be divided into three subtypes based on the expression patterns of the surface molecules, CXCR3 and CCR6: cTfh1 (CXCR3+CCR6−), cTfh2 (CXCR3−CCR6−), and cTfh17 (CXCR3−CCR6+). cTfh2 and cTfh17 have a stronger ability to promote B cell differentiation and antibody class switching (18). As shown in Figure 2A and B, we found that cTfh1 cells accounted for a smaller proportion of cTfh cells in the 1-year group than in the HC and Pre groups (14.44%±1.09% vs. 18.25%±1.28% and 18.58%±1.26%, P=0.027 and P=0.015, respectively). Furthermore, the 1-year group had significantly more cTfh17 (26.25%±1.58%) than the HC group (20.58%±1.38%, P=0.012) and the Pre group (19.60%±1.29%, P=0.002).
Next, we investigated the proliferative and anti-apoptotic abilities of cTfh cells in these three groups by examining the expression of the nuclear proteins Ki67 (a mitotic marker) and Bcl-2 (an apoptosis inhibitor). As shown in Figure 2C and D, the percentages of Ki67+ cTfh cells in the HC, Pre, and 1-year groups were 4.54%±0.27%, 4.36%±0.30%, and 2.90%±0.23%, respectively. We also found that the proliferative capacity of cTfh cells was correlated significantly with the proportion CD4+CXCR5+PD-1+ T cells (Figure 2C, r=0.49, P=0.002). No significant difference was found in the Bcl2-expression levels between groups, suggesting that cTfh cells in the patients of these groups exhibited comparable anti-apoptotic abilities.
We then compared the levels of circulating T follicular regulatory cells (cTfr), which are positive for both CXCR5 and Foxp3, in peripheral blood samples from patients in each group. The cTfr/cTfh ratio was significantly lower in the 1-year group than in the HC and Pre groups (Figure 2E, 14.85%±0.74% vs. 19.27%±0.94% and 18.73%±1.09%, P<0.001 and P=0.017, respectively). No significant difference was observed in the proportion of cTfr cells among total CD4+ T cells between these groups (Figure 2E). We found that the proportion of cTfh cells were negatively correlated with the cTfr/cTfh ratio (r=−0.371, P=0.018) and cTfr/CD4+ T cell ratio (r=−0.43, P=0.006) in the 1-year group, but were not related to the number of Treg (CD4+Foxp3+) cells (Figure 2F).
Stimulation of B cell differentiation by cTfh cells in vitro
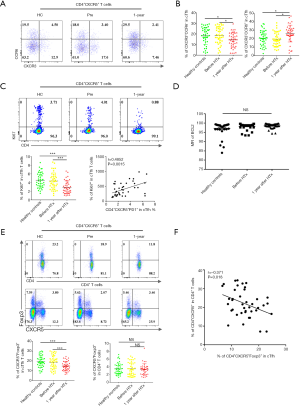
To examine whether the elevation of cTfh cells could stimulate B cell differentiation in vitro, we isolated CD4+CXCR5− T cells, CD4+CXCR5+ cells, and CD19+CD27+ mB cells via FACS. These cells were uniformly translucent, and the purity obtained by flow cytometry exceeded 95% (Figure 3A,B). We co-cultured these cells based on the following groupings: mB alone, the CD4+CXCR5+ + mB group, and the CD4+CXCR5−+ mB group. After 6 days of culture, the percentage of CD19+CD38hi plasmablasts per well was obtained using flow cytometry. The proportion of mB-derived plasmablasts in the CD4+CXCR5+ + mB group accounted for 37.63%±3.22% of the CD19+ B cells, which was significantly higher than the CD4+CXCR5− + mB group, in which the proportion was 21.50%±4.13% (Figure 3C,D, P=0.001), indicating that cTfh cells could promote the differentiation of mB cells into plasmablasts.
Distribution of B cell subpopulations and the correlation between cTfh cells and B cells in chronic-phase recipients after heart transplantation
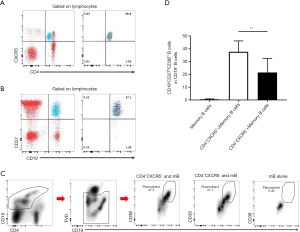
Based on our in vitro experimental results, we examined whether cTfh cells could promote B cell differentiation in chronic-phase recipients after heart transplantation. Flow cytometry was used to delineate different B cell subsets as shown in Figure 4A and B, including CD19+CD27−IgD+ naïve B cells, CD19+CD27−IgD+CD38++ transitional B cells, CD19+CD27+IgD+ non-class-switched memory B cells, CD19+CD27+IgD− class-switched memory B cells, and CD19+IgD−CD27hiCD38hi plasmablasts (19). Among these subsets, the proportion of naïve B cells and transitional B cells in CD19+ B cells was significantly decreased in the 1-year group compared with the HC and Pre groups. In addition, the 1-year group had a higher number of class-switched memory B cells compared with the HC and Pre groups (20.50%±1.49% vs. 16.31%±1.32% and 14.19%±1.20%, P=0.039 and P=0.002, respectively). This finding suggests that the abundance of memory B cells gradually increased, whereas naïve B cells gradually decreased over time compared with pre transplantation group.
Through correlation analysis, we found that the CD4+CXCR5+PD-1+ cTfh/CD4+ T cell ratio (r=0.329, P=0.037) and the CD4+CXCR5+PD-1+ cTfh/cTfh ratio (r=0.417, P=0.007) were closely related to the percentage of peripheral plasmablasts (Figure 4C), as was the proliferative capacity of cTfh cells (r=0.384, P=0.014). These observations suggest that cTfh cell activation and proliferation can promote plasma cell-like changes in peripheral blood B cells.
Cytokine concentrations and correlations with cTfh cells and B cell subsets between groups
IL-21 is a cytokine produced by cTfh cells that regulates B cell differentiation and proliferation as well as promoting auto-antibody production. Compared with the HC and Pre groups, the IL-21 protein and mRNA expression levels in PBMCs were significantly higher in the 1-year group (Figure 5A). IL-21 production was positively correlated with the proportions of cTfh cells (r=0.407, P=0.009) and CD4+CXCR5+PD-1+ cTfh cells (r=0.394, P=0.018) among CD4+ T cells.
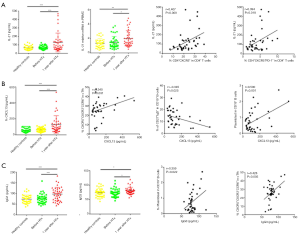
The CXCR5 ligand CXCL13 can be secreted by immune cells, endothelial cells, dendritic cells, and macrophages. The CXCL13−CXCR5 axis can promote the migration of cTfh cells and B cells to lymph node follicles and enhance the responses of these two cell populations. We measured the plasma CXCL13 levels in the three groups (Figure 5B). The 1-year group had significantly higher expression levels of the CXCL13 (130.80±17.26 pg/mL) compared to the HC and Pre groups (59.29±4.00 and 63.43±3.99 pg/mL, P<0.0001, respectively). We also found that plasma CXCL13 levels were positively correlated with the percentage of cTfh17 cells (r=0.340, P=0.031) and plasmablasts (r=0.500, P=0.001), but negatively correlated with the percentage of non-class-switched memory B cells (r=−0.345, P=0.025). Collectively, these observations suggest that the increase in plasma CXCL13 was most likely related to the transformation of cTfh cells to cTfh17 cells, and the migration of memory B cells to the lymph nodes.
We also measured the concentrations of IgG1 and IgG3 in the 1-year group using ELISA (Figure 5C). Both IgG subsets were significantly increased in the 1-year group, and IgG3 concentration was positively correlated with the proportion of plasmablasts among CD19+ B cells (r=0.359, P=0.022) and cTfh17 cells (r=0.428, P=0.005).
Presence of cTfh cells in patients with CAV
CAV is a major manifestation of the impaired immune system in chronic-phase recipients after heart transplantation and is the main heart transplantation-related complication affecting long-term survival (20). We examined coronary tissue samples from patients who underwent repeat heart transplantation due to chronic immune rejection. Compared to normal coronary tissue samples, graft vascular stenosis was evident, and was concentric due to the combination of intimal hyperplasia and insufficient external remodeling (Figure 6A).
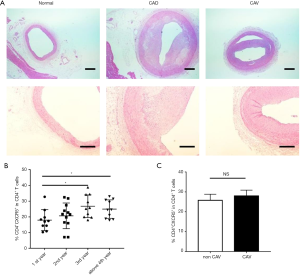
We then analyzed the clinical data of patients in the three treatment groups based on the postoperative duration. We found that the proportion of cTfh cells was higher in patients during the third and fourth years after heart transplantation compared to the first year after surgery (Figure 6B). The incidence rate of CAV increases with increasing time after heart transplantation, and our results showed that compared with recipients without angiogram-diagnosed CAV, patients with CAV did not have higher percentages of cTfh cells (Figure 6C).
Discussion
Chronic rejection is a major cause of organ transplant failure over time. Currently, limited therapeutic means are available for preventing chronic graft rejection (21). Multiple immunocompetent cells (including innate immune cells and adaptive immune cells) participate in the chronic immune response after heart transplantation. As the key factor linking natural and humoral immunity, T cells indirectly recognize donor major histocompatibility complex (MHC) molecules after transplantation and play important roles in the development of chronic immune damage (22).
Consistent with previous reports (13,23,24), in this study, we found that the proportion of cTfh cells and CD4+CXCR5+PD-1+ cTfh cells among CD4+ T cells was increased in the 1-year group compared with the HC and Pre groups. Additionally, the transcription factor Bcl-6 was not expressed on cTfh cells in each group, suggesting that these cells were not the memory form of lymph node Tfh cells reported previously (23). Our findings were in agreement with other previous transplantation-related reports (11,25). Furthermore, the proliferative capacity of cTfh cells in the 1-year group was significantly lower than that in the Pre group, which was likely due to the application of an immunosuppressant. cTfh might exist in the peripheral blood as the memory status of GC Tfh, thus this could also account for the fact that they had lower expression of Ki67. Previous findings suggested that Tfh cells expressing PD-1 and ICOS exhibit stronger function of promoting B cell differentiation (26). In line with this observation, we found that cTfh cell activation and proliferative capacity were closely related. Also this changes were not due to the blood concentration changes of tacrolimus, we found neither the cTfh proportion nor the CD4+CXCR5+PD-1+ T cell proportion was related with the tacrolimus (data not shown). This also shown that in the chronic phase of heart transplantation where the alloantigen persisted, cTfh instead of Th1 cells plays vital roles in long-term T cell response.
By profiling the cTfh cell subtypes, we found that the proportion of cTfh17 cells was increased, and the proportion of cTfh1 cells was decreased, whereas cTfh2 cells exhibited enhanced anti-apoptotic ability in the 1-year group (data not shown). Collectively, these findings suggest that in chronic-phase recipients after heart transplantation, transplantation-related injury can trigger an increase in the number of cTfh17 cells and enhance the survival of cTfh2 cells. These phenotypic changes from cTfh1 to cTfh17 are highly likely to drive more B cell differentiation towards plasmablasts, followed by increased antibody production (27,28). However, the exact relationship between these changes still need further in vitro sorting and Tfh17 and B cell coculture experiment validation.
In contrast to cTfh cells, cTfr cells prevent the production of alloreactive B cells (29-31). The loss of peripheral blood cTfr cell function has also been etiologically linked to autoimmune disease (32). Consistent with this, we found a negative correlation between peripheral blood cTfr cells and cTfh cells, suggesting that cTfr cells may be important regulators of cTfh cell levels, not only in the GC, but also in the peripheral blood. Meanwhile, for the HC and Pre group, we also witnessed cTfr/cTfh negatively correlated with cTfh/CD4+ T cells (data not shown), which further illustrated no matter in the no heart transplant status but also in the transplant status, cTfr might also participate in the process of cTfh response. However, further in vitro coculture experiment of cTfh and cTfr are needed to provide direct evidence of cTfr regulation on cTfh. Under normal conditions, cTfh cells secrete IL-21 (33,34), which promotes plasma cell formation in both humans and mice (35,36). In this study, we found that IL-21 mRNA levels in both PBMCs and plasma were up-regulated in chronic-phase recipients after heart transplantation. This finding suggests that IL-21 may play a vital role in cTfh cell-mediated immune injury. However, the downstream effectors of IL-21 have yet to be elucidated. CXCL13, a ligand for CXCR5, plays an important role in promoting the migration of cTfh and B cells into lymph nodes (37,38). The concentration of plasma CXCL13 in the 1-year group was significantly higher than that in the HC and Pre groups, suggesting that lymphocytes or peripheral immune cells secreted more CXCL13 in chronic-phase recipients after heart transplantation. An increase in plasma CXCL13 often results in a decreased proportion of cTfh cells among CD4+ T cells by promoting cTfh cells to migrate to the lymphoid organs (13). However, in the 1-year group, we found an increase in both circulating cTfh cells and CXCL13 levels, indicating that cTfh cells were strongly mobilized. In the future, we could block CXCL13-CXCR5 axis in vitro culture experiment to further test its functions on Tfh mobilization and recruiting.
The implication of antibody-mediated rejection (AMR), mediated by B cells in graft vascular disease caused by chronic immune injury after transplantation, has been well documented (39). Also, cTfh cells have been shown to play an important role in AMR development (40). In this study, we co-cultured cTfh and mB cells collected from HC peripheral blood, and found that cTfh cells promoted mB cell differentiation into plasmablasts. Our findings support the notion that the functional relationship between T and B cells potentially contributes to AMR development. In this study, we also unveiled a significant correlation between the number of plasmablasts and the activation and proliferative ability of cTfh cells, and that the cTfh cell proliferative capacity was related to the proportion of transitional B cells no matter in the 1 year group but also in the HC and Pre group. We speculate that cTfh cells not only directly promote B cell maturation, but also secrete cytokines to activate B cells localized in lymphoid organs to mediate humoral immune responses. Through GC responses, memory B cells and plasmablasts are produced, and the decreased percentage of plasmablasts may reflect their migration to the bone marrow for long-term antibody production. The higher percentage of class-switched memory B cells is in accordance with the elevated cTfh cell proportions. In addition, conversion of cTfh subtype from cTfh1 to cTfh17 may be linked with IgG3 secretion from plasmablasts.
We also examined the potential correlation between CAV (which is the major complication during chronic immune injury of allografts) and changes in the cTfh subtypes, but did not observe any definite correlations. Thus, our observations are not consistent with those of previous reports (41). This discrepancy may due to the small sample size of our study, or other immune cells or clinical factors affecting the number of cTfh cells. Hence, the correlation between the cTfh subtypes and CAV in chronic-phase recipients after heart transplantation needs to be further clarified in future studies. Also, due to the lack of enough re-heart transplant CAV tissues, whether Tfh could directly infiltrated the tissue remains to be evaluated by myocardium digestion and flow analysis.
Conclusions
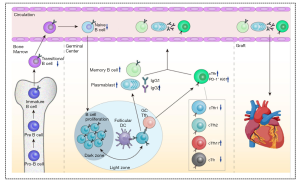
All the phenomenon is summarized in the Figure 7. We demonstrated that chronic-phase recipients after heart transplantation had increased proportions of CD4+CXCR5+ and CD4+CXCR5+PD-1+ cTfh cells among CD4+ T cells, with a subtype conversion observed from cTfh1 to cTfh17. The increased levels of cTfh cells were positively correlated with the activation and transformation of B cells to plasmablasts and class-switched memory B cells, as well as greater IgG production. cTfh-related soluble factors (chemokines and cytokines) such as CXCL13 and IL-21 may also participate in the immunopathogenesis of chronic immune injury. Thus, our study suggests that cTfh cells are an important factor in the long-term immune rejection observed in chronic-phase recipients after heart transplantation.
Acknowledgments
This project is attributed to the Department of Cardiovascular Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology.
Funding: This work was supported by the National Key Research and Development Program (No. 2016YFA0101100) and the National Natural Science Foundation of China (81700339, 82001701).
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-3027
Peer Review File: Available at http://dx.doi.org/10.21037/atm-20-3027
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-3027). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The protocol for the research project has been approved by the Ethics Committee of Tongji Medical College of Huazhong University of Science and Technology (IORG No: IORG0003571) and conforms to the provisions of the Helsinki Declaration as revised in 2013. Informed consent was obtained from all patients and healthy control (HC) subjects.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lund LH, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-second Official Adult Heart Transplantation Report--2015; Focus Theme: Early Graft Failure. J Heart Lung Transplant 2015;34:1244-54. [Crossref] [PubMed]
- Costello JP, Mohanakumar T, Nath DS. Mechanisms of chronic cardiac allograft rejection. Tex Heart Inst J 2013;40:395-9. [PubMed]
- Franchimont D. Overview of the actions of glucocorticoids on the immune response: a good model to characterize new pathways of immunosuppression for new treatment strategies. Ann N Y Acad Sci 2004;1024:124-37. [Crossref] [PubMed]
- Lindenfeld J, Page RL, Zolty R, et al. Drug therapy in the heart transplant recipient: Part III: common medical problems. Circulation 2005;111:113-7. [Crossref] [PubMed]
- Meier-Kriesche HU, Li S, Gruessner RW, et al. Immunosuppression: evolution in practice and trends, 1994-2004. Am J Transplant 2006;6:1111-31. [Crossref] [PubMed]
- Cruzado JM, Bestard O, Grinyo JM. New immunosuppressive protocols with the advent of novel biological drugs. Transplantation 2009;88:S20-3. [Crossref] [PubMed]
- Wedel J, Bruneau S, Kochupurakkal N, et al. Chronic allograft rejection: a fresh look. Curr Opin Organ Transplant 2015;20:13-20. [Crossref] [PubMed]
- Schaerli P, Willimann K, Lang AB, et al. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med 2000;192:1553-62. [Crossref] [PubMed]
- Breitfeld D, Ohl L, Kremmer E, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med 2000;192:1545-52. [Crossref] [PubMed]
- Kim CH, Rott LS, Clark-Lewis I, et al. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J Exp Med 2001;193:1373-81. [Crossref] [PubMed]
- Simpson N, Gatenby PA, Wilson A, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum 2010;62:234-44. [Crossref] [PubMed]
- de Graav GN, Dieterich M, Hesselink DA, et al. Follicular T helper cells and humoral reactivity in kidney transplant patients. Clin Exp Immunol 2015;180:329-40. [Crossref] [PubMed]
- Forcade E, Kim HT, Cutler C, et al. Circulating T follicular helper cells with increased function during chronic graft-versus-host disease. Blood 2016;127:2489-97. [Crossref] [PubMed]
- Im SJ, Hashimoto M, Gerner MY, et al. Defining cd8+ t cells that provide the proliferative burst after pd-1 therapy. Nature 2016;537:417-21. [Crossref] [PubMed]
- Billingham ME, Cary NR, Hammond ME, et al. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. The International Society for Heart Transplantation. J Heart Transplant 1990;9:587-93. [PubMed]
- Mehra MR, Crespo-Leiro MG, Dipchand A, et al. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J Heart Lung Transplant 2010;29:717-27. [Crossref] [PubMed]
- Ueno H, Banchereau J, Vinuesa CG. Pathophysiology of T follicular helper cells in humans and mice. Nat Immunol 2015;16:142-52. [Crossref] [PubMed]
- Akiyoshi T, Hirohashi T, Alessandrini A, et al. Role of complement and NK cells in antibody mediated rejection. Hum Immunol 2012;73:1226-32. [Crossref] [PubMed]
- Bamford A, Hart M, Lyall H, et al. The influence of paediatric HIV infection on circulating B cell subsets and CXCR5(+) T helper cells. Clin Exp Immunol 2015;181:110-7. [Crossref] [PubMed]
- Pober JS, Jane-wit D, Qin L, et al. Interacting mechanisms in the pathogenesis of cardiac allograft vasculopathy. Arterioscler Thromb Vasc Biol 2014;34:1609-14. [Crossref] [PubMed]
- Sawinski D, Trofe-Clark J, Leas B, S, et al. Calcineurin inhibitor minimization, conversion, withdrawal, and avoidance strategies in renal transplantation: a systematic review and meta-analysis. Am J Transplant 2016;16:2117-38. [Crossref] [PubMed]
- Jiang S, Herrera O, Lechler RI. New spectrum of allorecognition pathways: implications for graft rejection and transplantation tolerance. Curr Opin Immunol 2004;16:550-7. [Crossref] [PubMed]
- De Bruyne R, Gevaert P, Van WM, et al. Raised immunoglobulin A and circulating T follicular helper cells are linked to the development of food allergy in paediatric liver transplant patients. Clin Exp Allergy 2015;45:1060-70. [Crossref] [PubMed]
- Szabó K, Papp G, Szanto A, et al. A comprehensive investigation on the distribution of circulating follicular T helper cells and B cell subsets in primary Sjogren's syndrome and systemic lupus erythematosus. Clin Exp Immunol 2016;183:76-89. [Crossref] [PubMed]
- Bentebibel SE, Lopez S, Obermoser G, et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med 2013;5:176ra32. [Crossref] [PubMed]
- Locci M, Havenar-Daughton C, Landais E, et al. Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity 2013;39:758-69. [Crossref] [PubMed]
- Morita R, Schmitt N, Bentebibel SE, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 2011;34:108-21. [Crossref] [PubMed]
- Boswell KL, Paris R, Boritz E, et al. Loss of circulating CD4 T cells with B cell helper function during chronic HIV infection. PLoS Pathog 2014;10:e1003853. [Crossref] [PubMed]
- Linterman MA, Pierson W, Lee SK, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med 2011;17:975-82. [Crossref] [PubMed]
- Chung Y, Tanaka S, Chu F, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med 2011;17:983-8. [Crossref] [PubMed]
- Wollenberg I, Agua-Doce A, Hernandez A, et al. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J Immunol 2011;187:4553-60. [Crossref] [PubMed]
- Dhaeze T, Peelen E, Hombrouck A, et al. Circulating Follicular Regulatory T Cells Are Defective in Multiple Sclerosis. J Immunol 2015;195:832-40. [Crossref] [PubMed]
- Nurieva RI, Chung Y, Hwang D, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 2008;29:138-49. [Crossref] [PubMed]
- Chtanova T, Tangye SG, Newton R, et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol 2004;173:68-78. [Crossref] [PubMed]
- Bryant VL, Ma CS, Avery DT, et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol 2007;179:8180-90. [Crossref] [PubMed]
- Kuchen S, Robbins R, Sims GP, et al. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T cell-B cell collaboration. J Immunol 2007;179:5886-96. [Crossref] [PubMed]
- Legler DF, Loetscher M, Roos RS, et al. B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J Exp Med 1998;187:655-60. [Crossref] [PubMed]
- Schiffer L, Kumpers P, Davalos-Misslitz AM, et al. B-cell-attracting chemokine CXCL13 as a marker of disease activity and renal involvement in systemic lupus erythematosus (SLE). Nephrol Dial Transplant 2009;24:3708-12. [Crossref] [PubMed]
- Loupy A, Toquet C, Rouvier P, et al. Late failing heart allografts: pathology of cardiac allograft vasculopathy and association with antibody-mediated rejection. Am J Transplant 2016;16:111-20. [Crossref] [PubMed]
- Chen W, Bai J, Huang H, et al. Low proportion of follicular regulatory T cell in renal transplant patients with chronic antibody-mediated rejection. Sci Rep 2017;7:1322. [Crossref] [PubMed]
- Roldán C, Mirabet S, Cecilia C, et al. CD4+CD45RO+CD25-/lowCD127+: CD4+CD45RO+CD25hiCD127-/low ratio in peripheral blood: a useful biomarker to detect cardiac allograft vasculopathy in heart transplanted patients. Transplantation 2015;99:1521-8. [PubMed]


