
This article has an erratum available at: http://dx.doi.org/10.21037/atm-2021-2 the article has been update on 2021-02-01 at here.
Cerebrotendinous xanthomatosis with peripheral neuropathy: a clinical and neurophysiological study in Chinese population
Introduction
Cerebrotendinous xanthomatosis (CTX), is a rare autosomal-recessive disorder of bile acid metabolism, caused by an inborn deficiency of the mitochondrial sterol 27-hydroxylase encoded by CYP27A1 gene (OMIM *606530) (1). The lack of sterol 27-hydroxylase blocks the pathway from chwolesterol to chenodeoxycholic acid (CDCA), with diversion then to cholestenol, resulting in accumulation of cholesterol and cholestenol in tissues throughout the body, particularly in the central nervous system (CNS), lens, and Achilles tendons (2,3).
Clinically, the hallmarks of CTX include infant-onset chronic intractable diarrhea, juvenile-onset bilateral cataracts, tendinous xanthomas, and progressive neurologic dysfunction characterized by pyramidal signs, cerebral ataxia, cognitive impairment and peripheral neuropathy (4,5). Neonatal CTX was easily overlooked and often had a worse prognosis (6,7). Diagnosis was often made in adults or teenagers when typical clinical spectrum was fully developed with a long diagnostic delay (8). Elevated plasma cholestanol aid in diagnosis but was not available to detect in China (9). Treatment with CDCA (750 mg/d) normalizes the level of the bile acid precursor, helps maintain cholesterol homeostasis and improves neurological and non-neurological symptoms (10,11).
Peripheral neuropathy was a frequently reported feature of CTX from sporadic cases and series determined by EMG or biopsy. However, there remains controversies regarding whether neuropathy in CTX is demyelinating or axonal in origin. The most prominent neuromuscular feature documented in Dutch CTX patients was sensory motor polyneuropathy and muscle was not specifically involved (12). As for Chinese CTX cases, the neuropathological and electrophysiological features were discussed rarely but seemed to reflect a potential different profile from foreign cases.
In China, CTX was likely far under-diagnosed based on a recent epidemiological study and the enormous population (6). Poor recognition and unavailable cholestenol detection might be the cause. Considering the treatable nature and potential high incidence, we reported our own cases to broaden clinical and genetic spectrum of CTX, and reviewed literature to discuss CTX-related peripheral neuropathy in China. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-2746).
Methods
Subjects and clinical evaluation
Our six CTX patients were diagnosed from January 2018 to December 2019. Their medical records were carefully reviewed. Brain and Achilles’s tendon magnetic resonance imaging (MRI), laboratory tests, electromyography (EMG) analysis results were collected. Subjects were followed up every 6 months by routine appointment in out-patient clinic. For patient lost follow-up, information before the last follow-up was used. The diagnostic criteria in this study included clinical symptoms, genetic analysis and exclude differential diagnosis, adapted by the criteria previously established by a Japanese nationwide survey (13). The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Qilu Hospital, Shandong University (No. KYLL-2016-229). Written informed consent was obtained from the patient for publication of this study and any accompanying images.
Pathological examination
Right sural nerve biopsy was performed in 3 patients. The stained frozen and semithin sections were investigated under light microscopy and electron microscopy individually.
Gene analysis
The mutations in the CYP27A1 gene using PCR and DNA sequencing were detected in all patients’ blood samples (Running-Gene, Beijing, China). Conformational studies and familial segregation analysis were commonly performed.
Literature review
To delineate features of peripheral neuropathy in Chinese CTX cases, we reviewed all published cases and series study in Chinese population from PubMed during 2002 to 2020. Together 21 genetic proven cases with detailed data of EMG findings were collected and summarized in this study (including our cases) (14-22). For each case reported, we documented the clinical symptoms, EMG profiles, genetic data and nerve pathological descriptions if information were available.
Statistical analysis
Statistical collating and analysis were performed using SPSS 23.0 (SPSS Inc., Armonk, NY, USA). Clinical data of each category were listed directly and proportions of each category were also displayed.
Results
Clinical features
All 6 patients came from different non-consanguineous families. There were 5 males and 1 female patients. Their detailed clinical information was listed in Table 1. Only Patient 3 had positive family history, whose sister presented the same symptoms as him. All patients were diagnosed in their 20s or 30s with a diagnose delay ranging from 10 to 27 years. Four patients (66.67%) presented chronic diarrhea since early childhood as initial symptoms. Lower limbs weakness and mental retardation was first manifestation of the other 2 cases. Five patients developed bilateral cataracts before 15 years old and 3 of them underwent bilateral cataract extraction. Pes cavus, a commonly hereditary sign, was found in 5 patients. Patient 4 had undergone orthomorphia for pes cavus three times. He also suffered frequent bone fracture because of unsteady gait and osteoporosis. Achilles’s tendon xanthomas were disclosed in only 3 patients. The presence of characteristic xanthomas seemed not related to the severity of clinical symptoms. For example, Patient 2 with typical tendon xanthomas showed slight neurological dysfunction while Patient 3 presented typical CTX manifestations like mental decline, pyramidal signs, ataxia and severe neuropathy without any xanthomas. Furthermore, Patients 4 and 6 developed gallstones and related abdominal pain, the latter underwent cholecystectomy. All patients exhibited cognitive decline confirmed by decreased MMSE scores. Spastic paraplegia and cerebellar ataxia were frequently found in 5 patients. Only 2 patients presented epilepsy. Three patients developed bulbar palsy. Symptoms of patient 1 were aggravated after puberty at 25 years old. All but patient 2 showed an elevated blood total bilirubin and no patients harbored hypercholesterolemia.
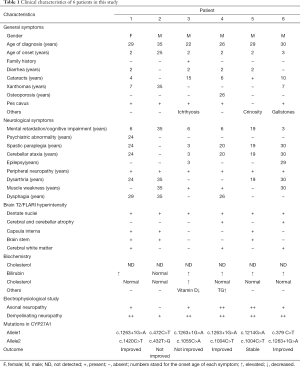
Full table
Neuroimaging findings
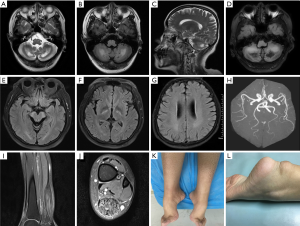
All 6 patients underwent brain MRI illustrated by Figure 1. The main abnormal findings included bilateral symmetrical hyperintensity lesions of bilateral dentate nucleus, cerebral peduncle and posterior limb of the internal capsule on T2W and FLAIR imaging. Atrophy of the cerebrum and cerebellum can also be noticed, generally mild or moderate. Patients 1, 4, 5 showed some decreased signal on T1W/FLAIR in bilateral cerebellar hemisphere. Brain MRA in Patient 1 confirmed slight premature atherosclerosis despite normal serum cholesterol concentrations. Three patients (Patients 1, 2 and 6) evaluated ankle MRI and showed fusiform thickening and heterogeneous signals in the Achilles tendon.
Electrophysiological and pathological findings
Electrophysiological study revealed motor or motor sensory demyelinating predominant polyneuropathy in all 6 cases. Decreased nerve conduction velocity (NCV) and prolonged F response latencies were prominent features, particularly in lower limbs’ motor nerves, indicating a length dependent pattern. Slowed motor NCV below 75% normal level and prolonged distal latencies over 130% indicated a conspicuous primary demyelinating process. In Patient 4 and 5, no motor or sensory conduction could be elicited in lower limbs. In some patients, length dependent axonal involvement was also existed, characterized by a decreased amplitude of sensory and motor nerves. As for needle EMG, Patients 1, 4, 5 disclosed evidence of moderate chronic neurogenic injury, characterized by decreased recruitment patterns, increased polyphasic waves, prolonged duration and giant amplitude of motor unit action potentials (MUAPs).
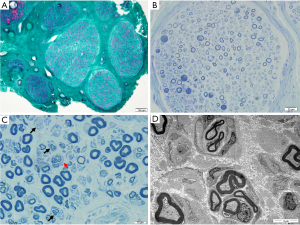
Three patients underwent sural nerve biopsy with confirmed peripheral neuropathy (Table 2 and Figure 2). Nerve biopsy of patient 2 showed slightly loss of myelinated fibers, about 10%. Some fibers with relatively thick myelin sheaths can be seen, exhibiting massive red in MGT staining (Figure 2A). In Patient 3, semithin sections showed a decreased density of large myelinating fibers by about 20%. Extensive onion bulbs were formed around demyelinated fibers, suggesting a chronic process of demyelination and remyelination. Regeneration cluster of small myelinated fibers (sprouting) can be frequently seen (Figure 2C,D). As for Patient 4, large-diameter, myelinated fibers were markedly decreased by about 40%. Some fibers presented thin myelin sheath. Semithin section revealed evidence of acute axonal degeneration with myelin bodies and chronic degeneration with clusters of regenerating fibers (Figure 2B). Onion bulb was not apparent. In accordance with electrophysiological data, sural nerve biopsy showed histological features of demyelinating and axonal peripheral neuropathy, with myelin sheath more seriously involved in Patient 3 and axon also seriously damaged in Patient 4. Electron microscopy confirmed onion bulbs formation in Patient 3.
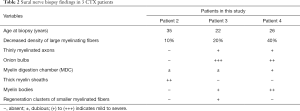
Full table
Molecular genetic analysis
The gene sequencing of all 6 patients revealed 8 variants in CYP27A1 gene, including 3 novel mutations (Table 3). Patient 1,4,5 and 6 identified 5 reported pathogenic mutations and confirmed the diagnosis, including. c.1263+1G>A, c.1420C>T, c.1004C>T, c.1214 G>A and c.379C>T in CYP27A1 (9,23-27). Among these detected variants, c.1263+1G>A seemed to be a very hot variant since 4 of our patients had detected this allele.

Full table
Patient 2 was identified compound heterozygous of novel mutations c.432 T>C (p.Y144X) and c.472 C>T (p.R158C). c.432 T>C (Y144X) generated a premature termination codon and caused protein truncation or nonsense-mediated mRNA decay. It was classified as likely pathogenic according to ACMG guidelines, although registered as a likely benign variant in ClinVar database. c.472 C>T (p.R158C) was a missense mutation located outside the functional domain interacting with two protein cofactors of sterol 27-hydroxylase. However, p.R158 residue was well-conserved in many species and substitution of this residue by a novel cysteine was predicted to alter the structure of the enzyme. It was predicted as deleterious according to PolyPhen-2 (score: 1.000) and disease causing in Mutation taster with the probability of >0.99. These two novel mutations were likely to cause CTX when compound heterozygous.
Patient 3 carried compound heterozygous of a novel mutation c.1055C>A (p.S352X) and a reported variant c.1263+1G>A. The former was a nonsense mutation predicted as disease causing according to mutation taster. The region p.Ser352 was located very closed to the adrenodoxin cofactor binding site (residues 384–398) and the premature stop codon resulted in truncated protein and further loss of the functional domain (28). This allele was not found in ClinVar database. As nonsense mutations were often pathogenic, it was considered to cause disease when in combination with another severe deficiency allele like c.1263+1G>A.
Follow-up
All patients were advised to initiate CDCA at a dose of 750 mg/d as well as low-cholesterol diet after diagnosis. Since CDCA was not so available in China, Patient 2 used UDCA and atorvastatin instead (Table 4). Treatment duration was at least half a year and patients were followed up for 2 years. Data was missing in Patient 2 after 1-year follow-up. Even though 4 patients with good adherence of CDCA relieved some of the symptoms or remained clinically stable, some symptoms continued progressing chronically. No adverse effect was observed. Patient 1 showed improvement in slow responses, stagger gait and psychiatric symptoms after 6 months. But she became occasionally choking and complained lower limb weakness at 2-year follow-up. Patient 2 still presented slow response and memory loss, and developed mild dysphagia and dysarthria under UDCA and atorvastatin. Patient 3 remained clinical stable in gait imbalance and cognitive impairment. His diarrhea relieved, weight gained and speech became more understood. Patient 4 also showed decreased diarrhea, weight gain and energetic feeling at one-year follow-up. Unfortunately, he continued to deteriorate in ataxia and suffered accidental falls. Muscle atrophy in lower limbs, dysphagia and tremor were newly developed symptoms. Patient 5 took CDCA for a short duration due to poor adherence. Unsteady gait continued to decline. Patient 6 improved his ataxia gait and recognition function (MMSE 8 to 11) after taking CDCA for a year. Evaluated modified Rankin Scale (mRS) at last follow-up were unchanged in 3 patients, decreased in 1 patient, and increased in 2 patients. It seemed CDCA had a limited effect once neurological symptoms were fully established since it could not prevent neurological progression especially in bulbar palsy. Unfortunately, none of our cases reevaluated NCV/EMG.
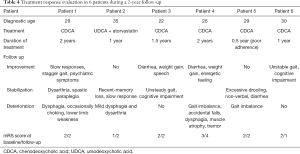
Full table
Literature review of CTX patients with peripheral neuropathy in China
To achieve a better understanding of CTX-related peripheral neuropathy, we reviewed literature and summarize 21 cases from PubMed, including our cases. Their clinical data and description of peripheral neuropathy were analyzed (Table 5). Demyelinating dominant peripheral neuropathy was found in 10 cases, axonal in 3 cases and mixed axonal and demyelinating polyneuropathy in 3 cases. Four cases reported by Chen (14) showed normal NCV and case reported by Gao (20) didn’t mention the specific type of peripheral neuropathy. Overall, 4 cases were motor predominant and 12 cases were sensorimotor. Thirteen cases showed a length dependent pattern with distal part more seriously involved. The profile of neuropathy features seemed not related to the genotype, disease duration or onset age. In Chinese patients, demyelinated polyneuropathy in a length dependent pattern was a prominent feature of CTX-related neuropathy. Mild NCV abnormal tended to be demyelinated changes like slowed conduction velocity and prolonged distal latency, and once the neuropathy fully developed, severe demyelinating as well as axonal changes often coexisted, leading absence response in the lower limbs.
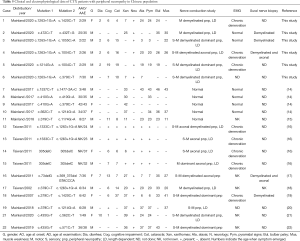
Full table
Discussion
CTX was once considered as an exceedingly rare hereditary disease with an estimated rate of less than 5/100,000 worldwide (29), but seemed far under-diagnosed based on a recent epidemiological study (3). The great heterogeneity of clinical spectrum and long period between symptoms emerged made early diagnosis a challenge. Based on our patients, xanthomas was not an indispensable sign because patients with typical neurological disorders could be in absence of tendon mass. Another indicative symptom was early-onset diarrhea. It seemed more frequently reported by Caucasian than Chinese population in a rate of 92% vs. 3.8% (22). However, our cases showed a relatively high proportion of chronic diarrhea (4/6), which could be relieved by long-term administration of CDCA. This discrepancy might result from genetic background differences, recall bias or inadequate medical history collecting. Furthermore, pes cavus was very common in some hereditary status and should be carefully examined in CTX. In addition, many CTX patients underwent cataract surgery before reaching the final diagnosis, providing ophthalmologists an opportunity to aid in early diagnosis. It was estimated that children with bilateral cataracts showed an astonishing prevalence of CTX of 1.8% (30,31). Failure to realize those non-neurological symptoms were part of a broad clinical spectrum of an underlying metabolic disorder was a main cause of diagnostic delay. It is important for clinicians from different specialties to create great awareness of and better screening for CTX. Early identification strategy described by Duell was helpful in judging the diagnosis (32).
Cognitive impairment, pyramidal signs, cerebral ataxia and peripheral neuropathy were prominent neurological disorder. As disease progressed, dysphagia, dysarthria, ambulatory difficulties and muscle weakness continue to aggravate, and finally lead to total dependence or even death, to some extent very similar to end-stage ALS (amyotrophic lateral sclerosis). Coincidentally, CYP27A1 had been identified as a possible candidate gene for ALS in a large and comprehensive genome-wide screening (33). It seemed plausible that CYP27A1 deficiency causes a reduction of liver X receptor (LXR) ligands and a decrease of motor neuron (MN) survival signaling (33-38). Specific cholestenoic acids might act as potential MN protectors and neuroinflammation modulators, shedding light on the management of neurodegenerative disease. In our cases, CDCA can alleviate some of neurological and non-neurological symptoms but the effect was somehow limited. Early diagnosis is important because initiating supplement of CDCA at asymptomatic stage could prevent disease progression (5). Treatment after 25 years old had worse outcome and limited improvement in ambulation and cognition (32). Hopefully, measures aiding in early diagnosis were in sight. For example, dried blood spot (DBS) screening assay excellently distinguished newborn CTX patients based on different ratio between cholestanetetrol glucuronide (tetrol) and taurochenodeoxycholic acid (t-CDCA) (39). Prominence of sulfated and sulfated/glucuronidated BAlc conjugates in urine is new biomarker facilitating rapid diagnosis in infancy (40).
Brain MRI showed extremely high diagnostic value for CTX. Hyperintensity in dentate nuclei was probably the initial result of abnormal lipid storage, while hyposignal in T2FLAIR indicated vacuolation and calcification caused by cholestanol-induced apoptosis, predominantly in the late stage (41,42). Diverse involvement of cerebellum indicated different state of natural disease progression, which started in the dentate nucleus and then extended to the surrounding white matter (43). Patient 1,4 and 5 in our series showed dentate hypointensity changes, and they all developed to bulbar palsy—a clinically advanced sign, consisting with the above hypothesis. In some cases, diffuse or focal hyperintense ischemic-like lesions (T2W/T2FLAIR) were found in subcortical and periventricular white matter, reflecting a process of demyelination or gliosis in the CNS (43). Premature atherosclerosis detected by MRA might be a result of reduced reverse cholesterol transport medicated by CYP27A1 deficiency.
In the literature, etiology of peripheral neuropathy in CTX was a matter of debate. Reported CTX cases utilized different methods-NCV/EMG/or nerve biopsy/or muscle biopsy to confirm and describe different type of neuropathy. Some authors believed the peripheral neurology was demyelinating in nature with evidence of recurrent demyelination and remyelination (44-46). Others considered length-dependent axonal neuropathy as the major pathological abnormality (12,47-49). However, large published case series and literature review tend to support the latter point of view. For example, a study from Netherlands included 58 patients and established axonal neuropathy in the majority of patients in whom an EMG was performed (5). A research from Italy identified polyneuropathy in 26 CTX patients and 76.9% of them was predominantly axonal (38.4% sensory-motor axonal and 38.4% motor axonal) (50). And another study in which 10 patients were observed through EMG and sural nerve biopsies found that the most prominent neuromuscular abnormality was sensorimotor axonal polyneuropathy (12). Furthermore, researchers from Taiwan focused on CTX-related neuropathy and concluded axonal degeneration was the most common type (16). In contrast, demyelinating dominant neuropathy was mostly reported by sporadic cases or small series. In this study, CTX-related neuropathy from 21 Chinese cases exhibited a different picture. Our study results were consistent with those of Pilo et al. (51) that sensorimotor demyelinating neuropathy was the prominent feature. When lesions of peripheral neuropathy were relatively slight, motor nerves were more vulnerable to demyelinate in a length dependent pattern. Axonal and demyelinating degeneration in sensory and motor nerves often coexisted in severe cases.
It is proposed that polyneuropathy in CTX can be divided into 3 types: axonal, demyelinated and mixed polyneuropathy (19). In our cases, patient 2 and 3 belonged to the 2nd type. Patient 4 showed both myelin and axon affected and pertained to be the 3rd type. Another 2 Chinese cases undergone nerve biopsy showed demyelinated and mixed polyneuropathy respectively (17,19). No genotype and neuropathy type relationship was found. The mechanism of divergence remains unclear. Several reasons need to be considered. First, sample size was relatively small compared to broad case series. Second, different genetic background between mainland China and Taiwanese and Caucasian populations might be a reason. In addition, pathophysiological mechanism underlies the development of neuropathy. Brimming with cholesterol, myelin sheath insulates and facilitates axonal conduction but also becomes vulnerable in this sterol metabolic disorder. Replacing large amounts of cholesterol with cholestanol that have different structure and electrical conductivity would conceivably have significant effects upon function of myelin sheath. As cerebral white matter was primarily involved in CNS (42,52), we considered it plausible to include a demyelinating process in peripheral neuropathy. Besides, CYP27A1 is a mitochondrial enzyme related to energy metabolism. Like other metabolic disorders, CTX has features of distal, sensorimotor axonal polyneuropathy. Either axons or myelin involved predominantly varies as disease progress, exhibiting different types of polyneuropathy. Also, inter-practitioner inconsistency in performing and analyzing NCV/EMG data should also be taken into consideration. Further studies with large sample size are needed.
There are several shortcomings in our study. First, reported CTX cases might be subjected to recall bias. The variation of inter-practitioner must relate to differences in NCV test performance. Secondly, the present 6 cases have not detected serum cholestenol level because this test was not available at present in China. Thirdly, no functional verification of the three novel mutations was done. Further research and follow-up studies are needed to conform these results.
Conclusions
Patients presented with cognitive decline, spastic paraplegia, cerebellar ataxia should be suspicious of CTX, especially accompanied with xanthomas, cataract, diarrhea and pes cavus. Dentate nuclei hyperintensity is a valuable MRI hallmark. CTX-related peripheral neuropathy was predominant sensorimotor demyelinating type, with an evident length dependent pattern and increased vulnerability in motor nerves. Demyelinating and axonal degeneration tend to coexist in severe neuropathy. Three novel mutations c.1055C>A, c.432T>G, c.472T>G are detected and predicted pathogenic, providing new data for mutation spectrum and molecular diagnosis of CTX. Oral CDCA therapy early on can provide clinical stability and may also ameliorate existing symptoms, but it could not cease disease progression completely.
Acknowledgments
We deeply thank our patients and their family for their cooperation and participation. We also thank physicians Dong Zhang, Kexu Sui and Kai Shao who devoted in performing the electrophysiological tests and providing help for the complicated pathological examinations.
Funding: The study was supported by grants from the Youth Program of National Natural Science Foundation of China (grant number 8170050970).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-2746
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-2746
Peer Review File: Available at http://dx.doi.org/10.21037/atm-20-2746
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-2746). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Qilu Hospital, Shandong University (No. KYLL-2016-229). Written informed consent was obtained from the patient for publication of this study and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cali JJ, Hsieh CL, Francke U, et al. Mutations in the bile acid biosynthetic enzyme sterol 27-hydroxylase underlie cerebrotendinous xanthomatosis. J Biol Chem 1991;266:7779-83. [PubMed]
- Panzenboeck U, Andersson U, Hansson M, et al. On the mechanism of cerebral accumulation of cholestanol in patients with cerebrotendinous xanthomatosis. J Lipid Res 2007;48:1167-74. [Crossref] [PubMed]
- Nie S, Chen G, Cao X, et al. Cerebrotendinous xanthomatosis: a comprehensive review of pathogenesis, clinical manifestations, diagnosis, and management. Orphanet J Rare Dis 2014;9:179. [Crossref] [PubMed]
- Bhattacharyya AK, Lin DS, Connor WE. Cholestanol metabolism in patients with cerebrotendinous xanthomatosis: absorption, turnover, and tissue deposition. J Lipid Res 2007;48:185-92. [Crossref] [PubMed]
- Verrips A, Hoefsloot LH, Steenbergen GC, et al. Clinical and molecular genetic characteristics of patients with cerebrotendinous xanthomatosis. Brain 2000;123:908-19. [Crossref] [PubMed]
- Lee MH, Hazard S, Carpten JD, et al. Fine-mapping, mutation analyses, and structural mapping of cerebrotendinous xanthomatosis in U.S. pedigrees. J Lipid Res 2001;42:159-69. [PubMed]
- Zubarioglu T, Kiykim E, Yesil G, et al. Early diagnosed cerebrotendinous xanthomatosis patients: clinical, neuroradiological characteristics and therapy results of a single center from Turkey. Acta Neurol Belg 2019;119:343-50. [Crossref] [PubMed]
- Gong JY, Setchell KDR, Zhao J, et al. Severe neonatal cholestasis in cerebrotendinous xanthomatosis: genetics, immunostaining, mass spectrometry. J Pediatr Gastroenterol Nutr 2017;65:561-8. [Crossref] [PubMed]
- Sekijima Y, Koyama S, Yoshinaga T, et al. Nationwide survey on cerebrotendinous xanthomatosis in Japan. J Hum Genet 2018;63:271-80. [Crossref] [PubMed]
- Björkhem I. Cerebrotendinous xanthomatosis. Curr Opin Lipidol 2013;24:283-7. [Crossref] [PubMed]
- Berginer VM, Salen G, Shefer S. Long-term treatment of cerebrotendinous xanthomatosis with chenodeoxycholic acid. N Engl J Med 1984;311:1649-52. [Crossref] [PubMed]
- Verrips A, van Engelen BG, ter Laak H, et al. Cerebrotendinous xanthomatosis. Controversies about nerve and muscle: observations in ten patients. Neuromuscul Disord 2000;10:407-14. [Crossref] [PubMed]
- Chen Q, Liu W, Jiang B, et al. Fluoxetine-responsive depression in a Chinese cerebrotendinous xanthomatosis. Gen Hosp Psychiatry 2012;34:578.e1-4. [Crossref] [PubMed]
- Chen C, Zhang Y, Wu H, et al. Clinical and molecular genetic features of cerebrotendinous xanthomatosis patients in Chinese families. Metab Brain Dis 2017;32:1609-18. [Crossref] [PubMed]
- Zhang L, Zhang L, Nian N, et al. Analysis of a cerebrotendinous xanthomatosis case with mental retardation as the initial symptom. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2016;33:476-80. [PubMed]
- Chen SF, Tsai NW, Chang CC, et al. Neuromuscular abnormality and autonomic dysfunction in patients with cerebrotendinous xanthomatosis. BMC Neurol 2011;11:63. [Crossref] [PubMed]
- Tian D, Zhang ZQ. 2 Novel deletions of the sterol 27-hydroxylase gene in a Chinese Family with Cerebrotendinous Xanthomatosis. BMC Neurol 2011;11:130. [Crossref] [PubMed]
- Lee MJ, Huang YC, Sweeney MG, et al. Mutation of the sterol 27-hydroxylase gene (CYP27A1) in a Taiwanese family with cerebrotendinous xanthomatosis. J Neurol 2002;249:1311-2. [Crossref] [PubMed]
- Wang Z, Yuan Y, Zhang W, et al. Cerebrotendinous xanthomatosis with a compound heterozygote mutation and severe polyneuropathy. Neuropathology 2007;27:62-6. [Crossref] [PubMed]
- Gao Y, Chen SW, Ren RJ, et al. Heterozygous-mutation induced cerebrotendinous xanthomatosis and correlation of genotype with clinical phenotype. J Intern Med Concepts Prac 2018;13:296-300.
- Tang Y, Liu Y, Li D, et al. A novel mutation in the CYP27A1 gene in a family with cerebrotendinous xanthomatosis. Int J Neurosci 2020. [Crossref] [PubMed]
- Tao QQ, Zhang Y, Lin HX, et al. Clinical and genetic characteristics of Chinese patients with cerebrotendinous xanthomatosis. Orphanet J Rare Dis 2019;14:282. [Crossref] [PubMed]
- Vanrietvelde F, Lemmerling M, Mespreuve M, et al. MRI of the brain in cerebrotendinous xanthomatosis (van Bogaert-Scherer-Epstein disease). Eur Radiol 2000;10:576-8. [Crossref] [PubMed]
- Garuti R, Lelli N, Barozzini M, et al. Cerebrotendinous xanthomatosis caused by two new mutations of the sterol-27-hydroxylase gene that disrupt mRNA splicing. J Lipid Res 1996;37:1459-67. [PubMed]
- Yoshinaga T, Sekijima Y, Koyama S, et al. Clinical and radiological findings of a cerebrotendinous xanthomatosis patient with a novel p.A335V mutation in the CYP27A1 gene. Intern Med 2014;53:2725-9. [Crossref] [PubMed]
- Suh S, Kim HK, Park HD, et al. Three siblings with Cerebrotendinous Xanthomatosis: a novel mutation in the CYP27A1 gene. Eur J Med Genet 2012;55:71-4. [Crossref] [PubMed]
- Abe R, Sekijima Y, Kinoshita T, et al. Spinal form cerebrotendinous xanthomatosis patient with long spinal cord lesion. J Spinal Cord Med 2016;39:726-9. [Crossref] [PubMed]
- Kim KS, Kubota S, Kuriyama M, et al. Identification of new mutations in sterol 27-hydroxylase gene in Japanese patients with cerebrotendinous xanthomatosis (CTX). J Lipid Res 1994;35:1031-9. [PubMed]
- Moghadasian MH, Salen G, Frohlich JJ, et al. Cerebrotendinous xanthomatosis: a rare disease with diverse manifestations. Arch Neurol 2002;59:527-9. [Crossref] [PubMed]
- Pilo-de-la-Fuente B, Jimenez-Escrig A, Lorenzo JR, et al. Cerebrotendinous xanthomatosis in Spain: clinical, prognostic, and genetic survey. Eur J Neurol 2011;18:1203-11. [Crossref] [PubMed]
- Tibrewal S, Duell PB, DeBarber AE, et al. Cerebrotendinous xanthomatosis: early diagnosis on the basis of juvenile cataracts. J AAPOS 2017;21:505-7. [Crossref] [PubMed]
- Duell PB, Salen G, Eichler FS, et al. Diagnosis, treatment, and clinical outcomes in 43 cases with cerebrotendinous xanthomatosis. J Clin Lipidol 2018;12:1169-78. [Crossref] [PubMed]
- Diekstra FP, Saris CG, van Rheenen W, et al. Mapping of gene expression reveals CYP27A1 as a susceptibility gene for sporadic ALS. PLoS One 2012;7:e35333. [Crossref] [PubMed]
- Babiker A, Andersson O, Lund E, et al. Elimination of cholesterol in macrophages and endothelial cells by the sterol 27-hydroxylase mechanism. Comparison with high density lipoprotein-mediated reverse cholesterol transport. J Biol Chem 1997;272:26253-61. [Crossref] [PubMed]
- Courtney R, Landreth GE. LXR regulation of brain cholesterol: from development to disease. Trends Endocrinol Metab 2016;27:404-14. [Crossref] [PubMed]
- Mouzat K, Raoul C, Polge A, et al. Liver X receptors: from cholesterol regulation to neuroprotection-a new barrier against neurodegeneration in amyotrophic lateral sclerosis? Cell Mol Life Sci 2016;73:3801-8. [Crossref] [PubMed]
- Theofilopoulos S, Griffiths WJ, Crick PJ, et al. Cholestenoic acids regulate motor neuron survival via liver X receptors. J Clin Invest 2014;124:4829-42. [Crossref] [PubMed]
- Abdel-Khalik J, Yutuc E, Crick PJ, et al. Defective cholesterol metabolism in amyotrophic lateral sclerosis. J Lipid Res 2017;58:267-78. [Crossref] [PubMed]
- Vaz FM, Bootsma AH, Kulik W, et al. A newborn screening method for cerebrotendinous xanthomatosis using bile alcohol glucuronides and metabolite ratios. J Lipid Res 2017;58:1002-7. [Crossref] [PubMed]
- Gong JY, Setchell KDR, Zhao J, et al. Severe neonatal cholestasis in cerebrotendinous xanthomatosis: genetics, immunostaining, mass spectrometry. J Pediatr Gastroenterol Nutr 2017;65:561-8. [Crossref] [PubMed]
- Andersson S, Gustafsson N, Warner M, et al. Inactivation of liver X receptor beta leads to adult-onset motor neuron degeneration in male mice. Proc Natl Acad Sci U S A 2005;102:3857-62. [Crossref] [PubMed]
- Mignarri A, Dotti MT, Federico A, et al. The spectrum of magnetic resonance findings in cerebrotendinous xanthomatosis: redefinition and evidence of new markers of disease progression. J Neurol 2017;264:862-74. [Crossref] [PubMed]
- Barkhof F, Verrips A, Wesseling P, et al. Cerebrotendinous xanthomatosis: the spectrum of imaging findings and the correlation with neuropathologic findings. Radiology 2000;217:869-76. [Crossref] [PubMed]
- Dotti MT, Federico A, Signorini E, et al. Cerebrotendinous xanthomatosis (van Bogaert-Scherer-Epstein disease): CT and MR findings. AJNR Am J Neuroradiol 1994;15:1721-6. [PubMed]
- Argov Z, Soffer D, Eisenberg S, et al. Chronic demyelinating peripheral neuropathy in cerebrotendinous xanthomatosis. Ann Neurol 1986;20:89-91. [Crossref] [PubMed]
- Ohnishi A, Yamashita Y, Goto I, et al. De- and remyelination and onion bulb in cerebrotendinous xanthomatosis. Acta Neuropathol 1979;45:43-5. [Crossref] [PubMed]
- Donaghy M, King RH, McKeran RO, et al. Cerebrotendinous xanthomatosis: clinical, electrophysiological and nerve biopsy findings, and response to treatment with chenodeoxycholic acid. J Neurol 1990;237:216-9. [Crossref] [PubMed]
- Pop PH, Joosten E, van Spreeken A, et al. Neuroaxonal pathology of central and peripheral nervous systems in cerebrotendinous xanthomatosis (CTX). Acta Neuropathol 1984;64:259-64. [Crossref] [PubMed]
- Soffer D, Benharroch D, Berginer V. The neuropathology of cerebrotendinous xanthomatosis revisited: a case report and review of the literature. Acta Neuropathol 1995;90:213-20. [Crossref] [PubMed]
- Ginanneschi F, Mignarri A, Mondelli M, et al. Polyneuropathy in cerebrotendinous xanthomatosis and response to treatment with chenodeoxycholic acid. J Neurol 2013;260:268-74. [Crossref] [PubMed]
- Pilo B. Neurophysiological study in cerebrotendinous xanthomatosis. Muscle Nerve 2011;43:531-6. [Crossref] [PubMed]
- Amador MDM, Masingue M, Debs R, et al. Treatment with chenodeoxycholic acid in cerebrotendinous xanthomatosis: clinical, neurophysiological, and quantitative brain structural outcomes. J Inherit Metab Dis 2018;41:799-807. [Crossref] [PubMed]


