
Lipoxin A4 protects rat skin flaps against ischemia-reperfusion injury through inhibiting cell apoptosis and inflammatory response induced by endoplasmic reticulum stress
Introduction
Flap transplantation is the most common, useful, and irreplaceable means for repairing tissue defect and abnormality and organ reconstruction during orthopedic surgery, and the survival of the skin flaps after transplantation is the key to the success of the operation. During the transplantation, the flap is wholly or partly necrosis through ischemia-reperfusion (I/R) injury, which seriously affects the surgical outcome (1,2). Many studies have shown that the I/R injury of skin flap is a complex pathophysiological process involving many cells and many factors (3,4). The specific mechanism has not been fully explained.
Apoptosis is an essential way of cell death after I/R injury associated with the severity of tissue ischemia and the time of reperfusion (5). Apoptotic cells during I/R injury may increase secondary necrosis of the flap tissue. ROS produced during I/R injury can destroy cell DNA and mitochondria, and the lipid peroxidation induced by cell membrane can affect signal transduction (6). Also, protein cross-linking results in the loss of protein function and affects nuclear gene transcription, inducing apoptosis. Calcium overload can also induce apoptosis through causing inactivation of Bcl-2, activating various calcium-dependent endonucleases and neutral proteases, degrading DNA, and mediating the TNF signaling pathway (7,8). Many cytokines, including TNF, IL-6, IL-8, and TGF and growth factors including VEGR, PDGR, EGF, and MCSF, are produced during I/R injury and induce apoptosis (9,10).
Endoplasmic reticulum (ER) is the site of protein synthesis and folding, synthesis of lipids and sterols, and maintenance of calcium homeostasis. Genetic or environmental damage can cause an imbalance of intracellular calcium homeostasis, oxidative stress, nutritional deficiency, glycation inhibition, and protein misfolding, breaking the ER function and inducing ER stress (11,12). Various factors including ischemia and hypoxia can cause the dysfunction of ER and cause ER stress, involved in the multistep process of pathological changes in I/R (13). ER stress induces apoptosis by activating downstream apoptotic factors, including CHOP/GADD153, ASK1/JNK, Caspases, and Bcl-2 (14). ER stress activates inflammatory response through unfolding protein response (UPR), leading to the activation of the NF-κB signaling pathway, which promotes the expression of pro-inflammatory cytokines (15).
Lipoxin A4 (LXA4) is one of the most important members of the Lipoxin family, which are endogenous lipoxygenases derived from eicosanoids and have significant anti-inflammatory and pro-resolution properties (16). It has been proved that LXA4 can protect the brain (17), lung (18), heart (19), kidney (20), stomach (21), and intestine (22) from I/R injury through inhibiting expression of pro-inflammatory factors, ROS production and ER stress apoptotic pathway. However, little is known about the role and underlying mechanisms of LXA4 protects skin flap against I/R injury. In the present study, we use the I/R model in rat skin flaps to investigate the effects of LXA4 and the underlying mechanisms associated with ER stress-induced apoptosis and inflammatory response and provide a promising therapeutic method of skin flap I/R injury. We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-5549).
Methods
Experimental I/R model
The present study was performed in strict accordance with the guidelines on ethical care for experimental animals and approved by the Animal Research Committee of Xiaoshan Traditional Chinese Medical Hospital. A total of fifty-four healthy male Wister rats (10-week-old; 280–300 g) obtained from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China) were in line with the specific pathogen-free (SPF) conditions and housed in groups of three and given five days to acclimate to the housing facility. They have a dark/light cycle of 12/12 hours in 595×380×200 mm cages (Techniplast UK, 1354G Eurostandard Type IV) at a fixed temperature of 22–23 °C with water and food freely. During housing, animals were monitored twice daily for health status. No adverse events were observed. Rats were randomly divided into three groups: sham-operated group (n=18), I/R model group (n=18) and I/R + LXA4 (Haoran Biological Technology CO., LTD., Shanghai, China) group (n=18).
The preparation of abdominal island skin flap, which size was 6 cm × 3 cm, was created according to the method of Petry’s (23). There are no other treatments after creating the skin flap according to the method above in the sham-operated group. In the I/R model group, after the skin flap formed according to the method above, the proximal end of the point that the superficial epigastric artery arose from the femoral artery was occluded by a vascular clamp and the vascular clamp was taken out in another operation after 8 hours as previously described (24). In the I/R + LXA4 group, 24 hours before surgery, intravenous tail injection of rats with LXA4 (100 µg/kg) once every 8 hours and the rest of the operation as the I/R model group of experimental. In each group, a 1.0×0.5×0.2 cm skin flap tissue sample was removed at 12, 24 and 48 hours following the surgical procedure or I/R for following experiments, except for the transmission electron microscope assay, in which a 1.0×2.0×2.0 cm skin flap tissue sample was removed (n=6 per time point).
Histological assessment
When the experiment ended, these rats got anesthetized with overdosed 10% chloral hydrate (450 mg/kg) via intraperitoneal injection before euthanasia by cervical dislocation with no signs of peritonitis being observed. The harvest was conducted to the skin flaps of the rat, which was fixed within 10% formalin, got dehydration, and then was embedded into paraffin. Then, sections with a thickness of 4 µm are cut and then stained with hematoxylin and eosin (HE). The Olympus BX51 microscope with equipment of a camera of Olympus DP71 CCD from Olympus Corporation in Japan was used to capture digital images (magnification ×200). Also, samples were fixed within 3% glutaraldehyde for 3 hours and 1% osmic acid for 1 hour, got dehydration, and then were embedded into paraffin. Then, sections with a thickness of 70 nm were cut, and then the transmission electron microscope was used (JEM1230; JEOL LTD, Japan) to capture digital images (magnification ×10,000). A blind manner was used to perform analysis on all images.
TUNEL staining
Sectioned slides were digested for 40 minutes, followed by the incubation with 50 µL TUNEL buffer at 37 °C for 1 hour and 50 µL peroxidase at 37 °C for 30 minutes, respectively. The slides were stained with 3,3'-diaminobenzidine (DAB) for 10 minutes. Samples were visualized by using a microscope, and the apoptotic cells were counted using the imaging mass spectrometry (IMS) cell imagine analysis system software version 6.0 [JRDUN Biotechnology (Shanghai) Co., Ltd., China].
Measurement of lactate dehydrogenase (LDH), glutathione (GSH), malondialdehyde (MDA), superoxide dismutase (SOD), tumor necrosis factor α (TNF-α) and endothelin (ET) level
The contents of LDH, GSH, MDA, SOD, TNF-α and ET were measured by the Lactate dehydrogenase assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), Reduced glutathione (GSH) assay kit (Nanjing Jiancheng Bioengineering Institute), MDA assay kit (TBA method; Nanjing Jiancheng Bioengineering Institute), SOD assay kit (Nanjing Jiancheng Bioengineering Institute), rat TNF-α ELISA Kit (Bio-swamp Life Science, Shanghai, China), and the Endothelin Radioimmunoassay Kit (Beijing North Institute of Biological Technology, Beijing, China) following protocols of the manufacturer, respectively.
Western blot assay
Whole protein was isolated out of snap-frozen aorta samples, RIPA lysis buffer supplemented with protease inhibitors. Proteins are quantified with a BCA protein quantification kit (BCA, Thermo, Shanghai, China) and run on 12% SDS-PAGE gel and blocked with 5% skim milk for 1 hour at 25 °C. The membrane was immunoblotted overnight at 4 °C with the first antibodies against Caspase-12, JNK, p-JNK, Bax, Bcl-2, active Caspase-3, NF-κBp65, GRP78, p-PERK, p-eIF2α, ATF4, CHOP, and GAPDH. Horseradish peroxidase-conjugated second antibodies were used to incubate membranes after they were washed (Beyotime Institute of Biotechnology, Inc., Shanghai, China) for 1 hour at 37 °C. TBST, with 20% Tween 20, was used to wash these membranes, and blots were observed visually through enhanced chemiluminescence (ECL, Thermo Scientific, Shanghai, China) and exposed to X-ray film and quantified in Chemi Doc XRS Imaging System, Bio-Rad (Hercules, CA, USA).
Statistical analysis
Results are expressed as mean the SD from 3 independent experiments. All statistical analysis was completed by using the GraphPad Prism software version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). Further, the statistical differences were calculated using a one-way ANOVA followed by Tukey’s post hoc test or unpaired, two-tailed Students’ t-tests. When P was less than 0.05, it had statistical significance.
Results
LXA4 promotes skin flap survival and attenuates I/R injury
The survival rate of skin flaps was markedly decreased by 14.9% in the I/R group compared with the sham group (Figure 1A,B). However, after pre-treatment of rats with LXA4, the survival rate was significantly increased by 11.6% and 16.6% in rats 24 and 48 hours post-I/R, respectively. These results suggest that LXA4 promotes skin flap survival in I/R-induced rats.
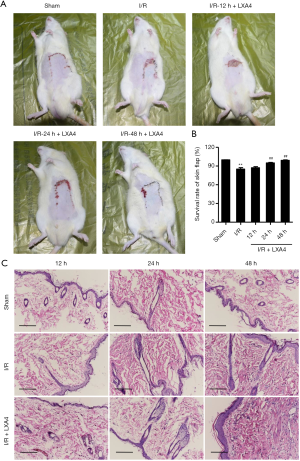
Histopathological changes of skin flaps during I/R and LXA4 pre-treatment showed that most of the cell bodies in the I/R group were shrank, pyknosis, and deep cytoplasm staining (Figure 1C). The morphological structure of skin flaps in the sham group is standard without the above changes. The abnormal cells in the LXA4 treatment groups were significantly lower than those in the I/R group, and the damage was mild (Figure 1C). Most of them showed slight cytoplasm staining, pyknosis, and nucleoli disappearance, and the damaged and inflammatory cells were rarely observed. These results suggest that LXA4 reduces skin flap injury in I/R-induced rats.
LXA4 inhibits I/R-induced expression of LDH, GSH, MDA, SOD, TNF-α and ET
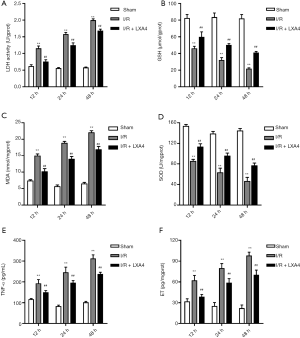
The concentration of LDH in the I/R group was markedly increased by 1.86-, 2.82- and 3.44-fold at 12, 24 and 48 hours post-I/R compared with the sham group (Figure 2A). The concentration of GSH in the I/R group was significantly decreased by 44.0%, 61.6% and 73.4% at 12, 24 and 48 hours post-I/R compared with the sham group (Figure 2B). The concentration of MDA in the I/R group was markedly increased by 1.04-, 2.36- and 2.52-fold at 12, 24 and 48 hours post-I/R compared with the sham group (Figure 2C). The concentration of SOD in the I/R group was significantly decreased by 44.6%, 54.7% and 68.2% at 12, 24 and 48 hours post-I/R compared with the sham group (Figure 2D). The concentration of TNF-α in the I/R group was significantly increased by 0.65-, 1.94-, and 2.06-fold at 12, 24, and 48 hours post-I/R compared with the sham group (Figure 2E). The concentration of ET in the I/R group was significantly increased by 0.94-, 2.14-, and 3.39-fold at 12, 24, and 48 hours post-I/R compared with the sham group (Figure 2F). However, the concentration of LDH, GSH, MDA, SOD, TNF-α and ET was significantly reversed by LXA4 treatment in rats at 12, 24, and 48 hours post-I/R compared with the I/R group (Figure 2A,B,C,D,E,F).
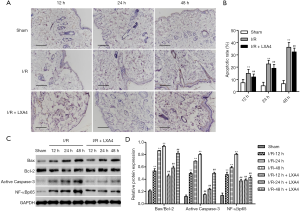
LXA4 inhibits I/R-induced apoptosis in skin flaps
The apoptotic rate in the I/R group was significantly increased by 1.05-, 3.75- and 4.61-fold at 12, 24 and 48 hours post-I/R compared with the sham group (Figure 3A,B). However, the apoptotic rate in LXA4 groups was significantly decreased by 19.9%, 15.8%, and 10.5% in rats at 12, 24 and 48 hours post-I/R compared with the I/R group. The apoptosis-related proteins, including Bax, Bcl-2 and Caspase-3, and NF-κBp65 were also evaluated by western blotting. The expression of active Caspase-3 and NF-κBp65 and Bax/Bcl-2 ratio in the I/R group was significantly increased compared with the sham group in a time-dependent manner, as shown in Figure 3C,D. However, the expression of active Caspase-3 and NF-κBp65 and Bax/Bcl-2 ratio in the LXA4 group was significantly decreased compared with the I/R group in a time-dependent manner.
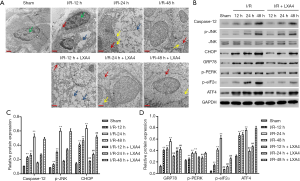
LXA4 inhibits I/R-induced ER stress in skin flaps
Morphological structure of cells during I/R and LXA4 pre-treatment showed incomplete cell structure and membrane, destroyed organelle structure and mitochondrial cristae, ER swelling, chromatin condensation, and plenty of vacuoles in the cytoplasm in the I/R group (Figure 4A). The structure of the cell in the sham group is still fine, with partly mass agglutination of chromatin, and organelle structure and mitochondrial cristae are partly destroyed. However, the morphological changes of the I/R group were significantly suppressed by LXA4 pre-treatment at 12, 24, and 48 hours post-I/R (Figure 4A). Since ER stress mediates three apoptotic pathways (14), Caspase-12, CHOP and JNK, these three markers of ER stress were therefore tested firstly. As shown in Figure 4B,C, the expression of Caspase-12, CHOP and p-JNK in the I/R group was increased compared with the sham group in a time-dependent manner. However, the expression of CHOP but not Caspase-12 and p-JNK in the LXA4 group was markedly decreased compared with the I/R group. Therefore, the ER stress-related proteins, including GRP78, p-PERK, p-eIF2α, and ATF4, were further evaluated by western blotting. As shown in Figure 4B,D, the expression of GRP78, p-PERK, p-eIF2α and ATF4 in the I/R group was increased compared with the sham group in a time-dependent manner. However, the expression of GRP78, p-PERK, p-eIF2α and ATF4 in the LXA4 group was markedly decreased compared with the I/R group at 48 hours post-I/R.
Discussion
I/R injury is one of the major factors leading to the increase of chronic rejection after skin flap transplantation. Previous studies showed that the success rate of free flap transplantation was 90–95% (25), and the necrosis rate of pedicle flap was 20–33% (26). The exact pathogenesis of I/R injury is not entirely clear, a complex pathophysiological process, and cell apoptosis plays a vital role in skin flap necrosis. LXA4 plays a vital role in inflammation, promoting inflammation, dissipation, and anti-proliferation (27). In the present study, LXA4 treatment inhibited rat skin flaps I/R injury, shown by inhibiting apoptosis, inflammatory response, and ER stress.
Compared with the I/R group, LXA4 treatment showed higher survival of skin flaps, slight cytoplasm staining, pyknosis, and nucleoli disappearance. In line with our findings, previous studies have shown the protective effect of LXA4 on I/R injury (19,22). LXA4 has been found to decrease the serum concentration of TNF-α and MDA in the myocardial I/R injury model, suggesting that LXA4 reduces the myocardial reperfusion injury through inhibiting the production of oxygen free radicals (19). Our results also found that LXA4 inhibited ET and NF-κBp65 expression, and increased SOD and GSH level in skin flap I/R rats. Increasing ET release during I/R led to obvious vasoconstriction, induced tissue ischemia, aggravated vascular dysfunction, and caused irreversible damage to cells. LXA4 inhibits NF-κB activation and downregulation of activated pro-inflammatory cytokines, including TNF-α, IL-1β, IL-6 and IL-8, suggesting that LXA4 may reduce inflammatory mediators and play a protective role by inhibiting phosphorylation of NF-κB (28).
In the I/R rats, we found significant morphological changes in apoptotic cells, including incomplete cell structure and membrane, destroyed organelle structure and mitochondrial cristae, ER swelling, chromatin condensation, and plenty of vacuoles in cytoplasm, which were consistent with the previous study (29). However, LXA4 treatment significantly inhibited cell apoptosis by decreasing the expression of the Bax/Bcl-2 ratio and Caspase-3. There is evidence showing that the family of Caspases and Bcl-2 was involved in the cell apoptosis during I/R injury, showing an essential role of those in the I/R injury (30,31).
Unfold protein response (UPR) can inhibit protein secretion in the ER, fold proteins and clear misfolded proteins involving GRP78, PERK and IRE1, increased in myocardial I/R models in vivo and in vitro (32,33). PERK is a transmembrane chaperone that activates eIF2α phosphorylation after activation, followed by increased ATF4 mRNA level and activation of CHOP, activating apoptotic signaling pathways in I/R injury (34). High expression of CHOP can inhibit the expression of Bcl-2 and cause the translocation of Bax from the cytoplasm to mitochondria (35). In the present study, LXA4 significantly inhibited increased expression of GRP78, p-PERK, p-eIF2α, ATF4, and CHOP in I/R rats. ER stress also activates NF-κB signaling and induces TNF-α expression in I/R model (36). These findings suggest that the anti-apoptosis and anti-inflammatory effect of LXA4 may occur from the inhibition of ER stress. In addition to ER stress mediated CHOP apoptotic pathway, Caspase-12 and JNK are other apoptotic pathways mediated by ER stress (14). LXA4 attenuates myocardial I/R injury via ER stress mediated downregulation of Caspase-12 (37), and exerts a neuroprotective effect in cerebral I/R injury via a JNK-independent signaling (16). In the present study, LXA4 attenuates skin flaps I/R injury via ER stress mediated CHOP apoptotic pathway but not Caspase-12 or JNK apoptotic pathway in rats. However, on a cautionary note, there are some limitations to our current study that should be pointed out. First, other apoptotic pathways and major factors mediated by ER should be further investigated in our future research. Second, the protective effects of LXA4 in clinical application demand further investigation.
Conclusions
In conclusion, we showed that ER stress causes tissue damage through induced cell apoptosis and inflammatory response in skin flap I/R rats. Besides, LXA4 presented a protective effect on skin flap tissues through inhibition of ER stress, leading to the reduced apoptotic cells and pro-inflammatory factor production. Our research indicates an important role of LXA4 in the treatment of skin flaps against I/R injury. Therefore, understanding the pharmacology of LXA4 is a key to developing new and more effective therapeutic strategies for treating skin flaps I/R injury.
Acknowledgments
Funding: This work was funded by the social development of major scientific and technological projects in the Xiaoshan District of Hangzhou City (2015203).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-5549
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-5549
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (http://dx.doi.org/10.21037/atm-20-5549). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The present study was performed in strict accordance with the guidelines on ethical care for experimental animals and approved by the Animal Research Committee of Xiaoshan Traditional Chinese Medical Hospital (No. XSZYY-2015-007).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Song K, Zhang M, Hu J, et al. Methane-rich saline attenuates ischemia/reperfusion injury of abdominal skin flaps in rats via regulating apoptosis level. BMC Surg 2015;15:92. [Crossref] [PubMed]
- Cetinkale O, Bilgic L, Bolayirli M, et al. Involvement of neutrophils in ischemia-reperfusion injury of inguinal island skin flaps in rats. Plast Reconstr Surg 1998;102:153-60. [Crossref] [PubMed]
- Wu X, Yu M, Li A. Protective effect of a nuclear factor-kappaB inhibitor on ischemia-reperfusion injury in a rat epigastric flap model. J Reconstr Microsurg 2008;24:351-9. [Crossref] [PubMed]
- Qi Z, Gao CJ, Wang YB, et al. Effects of hyperbaric oxygen preconditioning on ischemia-reperfusion inflammation and skin flap survival. Chin Med J (Engl) 2013;126:3904-9. [PubMed]
- Malý O, Zajak J, Hyšpler R, et al. Inhalation of molecular hydrogen prevents ischemia-reperfusion liver damage during major liver resection. Ann Transl Med 2019;7:774. [Crossref] [PubMed]
- Sanderson TH, Reynolds CA, Kumar R, et al. Molecular mechanisms of ischemia-reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol Neurobiol 2013;47:9-23. [Crossref] [PubMed]
- Chattopadhyay P, Chaudhury P, Wahi AK. Ca2+ concentrations are key determinants of ischemia-reperfusion-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. Appl Biochem Biotechnol 2010;160:1968-77. [Crossref] [PubMed]
- Vasques ER, Cunha JE, Coelho AM, et al. Trisulfate Disaccharide Decreases Calcium Overload and Protects Liver Injury Secondary to Liver Ischemia/Reperfusion. PLoS One 2016;11:e0149630. [Crossref] [PubMed]
- Yaidikar L, Thakur S. Punicalagin attenuated cerebral ischemia-reperfusion insult via inhibition of proinflammatory cytokines, up-regulation of Bcl-2, down-regulation of Bax, and caspase-3. Mol Cell Biochem 2015;402:141-8. [Crossref] [PubMed]
- Han J, Wang D, Yu B, et al. Cardioprotection against ischemia/reperfusion by licochalcone B in isolated rat hearts. Oxid Med Cell Longev 2014;2014:134862. [Crossref] [PubMed]
- Zhang HY, Wang ZG, Lu XH, et al. Endoplasmic reticulum stress: relevance and therapeutics in central nervous system diseases. Mol Neurobiol 2015;51:1343-52. [Crossref] [PubMed]
- Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res 2005;569:29-63. [Crossref] [PubMed]
- Guo W, Jiang T, Lian C, et al. QKI deficiency promotes FoxO1 mediated nitrosative stress and endoplasmic reticulum stress contributing to increased vulnerability to ischemic injury in diabetic heart. J Mol Cell Cardiol 2014;75:131-40. [Crossref] [PubMed]
- Zhu H, Zhu H, Xiao S, et al. Activation and crosstalk between the endoplasmic reticulum road and JNK pathway in ischemia-reperfusion brain injury. Acta Neurochir (Wien) 2012;154:1197-203. [Crossref] [PubMed]
- Verfaillie T, Garg AD, Agostinis P. Targeting ER stress induced apoptosis and inflammation in cancer. Cancer Lett 2013;332:249-64. [Crossref] [PubMed]
- Wu L, Miao S, Zou LB, et al. Lipoxin A4 inhibits 5-lipoxygenase translocation and leukotrienes biosynthesis to exert a neuroprotective effect in cerebral ischemia/reperfusion injury. J Mol Neurosci 2012;48:185-200. [Crossref] [PubMed]
- Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nature Reviews Immunology 2008;8:349. [Crossref] [PubMed]
- Scully M, Gang C, Condron C, et al. Protective role of cyclooxygenase (COX)-2 in experimental lung injury: evidence of a lipoxin A4-mediated effect. J Surg Res 2012;175:176-84. [Crossref] [PubMed]
- Zhao Q, Shao L, Hu X, et al. Lipoxin a4 preconditioning and postconditioning protect myocardial ischemia/reperfusion injury in rats. Mediators Inflamm 2013;2013:231351. [Crossref] [PubMed]
- Leonard MO, Hannan K, Burne MJ, et al. 15-Epi-16-(para-fluorophenoxy)-lipoxin A(4)-methyl ester, a synthetic analogue of 15-epi-lipoxin A(4), is protective in experimental ischemic acute renal failure. J Am Soc Nephrol 2002;13:1657-62. [Crossref] [PubMed]
- Peskar BM, Ehrlich K, Schuligoi R, et al. Role of lipoxygenases and the lipoxin A(4)/annexin 1 receptor in ischemia-reperfusion-induced gastric mucosal damage in rats. Pharmacology 2009;84:294-9. [Crossref] [PubMed]
- Han X, Yao W, Liu Z, et al. Lipoxin A4 Preconditioning Attenuates Intestinal Ischemia Reperfusion Injury through Keap1/Nrf2 Pathway in a Lipoxin A4 Receptor Independent Manner. Oxid Med Cell Longev 2016;2016:9303606. [Crossref] [PubMed]
- Petry JJ, Wortham KA. The anatomy of the epigastric flap in the experimental rat. Plast Reconstr Surg 1984;74:410-3. [Crossref] [PubMed]
- Liu B, Xu Q, Wang J, Lin J, Pei Y, Cui Y, Wang G, Zhu L. Recombinant human growth hormone treatment of mice suppresses inflammation and apoptosis caused by skin flap ischemia-reperfusion injury. J Cell Biochem 2019;120:18162-18171. [Crossref] [PubMed]
- Harder Y, Amon M, Laschke MW, et al. An old dream revitalised: preconditioning strategies to protect surgical flaps from critical ischaemia and ischaemia-reperfusion injury. J Plast Reconstr Aesthet Surg 2008;61:503-11. [Crossref] [PubMed]
- Moran SL, Serletti JM. Outcome comparison between free and pedicled TRAM flap breast reconstruction in the obese patient. Plast Reconstr Surg 2001;108:1954-60; discussion 1961-2. [Crossref] [PubMed]
- Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol 2007;25:101-37. [Crossref] [PubMed]
- Kure I, Nishiumi S, Nishitani Y, et al. Lipoxin A(4) reduces lipopolysaccharide-induced inflammation in macrophages and intestinal epithelial cells through inhibition of nuclear factor-kappaB activation. J Pharmacol Exp Ther 2010;332:541-8. [Crossref] [PubMed]
- Li HZ, Guo J, Gao J, et al. Role of dopamine D2 receptors in ischemia/reperfusion induced apoptosis of cultured neonatal rat cardiomyocytes. J Biomed Sci 2011;18:18. [Crossref] [PubMed]
- Li J, Han B, Ma X, et al. The effects of propofol on hippocampal caspase-3 and Bcl-2 expression following forebrain ischemia-reperfusion in rats. Brain Res 2010;1356:11-23. [Crossref] [PubMed]
- Liu G, Wang T, Wang T, et al. Effects of apoptosis-related proteins caspase-3, Bax and Bcl-2 on cerebral ischemia rats. Biomed Rep 2013;1:861-7. [Crossref] [PubMed]
- Guo XF, Yang XJ. Endoplasmic reticulum stress response in spontaneously hypertensive rats is affected by myocardial ischemia reperfusion injury. Exp Ther Med 2015;9:319-26. [Crossref] [PubMed]
- Yu Y, Sun G, Luo Y, et al. Cardioprotective effects of Notoginsenoside R1 against ischemia/reperfusion injuries by regulating oxidative stress- and endoplasmic reticulum stress- related signaling pathways. Sci Rep 2016;6:21730. [Crossref] [PubMed]
- Szegezdi E, Logue SE, Gorman AM, et al. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep 2006;7:880-5. [Crossref] [PubMed]
- Kim H, Tu HC, Ren D, et al. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell 2009;36:487-99. [Crossref] [PubMed]
- Wu Q, Wang Q, Guo Z, et al. Nuclear factor-kappaB as a link between endoplasmic reticulum stress and inflammation during cardiomyocyte hypoxia/reoxygenation. Cell Biol Int 2014;38:881-7. [Crossref] [PubMed]
- Zhao Q, Hu X, Shao L, Wu G, Du J, Xia J. LipoxinA4 attenuates myocardial ischemia reperfusion injury via a mechanism related to downregulation of GRP-78 and caspase-12 in rats. Heart Vessels 2014;29:667-78. [Crossref] [PubMed]
(English Language Editor: J. Chapnick)


