
Management of hyponatremia associated with acute porphyria—proposal for the use of tolvaptan
Introduction
Porphyrias are rare metabolic disorders caused by abnormal activity of one of the eight enzymes in the heme biosynthesis pathway and by the respective loss or gain of function mutations in the corresponding genes (Figure 1). Each porphyria has a specific biochemical profile of metabolite accumulation contributing to its diagnosis and typing (1-3). Acute hepatic porphyrias (AHPs) are characterized by neurovisceral attacks associated with high accumulation of early porphyrin precursors, δ-aminolaevulinic acid (ALA) and porphobilinogen (PBG), of hepatic origin. This group includes acute intermittent porphyria, hereditary coproporphyria and variegate porphyria and result from autosomal dominant loss-of-function mutations in the third, sixth and seventh enzymes of the heme pathway, respectively (Figure 1). An extremely rare autosomal recessive disorder of the second enzyme, ALA dehydratase porphyria (ADP), characterized by a marked increase in plasma and urine ALA is also included in AHPs.
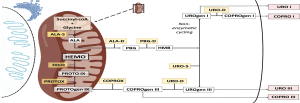
A marked enzymatic deficiency in ADP is related to its high penetrance and severe symptomatology with onset in childhood (4). In contrast, most gene carriers of the dominant form of AHPs are asymptomatic and precipitating factors are required to trigger acute attacks (5). These precipitating factors, both endogenous and exogenous (6), have in common the induction of the first and rate-limiting step in hepatic heme biosynthesis pathway, the ALA Synthetase (ALAS1), either directly after starvation (via peroxisome-proliferator-activated receptor γ coactivator 1α, PGC-1α) or through the suppression of the negative feedback mechanism caused by excessive heme consumption to form hemoproteins (mostly inducible cytochrome P450) or by increasing end-product degradation through heme oxygenase-1 (HO-1) induction.
Pathophysiology and management of the porphyric attack
Acute porphyria attacks are characterized by intense abdominal pain often accompanied by nausea, vomiting, hyponatremia, constipation, hypertension, tachycardia, insomnia and anxiety. Severe attacks can lead to neuropsychiatric disorders, seizures, sensory loss or motor neuropathy (including tetraparesis and life-threatening respiratory muscle failure) (2). Most acute neurovisceral attacks require hospital admission. The presence of seizures, motor neuropathy, and hyponatremia suggest severe disease that ideally should be managed in an intensive care unit.
The current goal of treatment for acute attacks is to reduce the activity of hepatic ALAS1, thus resulting in decreased production of early porphyrin precursors. The administration of intravenous human hemin, (heme arginate, Normosang® in Europe and crystallized hemin, Panhematin® in the US, both from Recordati) down-regulates hepatic ALAS1 transcription, inhibits ALAS1 mRNA translation and the translocation of the ALAS1 protein precursor into the mitochondria (1-3,7,8).
Mild attacks (without seizures, weakness, or hyponatremia and not requiring opioids) can sometimes be treated with a glucose overload (8,9) although this is less effective than hemin. Dose-dependent administration of glucose has also been shown to downregulate ALAS1 mRNA transcription in experimental conditions via PGC-1α (10). Subsequently, glucose infusions have been used to prevent mild attacks. However, this therapy has the disadvantage of aggravating hyponatremia owing to dilution, so this hydroelectrolytic abnormality must be corrected with extreme caution.
Hyponatremia and ADH during the porphyric attack
Mild to severe hyponatremia is a rather common phenomenon (occurring in 25–60% of cases) during an acute attack, as well as a marker of the severity of the crisis (11). Major motor seizure occurs in up to 20% of acute attacks and is often associated with severe hyponatremia. Although undoubtedly the etiology of the acute attack in hepatic porphyrias is the rise in plasma ALA and PBG levels, hyponatremia, as well as the development of a syndrome of inappropriate antidiuretic hormone secretion (SIADH), play a central role in the prognosis of the patient.
Although intestinal losses of Na+ during the crisis, or renal losses secondary to a salt-wasting ALA-dependent syndrome may play a role, hyponatremia in AHPs (12) is more likely to be caused by SIADH (13). In fact, elevated plasma levels of ADH in euvolemic conditions have been observed during acute attacks (14).
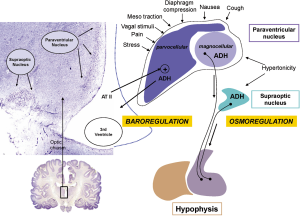
The etiology of SIADH in porphyric attacks is probably multifactorial. First, abdominal pain attributed to vascular spasms caused by ALA and PBG is a direct stimulus for the synthesis of ADH by the parvocellular portion of the paraventricular nucleus (Figure 2) (15). The excess of ADH also acts on the V1a receptors of the vasculature, increasing peripheral resistance and raising central blood pressure, and on the renal V2 receptors, preventing the kidney from adequately eliminating any water overload (16). Second, in the paralytic ileus that frequently accompanies acute attacks; intestinal sequestration of water and electrolytes stimulates the secretion of angiotensin II. The floor of the third ventricle is equipped with receptors for angiotensin II, which are capable of directly stimulating ADH secretion into the third ventricle through a mechanism known as ADH secretion by baroreception (Figure 2) (17).
When the attack begins to subside, there is rapid a reabsorption of a significant volume of water and electrolytes from the interstitial space or a third-space to the blood vessels. Sodium is rapidly excreted by the kidney when all natriuretic systems are activated. However, if there is an excess of ADH, water can cause a usually unexpected late hyponatremia. The iatrogenic component should not be forgotten if the intense pain is interpreted as an acute abdomen and an exploratory laparotomy is performed. Simple surgical manipulation is a direct stimulus for hypothalamic secretion of ADH. In addition, abdominal surgery produces a mean decrease in natremia of between 5 and 6 mmol/L, or greater when there is underlying SIADH (18).
Finally, the majority of deaths recorded during acute attacks of porphyria have been reported in relation to hypoxemia due to respiratory paralysis. The cerebral defense against the hypotonic edema, which follows the development of acute hyponatremia, is based on the activity of an astrocyte sodium pump. Hypoxia inhibits this pump; thus the prognosis of hyponatremia worsens when there are respiratory difficulties.
The “glucose effect” and the complications derived from its application in the treatment of acute attacks
High doses of glucose (>500 g/day) effectively suppress the hepatic ALAS1 promoter (19). The “glucose effect” in acute hepatic porphyrias was first described by Kaufman (20). To be useful, the minimum glucose infusion should reach 300–500 g/day. However, a rise in glycemia tends to produce a pseudo-hyponatremia that makes it difficult to adequately interpret what fraction of the reduction in natremia is due to SIADH or is secondary to the rise in glucose. Therefore, when hyponatremia is detected during a hepatic porphyria attack, it is necessary to correct the apparent hyponatremia by adjusting the glucose concentration (21) to reveal the real value of the natremia.
Corrected Nap = Nap measured + ((blood glucose (mg/dL) – 100)/63)
The volume of dextrose solution administered can be very high when 5% glucose is used to administer 300–500 g/day (6–10 L/day). Although the use of 10% glucose allows the dose to be reduced to 3–5 L/day, it remains a very high volume when the patient has SIADH because renal excretion of electrolyte-free water is impaired.
Proposal for the treatment of hyponatremia during an acute attack of porphyria
- Check for hypoxemia and proceed to its immediate correction.
- Exclude adrenal insufficiency as a contributing factor in the etiology of the hyponatremia. During the attack of acute porphyria, other abnormalities in the hypothalamic-pituitary axis have been reported, other than SIADH, such as growth hormone alteration, hyperprolactinemia and Adrenocorticotropic hormone (ACTH) deficit (22).
- If the patient tolerates oral treatment, 5–8 g of oral NaCl administered in two doses can be an option.
- When intravenous therapy is necessary, it is preferable to administer NaCl to a concentration higher than 0.9%, to avoid volume overload. The current recommendation is to use 3% NaCl, which is obtained by adding 30 mL of 20% NaCl to 250 mL of 0.9% NaCl solution. It is not advisable to use higher concentrations as they increase the risk of excessive hypercorrection and osmotic demyelination. The 3% NaCl infusion is started at 10 mL/h, and can be increased to up to 50 mL/h depending on the symptoms. This medication should be used with caution and close monitoring to prevent hypercorrection. The objective is not the complete correction of hyponatremia, but to avoid the risk of intracerebral compression due to hypotonic edema. In the case of a rapid progression of the neurological symptomatology, a bolus of 50 mL of saline 3% can be administered, which can be repeated after 10 minutes when no clinical improvement is observed.
- 126 mmol/L are reached in patients starting with natremias ≤125 mmol/L.
- Natremia has risen >5 mmol/L in one day.
- Usually the patient has high blood pressure (1,2), so salt overload can cause pulmonary congestion and hypoxemia. Therefore, a volume of 250 mL of 3% NaCl is recommended, in order to avoid the harmful effects caused by large quantities of NaCl.
- In patients treated with a glucose infusion and who are receiving between 3–10 L/day of water and glucose (depending on the concentration used), the kidney is capable of removing up to 20 L of water per day (1.5 L/h). However, it is required a minimum amount of 50 mOsm for the disposal of each liter of water. The induced glycosuria can reduce the excretion of free water, and there is also a minimum loss of Na+ of 25 mmol/L. Depending on the dextrose solution administered, the dilutive capacity of the kidney can be easily exceeded, even with a 3% NaCl infusion. Thus, the result is the development of hyponatremia.
- Water restriction in a patient who requires high volumes of infusion of dextrose solution is not an option.
- The last treatment option is the increase in free water clearance using tolvaptan (ATC code: C03XA01).
Natremia should be monitored every 2–4 h and the infusion should be suspended when:
The therapeutic goal in the first 24 h is to raise the natremia between 5 and 6 mmol/L, reaching 125 mmol/L when the baseline hyponatremia was lower or achieve 132 mmol/L when the natremia was between 130 and 125 mmol/L. However, these criteria in the treatment algorithms of acute hyponatremia exhibit two clinical peculiarities in the case of hyponatremia associated with an acute hepatic porphyria attack:
Use of tolvaptan in SIADH
Treatment with anticonvulsants can be challenging in AHPs, because many of the commonly used anti-convulsants (carbamazepines, hydantoins, valproate, etc.) cause up-regulation of hepatic ALAS1 and can precipitate and/or worsen acute porphyric attacks (23).
Tolvaptan is a selective ADH antagonist, which acts by binding to renal vasopressin V2 receptor (24). It is administered orally and produces specific aquaresis without changes in the Na+ balance. It is the only ADH inhibitor available in Europe. It has been shown to be useful in hyponatremia associated with SIADH of any etiology, and in hyponatremia associated with heart failure, liver disease (24) and post-operative situations resulting from different surgeries (25).
The use of tolvaptan in SIADH is recommended when the following criteria are met: (I) existence of true hyponatremia after correcting plasma natremia by glucose, protein or lipid levels (corrected Natremia <130 mmol/L); (II) urinary osmolarity >100 mOsm/kg. Hyponatremia with lower urinary osmolarities are considered secondary to excessive water intake; (III) a urinary Na+ concentration of >40 mmol/L. Lower concentrations of urinary Na+ are considered secondary to hypovolemia; and (IV) in those cases when thyroid and adrenal insufficiency has been adequately ruled out.
Tolvaptan is formulated in tablets of 15 and 30 mg, with daily doses ranging between 7.5 and 60 mg, always administered in a single dose (26). The duration of its effect is 8–12 h during which aquaresis increases significantly, thus it is usually administered in the morning. It is recommended to start treatment with 7.5 mg and then titrate the dose according to response. In most cases, the dose of 7.5 mg is adequate (26,27). Hypercorrection is exceptional and natremia is usually normalized in a period of 3 to 10 days. Given the irregularity of the response to the first dose, its use is not recommended in the first 24 h following the development of hyponatremia. Oral salt or the administration of 3% NaCl is the preferred option for that period of time.
Tolvaptan is metabolized by the cytochrome CYP3A4 in the liver. It is considered as a “Probably Not Porphyrinogenic” drug for porphyria (6), and was well tolerated during a porphyric attack, as reported in isolated cases. One of these cases has been reported recently (28). A 13-year-old Indian boy developed an acute porphyric attack after starting treatment with tuberculostatic. At first, laboratory studies showed hyponatremia at 114 mmol/L and urinary sodium and osmolality confirmed the diagnosis of SIADH. The patient received tolvaptan, antihypertensives, levetiracetam and opioids. His mental state and hyponatremia progressed favorably, but he developed an ascending areflexic paralysis of all four limbs with respiratory involvement. Then, high urinary excretion of PBG established the diagnosis of acute porphyria. As hemin was not available, treatment was based on IV glucose 10–20 mg/h in combination with tolvaptan that led to a progressive improvement in motor symptoms, with no further deterioration of natremia.
Natremia monitoring during the acute attack should be performed every 2–4 h, to detect rapid hypercorrections, especially when 3% hypertonic saline is being used. In case of hypercorrection, these instructions should be followed: (I) supply water; (II) suspend the 3% saline infusion; (III) omit the next dose of tolvaptan; (IV) infuse 5% glucose therapy at a rate of 6 mL/kg/h for 2 h; and then, measure the natremia; and (V) if no improvement occurs, administer desmopressin 1–2 mg subcutaneously or intravenously every 6 hours.
The duration of tolvaptan treatment depends on the nature of the SIADH and the underlying disease, and it is recommended to be used for no longer than 30 days (U.S. Food and Drug Administration). Tolvaptan can be suspended when plasma Na+ rises to a level between 132 and 140 mmol/L, without progressive dose reduction. In the case of acute hepatic porphyria, its indication will only be maintained for the duration of the acute condition. Usually the acute porphyria attacks last 5 to 7 days (29), and the persistence of symptomatic hyponatremia beyond the crisis is unusual. In other situations with chronic SIADH (senile hyponatremia, tumors.) maintenance treatment is frequently achieved with a dose of 7.5 mg every/48 or 72 h chronically (26).
It is likely that tolvaptan may be part of the treatment of acute porphyria attack, especially if there is hyponatremia and it is necessary to increase the clearance of free water to safely handle high volumes of dextrose solution. However, its effectiveness may be reduced in the presence of large glucose infusions with glucosuria that reduce the concentration gradient between the tubular lumen and the interstitium of the renal papilla. For this reason, urea does not work in porphyria hyponatremia when glucosated sera are used.
In conclusion, electrolyte imbalances, and especially hyponatremia, should be corrected and monitored during acute episodes of porphyria. Precautions against convulsions and seizures are recommended, especially in patients who develop hyponatremia. This often results from hypothalamic involvement and SIADH or excess gastrointestinal or renal sodium loss. Although patients who experience seizures during an acute attack seldom require anticonvulsant therapy; treatment is difficult since common anticonvulsants could be harmful in AHPs and, most importantly, they do not resolve the underlying hyponatremia. Thus, we propose the use of tolvaptan for the treatment of hyponatremia in acute porphyria under the strict conditions specified in this review.
Acknowledgments
In memoriam of Dr. Alberto Tejedor, sadly deceased during the COVID19 pandemic. May his memory guide our path in the management of hyponatremia and acid-base disorders.
Funding: This work was supported in part by grants from Spanish Institute of Health Carlos III (FIS) cofunded by European FEDER funds (grant numbers PI18/00860), the Spanish Fundación Eugenio Rodríguez Pascual, the Spanish Fundación Mutua Madrileña and the Spanish Fundación FEDER para la investigación de enfermedades raras. The founders had no role in the analysis or the development of conclusions. The investigators are solely responsible for the content and the decision to submit the manuscript for publication.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-1529). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspect of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Puy H, Gouya L, Deybach JC. Porphyrias. Lancet 2010;375:924-37. [Crossref] [PubMed]
- Bissell DM, Anderson KE, Bonkovsky HL. Porphyria. N Engl J Med 2017;377:862-72. [Crossref] [PubMed]
- Pischik E, Kauppinen R. An update of clinical management of acute intermittent porphyria. Appl Clin Genet 2015;8:201-14. [Crossref] [PubMed]
- Neeleman RA, van Beers EJ, Friesema EC, et al. Clinical Remission of Delta-Aminolevulinic Acid Dehydratase Deficiency Through Suppression of Erythroid Heme Synthesis. Hepatology 2019;70:434-6. [PubMed]
- Chen B, Whatley S, Badminton M, et al. International Porphyria Molecular Diagnostic Collaborative. an evidence-based database of verified pathogenic and benign variants for the porphyrias. Genet Med 2019;21:2605-13. [Crossref] [PubMed]
- Database. Drugs database and acute porphyrias. Available online: ; last entry: April 9th, 2020.http://www.drugs-porphyria.org/monograph2.php?id=5458
- Schmitt C, Lenglet H, Yu A, et al. Recurrent attacks of acute hepatic porphyria: major role of the chronic inflammatory response in the liver. J Intern Med 2018;284:78-91. [Crossref] [PubMed]
- Balwani M, Wang B, Anderson KE, et al. Acute hepatic porphyrias: Recommendations for evaluation and long-term management. Hepatology 2017;66:1314-22. [Crossref] [PubMed]
- Castelbón Fernández FJ, Solares Fernandez I, Arranz Canales E, et al. Protocol For Patients With Suspected Acute Porphyria. Rev Clin Esp 2020. [Epub ahead of print]. [PubMed]
- Handschin C, Lin J, Rhee J, et al. Nutritional regulation of hepatic heme biosynthesis and porphyria through PGC-1alpha. Cell 2005;122:505-15. [Crossref] [PubMed]
- Stein PE, Badminton MN, Rees DC. Update review of the acute porphyrias. Br J Haematol 2017;176:527-38. [Crossref] [PubMed]
- Jaramillo-Calle DA, Solano JM, Rabinstein AA, et al. Porphyria-induced posterior reversible encephalopathy syndrome and central nervous system dysfunction. Mol Genet Metab 2019;128:242-53. [Crossref] [PubMed]
- Meersseman W, Cassiman D, Goossens W, et al. An unusual cause of syndrome of inappropriate antidiuretic hormone secretion. Acta Clin Belg 2008;63:277-80. [Crossref] [PubMed]
- Eales L, Dowdle EB, Sweeney GD. The acute porphyric attack. I. The electrolyte disorder of the acute porphyric attack and the possible role of delta-aminolaevulic acid. S Afr Med J 1971.89-97. [PubMed]
- Buggy J, Fisher AE. Anteroventral third ventricle site of action for angiotensin induced thirst. Pharmacol Biochem Behav 1976;4:651-60. [Crossref] [PubMed]
- Greenberg A, Verbalis JG. Vasopressin receptor antagonists. Kidney Int 2006;69:2124-30. [Crossref] [PubMed]
- Kubo T, Numakura H, Endo S, et al. Angiotensin receptor blockade in the anterior hypothalamic area inhibits stress-induced pressor responses in rats. Brain Res Bull 2001;56:569-74. [Crossref] [PubMed]
- Caramelo C, Tejedor A, Criado C, et al. Fluid therapy in surgical patients: composition and influences on the internal milieu. Nefrologia 2008;28:37-42. [PubMed]
- Tschudy DP, Welland FH, Collins A, et al. The Effect of Carbohydrate Feeding on the Induction of Delta-Aminolevulinic Acid Synthetase. Metabolism 1964;13:396-406. [Crossref] [PubMed]
- Kaufman L, Marver HS. Biochemical defects in two types of human hepatic porphyria. N Engl J Med 1970;283:954-8. [Crossref] [PubMed]
- Katz MA. Hyperglycemia-induced hyponatremia--calculation of expected serum sodium depression. N Engl J Med 1973;289:843-4. [Crossref] [PubMed]
- Waxman AD, Berk PD, Schalch D, et al. Isolated adrenocorticotrophic hormone deficiency in acute intermittent porphyria. Ann Intern Med 1969;70:317-23. [Crossref] [PubMed]
- Anderson KE, Bloomer JR, Bonkovsky HL, et al. Recommendations for the diagnosis and treatment of the acute porphyrias. Ann Intern Med 2005;142:439-50. [Crossref] [PubMed]
- Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med 2006;355:2099-112. [Crossref] [PubMed]
- Nakamura Y, Kishimoto Y, Harada S, et al. Tolvaptan can limit postoperative paroxysmal atrial fibrillation occurrence after open-heart surgery. Surg Today 2020;50:841-8. [Crossref] [PubMed]
- Castello LM, Baldrighi M, Panizza A, et al. Efficacy and safety of two different tolvaptan doses in the treatment of hyponatremia in the Emergency Department. Intern Emerg Med 2017;12:993-1001. [Crossref] [PubMed]
- Cuesta M, Gomez-Hoyos E, Montañez C, et al. An initial dose of 7.5 mg Tolvaptan is safe and effective in the treatment of hyponatremia caused by SIADH. Endocrine Abstracts 2012;29:1149.
- Golla R, Mukherjee A, Gone RK, et al. Acute intermittent porphyria and anti-tuberculosis therapy. QJM 2020;113:207-8. [PubMed]
- Gouya L, Ventura P, Balwani M, et al. EXPLORE: A Prospective, Multinational, Natural History Study of Patients with Acute Hepatic Porphyria with Recurrent Attacks. Hepatology 2020;71:1546-58. [Crossref] [PubMed]


