
Beta-elemene inhibits differentiated thyroid carcinoma metastasis by reducing cellular proliferation, metabolism and invasion ability
Introduction
Thyroid carcinoma (TC) is one of the most common endocrine malignancies, and the incidence has increased worldwide more than any other cancer (1,2). In the past 30 years, the incidence has increased by more than 300% in the United States (1). With histology, TC is classified into four major types: papillary, follicular, medullary, and anaplastic (3). Among all the TC subtypes, differentiated thyroid cancer (DTC), which includes the papillary and follicular types, accounts for more than 90% of cases (4). Following standard surgical treatment in conjunction with radioiodine ablation and TSH suppression therapy, the 5-year disease-specific survival rate of TC is approximately 98% (5). However, despite its positive prognosis, regional, and cervical lymph node recurrence occurs in up to 13% DTC patients (6), resulting in a significant challenge to the long-term survival of DTC patients.
Otto Warburg first reported aerobic glycolysis in the 1950s (7). More recently, the theory of the Warburg effect has been applied to cancer cell metabolism (8). Although oxidative phosphorylation produces ATP more efficiently, aerobic glycolysis produces ATP more rapidly, which suits the metabolic needs of rapidly proliferating cancer cells (8,9). By this theory, DTC cells show higher rates of efficient glycolysis (10).
Elemene (1-methyl-1-vinyl-2,4-diisopropylcyclohexane) is a lipid-soluble anticancer drug extracted from the traditional Chinese medicinal herb Rhizoma zedoariae (11). The elemene extract comprises a mixture of beta (β)-, delta (δ)-, and gamma (γ)-elemene, with β-elemene as the main component, accounting for 60–72% of the three isoforms (12). β-Elemene, the active component of elemene, is effective against various tumors, including liver, lung, and breast cancer (13-15); however, the underlying mechanism remains to be fully elucidated. One study indicated the anticancer effects of β-elemene combined with rapamycin (16). However, the influence of β-elemene alone on DTC cells and the underlying mechanism are unclear. In this study, we investigated the antitumor effect of β-elemene on human DTC cells. Our results showed that β-elemene inhibited cell proliferation, promoted apoptosis, and arrested cell cycle progression.
Furthermore, β-elemene inhibited DTC cell invasion ability and reduced angiogenesis. β-elemene also significantly inhibited the respiratory and glycolytic ability of human DTC cells, which could form the basis of the mechanism antitumor effect of β-elemene. Finally, the antitumor effect of β-elemene was confirmed in vivo in a mouse xenograft model.
We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4460).
Methods
Cell culture
Thyroid carcinoma cells were supported in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) and cultured at 37 °C in a humidified atmosphere containing 5% CO2. Papillary thyroid carcinoma (PTC) cell lines (IHH-4, TPC-1, K1) and follicular thyroid carcinoma (FTC) cell line (FTC133) were incubated overnight and then exposed to β-elemene (0, 10, 20, 40, 60, 80, 120 and 160 µg/mL) for 24, 48 or 72 hours.
Reagents and antibodies
β-Elemene (98% purity) was obtained from Yuanda Pharmaceuticals (Dalian, China). Propidium iodide (PI), RNase, and glycine were bought from Sigma-Aldrich (St. Louis, MO, USA). The primary antibodies against cyclinE, cyclinB1, CDK1, CDK2, CDK6, caspase-8, cleaved caspase-9, BCL-2, VEGF, and β-actin and the HRP-conjugated goat anti-rabbit IgG secondary antibody was from Cell Signaling Technology (Beverly, MA, USA). The human DTC cell lines, IHH-4, TPC-1, K1, and FTC133, were obtained from the Health Science Research Resources Bank (Osaka, Japan). DMEM, FBS, and 0.25% trypsin-EDTA solution were bought from Gibco (Gaithersburg, MD, USA).
Cell viability assay
Cell viability or the effects of β-elemene on cell proliferation were measured using the CCK8 method. In brief, 4×103 cells/well (IHH-4, TPC-1, K1, and FTC133) were evenly distributed and cultured in 96-well plates overnight at 37 °C in a humidified atmosphere containing 5% CO2. After that, the cells were incubated for another hour at 37 °C with 10 µL CCK8. And the optical density of each well was measured at 450 nm with a microplate reader (Infinite® 200 PRO, Tecan).
Cell cycle analysis by flow cytometry
After treatment with various concentrations of β-elemene (0, 10, 20, 40, 60, 80, 120 and 160 µg/mL) for 24, 48 or 72 hours, the cells (1×106) were stained with PI following incubation with 0.2 mg/mL RNase for 30 minutes. Finally, flow cytometry analyzed the cells using a FACS Calibur (Becton–Dickinson, San Diego, CA, USA). Cell cycle phase distribution was analyzed with ModFit LT software (Verity Software House, USA).
Analysis of apoptosis
IHH-4, TPC-1, K1, and FTC133 cells were seeded at 1.5×105 cells/well in 6-well plates, incubated overnight, and then exposed to 0, 20, 40, or 60 µg/mL of β-elemene for 24 hours. Cells were collected and incubated with 1 µg/mL Annexin V-FITC (Becton–Dickinson) for 20 minutes in the dark. Finally, flow cytometry evaluated the samples, and the data were analyzed using FlowJo software.
Transwell assay of cell invasion ability
Transwell chambers were prepared by the addition of 40 µL ECM Gel (dissolved in serum-free medium at 1:7.5) per well in the upper chamber. The plates were incubated at 37 °C for 30 minutes to allow polymerization of the Matrigel. Cells treated with different concentrations of β-elemene (0, 20, 40, and 60 µg/mL) for 24 hours were harvested, resuspended in serum-free DMEM medium, and the cell density was adjusted to 1.5×105/mL. Cells (200 µL) were then added to the Transwell upper chamber, while 500 µL of medium containing 10% FBS was added to the lower chamber. The plates were incubated for 12 hours at 37 °C in a humidified atmosphere holding 5% CO2. After the Transwell chamber was removed, the cells were fixed with paraformaldehyde and stained with crystal violet. After observation under a Leica upright microscope, the Transwell chambers were photographed, and the numbers of cells in five randomly selected fields were view recorded.
Western blot analysis
IHH-4, TPC-1, K1 and FTC133 cells were treated with different concentrations (0, 20, 40 or 60 µg/mL) of β-elemene for 24 hours. The cells were washed twice with ice-cold PBS and harvested on ice. The total protein content was quantified using the Lowry method. Cell lysate proteins (50 µg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrophoretically transferred to nitrocellulose membranes (Immobilon-P; Millipore, USA). The membranes were blocked with 5% skimmed milk in TBS-T buffer [10 mmol/L Tris (pH 7.4), 150 mmol/L NaCl and 0.1% Tween-20] at room temperature for 2 hours and incubated overnight at 4 °C with the indicated primary antibodies against cyclinE, cyclinB1, CDK1, CDK2, CDK6, caspase-8, cleaved caspase-9, BCL-2 or VEGF. The membranes were then incubated with HRP-conjugated secondary antibodies for 2 h at room temperature. After extensive washing with TBS-T buffer, the proteins were visualized with an enhanced chemiluminescence reagent. The images were analyzed using NIH Image J software.
The respiratory and glycolytic ability of living cells
The XFe 96 Extracellular Flux analyzer (Seahorse Biosciences, Billerica, MA, USA) measures real-time uptake and release of metabolic end products. Each XFe 96-assay well holds a disposable sensor cartridge, embedded with 96 pairs of fluorescent biosensors (oxygen and pH), coupled to fiber-optic waveguides. This technology was used to measure oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) in cells treated with β-elemene. Mitochondrial respiratory ability and glycolytic ability of these cells were investigated using commercial kits (Seahorse Biosciences) according to the manufacturer’s instructions.
In vivo experiments
Twelve nude mice (aged 4 weeks, 18±2 g) were obtained from Shanghai SLAC Laboratory Animal Co. Ltd. (Shanghai, China). All animal experiments were approved by the Animal Ethics Committee of China Medical University. All procedures were performed following the Guide for the Care and Use of Laboratory Animals and complied with institutional ethical guidelines. Mice were injected subcutaneously with 5×106 IHH-4 cells and then randomized into two equal groups (n=6) before drug treatment was started. β-Elemene was formulated as emulsions and injected intraperitoneally. One group was treated with β-elemene emulsion (50 mg/kg) every three days for 15 days, while the control group was treated with blank emulsions alone as the vehicle. Tumors were measured using a ruler by the same person weekly post-treatment. Tumor size was determined by measurement of the maximum and minimum superficial diameters every other day. Tumor volumes were calculated according to the formula: V =1/2ab2, where a and b denote the maximum and minimum superficial diameter, respectively.
Statistical analysis
All values were expressed as the mean ± standard deviation (SD) of at least three independent experiments. Inter-group differences were evaluated using the Student’s t-test and ANOVA. P<0.05 was considered to show statistical significance.
Results
Inhibitory effects of β-elemene on the growth of human DTC cell lines
To evaluate the antitumor effects of β-elemene on DTC cells, we investigated the ability of β-elemene to inhibit cell viability in vitro using the CCK8-based colorimetric assay. Treatment of DTC cell lines with β-elemene at concentrations ranging from 10 to 160 µg/mL for 24, 48, and 72 hours showed that cell growth was inhibited in a dose and time-dependent manner (Figure 1). The half-maximal inhibitory concentrations (IC50) of β-elemene on IHH-4 cell growth were 42.2±1.53, 36.6±0.85, and 39.1±1.26 µg/mL at 24, 48, and 72 hours, respectively. The IC50 values of β-elemene on TPC-1, K1, and FTC133 cells showed a similar trend.

Effects of β-elemene on cell cycle progression of human DTC cell lines
After exposure to β-elemene, the populations of IHH-4, TPC-1, and FTC133 cells in the G1 phase were increased in a concentration-dependent manner, indicating that β-elemene induced cell cycle arrest in the G1 phase (Figure 2A). Following exposure to β-elemene, the populations of K1 in the G2 phase were increased, showing that β-elemene induced cell cycle arrest in the G2 phase. We measured the expression levels of cyclinE, CDK2, and CDK6 by western blot in the IHH-4, TPC-1, K1, and FTC133 cells treated with β-elemene (Figure 2B,C). In IHH-4, TPC-1, and FTC133 cells, the levels of cyclinE, CDK2, and CDK6 decreased in a dose-dependent manner after exposure to different concentrations of β-elemene. Meanwhile, the expression levels of cyclinB1 and CDK1 decreased in K1 cells.
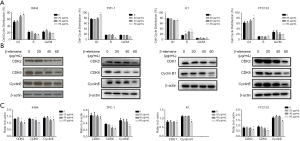
Effects of β-elemene on apoptosis in human DTC cell lines
β-Elemene induced apoptosis in IHH-4, TPC-1, K1, and FTC133 cells in a dose-dependent manner (Figure 3A). Caspase-8 and caspase-9 are markers of the extrinsic pathway and intrinsic pathways of apoptosis, respectively, while BCL-2 plays a critical role in the inhibition of cell apoptosis (17,18). As shown in Figure 3B,C, cleaved caspase-9 expression increased, and BCL-2 expression decreased in IHH-4, K1, and FTC133 cells following β-elemene treatment, while there was no significant change in caspase-8 protein expression. However, in the TPC-1 cell line, β-elemene treatment increased the expression of caspase-8 and cleaved caspase-9, while BCL-2 expression was reduced.
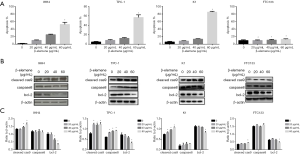
Effects of β-elemene on the invasiveness of human DTC cell lines
As shown in Figure 4A, β-elemene (0, 10, 20, and 40 µg/mL) significantly inhibited numbers of IHH-4, TPC-1, K1, and FTC133 cells that migrated through the Matrigel layer in Transwell assays in a dose-dependent manner (all P<0.05).
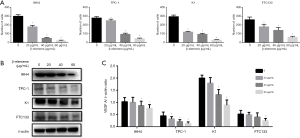
The effect of β-elemene on the expression of VEGF in human DTC cell lines
As shown in Figure 4B,C, VEGF expression in IHH-4, TPC-1, K1, and FTC133 cells decreased after treated with different concentrations of β-elemene in a dose-dependent manner.
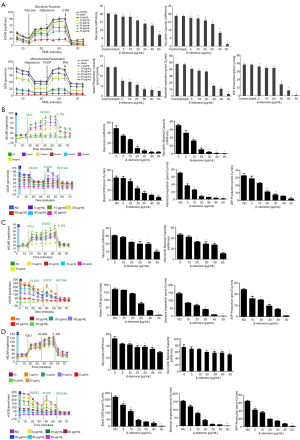
Effects of β-elemene on the respiratory and glycolytic ability of human DTC cells
Energy metabolism is crucial for cell proliferation and pathobiological behavior. We hypothesized that β-elemene alters energy metabolism in DTC cells, and these changes are manifested as reduced proliferation and increased apoptosis. To test this hypothesis, we measure the mitochondrial respiratory capacity and glycolytic capacity of DTC cells pretreated with different concentrations of β-elemene (0, 5, 10, 20, 30, 40, and 50 µg/mL) using an XFe 96 Extracellular Flux analyzers. After treatment with β-elemene at 20 µg/mL, the basal ECAR and maximal glycolytic capacity of IHH4 cells were reduced, showing that β-elemene inhibits IHH4 cell glycolysis at this dose (Figure 5A). Interestingly, β-elemene treatment at 5 µg/mL also reduced the basal OCR, maximal respiration and ATP production of IHH4 cells, indicating that β-elemene inhibits mitochondrial aerobic respiration in IHH4 cell at this dose (Figure 5A). The results showed that β-elemene inhibited the mitochondrial respiratory ability and glycolytic ability of IHH4 cells in a dose-dependent manner. Comparable results were obtained in analyses of TPC-1 (Figure 5B), K1 (Figure 5C), and FTC133 (Figure 5D).
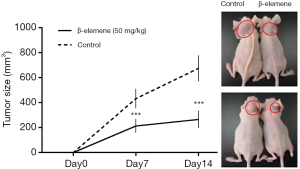
Anti-growth effects of β-elemene on IHH-4 human PTC cells in xenograft mice
At 7 and 14 days after treatment with β-elemene or blank emulsions, tumor volumes in untreated mice were significantly larger than those in the drug-treated mice, which confirmed β-elemene inhibited the growth of IHH-4 cells in vivo. Representative images of a tumor mass from a mouse treated with either β-elemene or blank emulsion are shown in Figure 6.
Discussion
The rapidly increasing incidence of TC is a cause for concern in many countries (19,20). Although most thyroid cancer patients have an excellent outcome, and well-differentiated carcinoma is often curable by the combination of surgery and radioiodine ablation, approximately 10% of these patients lose the ability to respond to radioiodine therapy, leading to recurrent disease and death (21-23). Thus, new effective therapeutic strategies are urgently required in such cases of advanced differentiated thyroid carcinoma (DTC) (21).
Accelerated glycolysis is a characteristic of carcinoma (22,23). Previous studies have shown a metabolic shift occurs in many tumors and correlates with negative prognoses (24-26). Our study showed that β-elemene significantly inhibited the respiratory and glycolytic ability of human DTC cells, with both basal ECAR and maximal glycolytic capacity reduced in a dose-dependent manner. These results show that β-elemene targets human DTC cell metabolism. However, to our prediction, β-elemene treatment did not result in the conversion of glycolysis to mitochondrial aerobic respiration. β-Elemene treatment reduced the basal OCR maximal respiration and ATP production of DTC cell lines, indicating that ß-elemene inhibits mitochondrial aerobic respiration. This suppression of mitochondrial aerobic respiration was observed at concentrations of β-elemene as low as 5 µg/mL, while a higher concentration (20 µg/mL) was required for suppression of glycolysis. Further studies must clarify the metabolism pathways influenced by the β-elemene treatment of DTC cells.
Rapid growth is a common feature of tumor cells. Thus, growth inhibition and cell cycle arrest are fundamental indexes of drug antitumor effects in vitro (27). In our study, we observed cell cycle arrest of IHH-4, TPC-1, and FTC133 cells in the G1 phase following treatment with when treated with β-elemene. This effect was accompanied by decreased expression of cyclinE, CDK2, and CDK6, three proteins are known to regulate the G1 phase. Similarly, β-elemene treatment resulted in K1 cell cycle arrest in the G2 phase, combined with decreased expression of cyclinB1 and CDK1, proteins that regulate the G2 phase of the cell cycle. This phenomenon reflected the antitumor effects of β-elemene on human DTC cells in vitro in a time- and dose-dependent manner.
Bcl-2 family proteins, which are essential regulators of cell apoptosis (28), can be divided into subcategories of anti-apoptotic proteins, including BCL-2, and pro-apoptotic proteins, including Bax. The ratio of anti-apoptotic proteins and pro-apoptotic protein defines the sensitivity of cells to apoptotic signals (29). Studies have shown β-elemene induces cell apoptosis via the mitochondrial caspase-dependent pathway. In lung cancer and prostate cancer, β-elemene can reduce BCL-2 expression and increase the expression of cytochrome C, ADP ribose polymerase (PARP), caspase 3, caspase 7, and caspase 9 (30). In our study, we found that β-elemene treatment of IHH-4, K1, and FTC133 cells reduced BCL-2 expression and increased cleaved caspase-9 expression. These observations showed that β-elemene induces apoptosis in IHH-4, K1, and FTC133 cells via a caspase-9-dependent pathway. TPC-1 cells, β-elemene treatment reduced BCL-2 expression, while cleaved caspase-9 and caspase-8 expression were increased, indicating that apoptosis was induced not only via a caspase-9-dependent pathway, but also through a caspase-8-dependent pathway. Thus, our findings supply evidence that the anticancer actions of β-elemene are mediated by its ability to regulate cell cycle progression and apoptosis in DTC cells.
The invasiveness nature of tumor cells is the main reason for the failure of cancer treatment. At present, it is widely believed that the adhesion of cancer cells to surrounding tissues is a critical factor leading to the migration and invasion of tumor cells (31). Therefore, clarification of the effect of β-elemene on the invasive ability of DTC cells is of great significance in the elucidation of its anticancer mechanism. In this study, we found β-elemene inhibited the invasiveness of IHH-4, TPC-1, K1, and FTC133 cell lines, with amounts of cells passing through Matrigel decreasing in a dose-dependent manner.
VEGF is an essential regulator of tumor angiogenesis, which is necessary to meet the increased requirement for oxygen and nutrients of rapidly proliferating tumor cells (32). Inhibited angiogenesis is likely to induce tumor dormancy, necrosis, or apoptosis due to insufficient oxygen and nutrients. VEGF is currently considered one of the most crucial factors regulating tumor angiogenesis, suggesting that reducing VEGF expression is vital for DTC treatment. Our results showed that VEGF protein expression was decreased after treatment of IHH4, TPC-1, K1, and FTC133 cells with different concentrations of β-elemene, implicating decreased VEGF expression in the mechanism by which β-elemene inhibits DTC proliferation.
Finally, we used a nude mouse tumor xenograft model to confirm the antitumor effects of β-elemene in vivo. At 7 and 14 days after treatment with β-elemene or blank emulsions, tumor volumes in untreated mice were significantly larger than those in drug-treated mice. Our results also showed that β-elemene treatment significantly decreased the tumor size and weight at all doses, thus confirming the antitumor effects of β-elemene in vivo.
In conclusion, our study provides the first evidence that β-elemene has significant inhibitory effects on cell proliferation in human PTC (IHH-4, TPC-1, K1) and FTC (FTC133) cell lines. The antitumor effects include inhibiting cell proliferation, promoting apoptosis, arresting cell cycle progression, inhibiting cell invasion ability, and reducing angiogenesis. We also demonstrated β-elemene inhibits the respiratory and glycolytic capacity of human DTC cells, which is, therefore, implicated in the mechanism underlying the antitumor effects of β-elemene. Moreover, β-elemene inhibited tumor growth in a nude mouse xenograft model. Thus, the evidence obtained in this study highlights the potential of β-elemene as a novel treatment for DTC.
Acknowledgments
We thank Renee Wang and LetPub Company for linguistic aid during the preparation of this manuscript. We thank Professor Guan from the department of endocrinology and metabolism, and the institute of endocrinology supplied the cell lines, including IHH-4, TPC-1, K1, and FTC133 cells.
Funding: This study was supported by grants from the National Natural Science Foundation, China (Grant# 81370893).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-4460
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-4460
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4460). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal experiments were approved by the Animal Ethics Committee of China Medical University. All procedures were performed following the Guide for the Care and Use of Laboratory Animals and complied with institutional ethical guidelines.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lim H, Devesa SS, Sosa JA, et al. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA 2017;317:1338-48. [Crossref] [PubMed]
- Takano T. Natural history of thyroid cancer Endocr J 2017;64:237-44. [Review]. [Crossref] [PubMed]
- Durante C, Grani G, Lamartina L, et al. The Diagnosis and Management of Thyroid Nodules: A Review. JAMA 2018;319:914-24. [Crossref] [PubMed]
- Lubitz CC, Sosa JA. The changing landscape of papillary thyroid cancer: Epidemiology, management, and the implications for patients. Cancer 2016;122:3754-9. [Crossref] [PubMed]
- Thyroid Cancer Statistics. Available online: http://seercancergov/statfacts/html/thyrohtml. Accessed June 15, 2016.
- Chang YW, Kim HS, Kim HY, et al. Should central lymph node dissection be considered for all papillary thyroid microcarcinoma? Asian J Surg 2016;39:197-201. [Crossref] [PubMed]
- Warburg O. On the origin of cancer cells. Science 1956;123:309-14. [Crossref] [PubMed]
- Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol 2011;27:441-64. [Crossref] [PubMed]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009;324:1029-33. [Crossref] [PubMed]
- Coelho RG, Cazarin JM, Cavalcanti de Albuquerque JP, et al. Differential glycolytic profile and Warburg effect in papillary thyroid carcinoma cell lines. Oncol Rep 2016;36:3673-81. [Crossref] [PubMed]
- Dosoky NS, Setzer WN. Chemical Composition and Biological Activities of Essential Oils of Curcuma Species. Nutrients 2018;10:1196. [Crossref] [PubMed]
- Long J, Liu Z, Hui L. Anti-tumor effect and mechanistic study of elemene on pancreatic carcinoma. BMC Complement Altern Med 2019;19:133. [Crossref] [PubMed]
- Pan Y, Wang W, Huang S, et al. Beta-elemene inhibits breast cancer metastasis through blocking pyruvate kinase M2 dimerization and nuclear translocation. J Cell Mol Med 2019;23:6846-58. [Crossref] [PubMed]
- Xu L, Guo T, Qu X, et al. beta-elemene increases the sensitivity of gastric cancer cells to TRAIL by promoting the formation of DISC in lipid rafts. Cell Biol Int 2018;42:1377-85. [Crossref] [PubMed]
- Guo Z, Liu Z, Yue H, et al. Beta-elemene increases chemosensitivity to 5-fluorouracil through down-regulating microRNA-191 expression in colorectal carcinoma cells. J Cell Biochem 2018;119:7032-9. [Crossref] [PubMed]
- Zhou J, He LL, Ding XF, et al. Combinatorial Antitumor Effect of Rapamycin and beta-Elemene in Follicular Thyroid Cancer Cells. Biomed Res Int 2016;2016:6723807. [Crossref] [PubMed]
- Elkin ER, Harris SM, Loch-Caruso R. Trichloroethylene metabolite S-(1,2-dichlorovinyl)-l-cysteine induces lipid peroxidation-associated apoptosis via the intrinsic and extrinsic apoptosis pathways in a first-trimester placental cell line. Toxicol Appl Pharmacol 2018;338:30-42. [Crossref] [PubMed]
- Rathore R, McCallum JE, Varghese E, et al. Overcoming chemotherapy drug resistance by targeting inhibitors of apoptosis proteins (IAPs). Apoptosis 2017;22:898-919. [Crossref] [PubMed]
- Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA 2006;295:2164-7. [Crossref] [PubMed]
- Pilli T, Prasad KV, Jayarama S, et al. Potential utility and limitations of thyroid cancer cell lines as models for studying thyroid cancer. Thyroid 2009;19:1333-42. [Crossref] [PubMed]
- Khatami F, Larijani B, Nikfar S, et al. Personalized treatment options for thyroid cancer: current perspectives. Pharmgenomics Pers Med 2019;12:235-45. [Crossref] [PubMed]
- Faubert B, Boily G, Izreig S, et al. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab 2013;17:113-24. [Crossref] [PubMed]
- Patra KC, Wang Q, Bhaskar PT, et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell 2013;24:213-28. [Crossref] [PubMed]
- Li L, Liang Y, Kang L, et al. Transcriptional Regulation of the Warburg Effect in Cancer by SIX1. Cancer Cell 2018;33:368-85.e7. [Crossref] [PubMed]
- Xu Q, Tu J, Dou C, et al. HSP90 promotes cell glycolysis, proliferation and inhibits apoptosis by regulating PKM2 abundance via Thr-328 phosphorylation in hepatocellular carcinoma. Mol Cancer 2017;16:178. [Crossref] [PubMed]
- Graziano F, Ruzzo A, Giacomini E, et al. Glycolysis gene expression analysis and selective metabolic advantage in the clinical progression of colorectal cancer. Pharmacogenomics J 2017;17:258-64. [Crossref] [PubMed]
- Leemans CR, Snijders PJF, Brakenhoff RH. The molecular landscape of head and neck cancer. Nat Rev Cancer 2018;18:269-82. [Crossref] [PubMed]
- Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science 1998;281:1322-6. [Crossref] [PubMed]
- Chen D, Zheng X, Kang D, et al. Apoptosis and expression of the Bcl-2 family of proteins and P53 in human pancreatic ductal adenocarcinoma. Med Princ Pract 2012;21:68-73. [Crossref] [PubMed]
- Wang SL, Yu XJ, Zhao ZK. Haplopappus gracillis Resources of Elemene in China: a Review. Zhongguo Zhong Xi Yi Jie He Za Zhi 2015;35:764-8. [PubMed]
- Wang TS, Sosa JA. Thyroid surgery for differentiated thyroid cancer - recent advances and future directions. Nat Rev Endocrinol 2018;14:670-83. [Crossref] [PubMed]
- Chekhonin VP, Shein SA, Korchagina AA, et al. VEGF in tumor progression and targeted therapy. Curr Cancer Drug Targets 2013;13:423-43. [Crossref] [PubMed]
(English Language Editor: J. Chapnick)


