
A narrative review of intraocular lens opacifications: update 2020
Introduction
Twenty million cataract surgeries are being performed annually worldwide defining it as the most common surgery (1). Nearly five million cataract surgeries were performed in Europe in 2017 (2). It was estimated that in the Unites States 9.5 million people would have pseudophakia by 2020 (3). The main purpose of intraocular lenses (IOLs) that are implanted during cataract surgery is to restore vision (1). Their performance depends on different factors, including surgical technique, possible complications, lens biomaterial and design, and host reaction (4). Posterior capsular opacification (PCO) occurs in 10% of patients two years after cataract surgery and is the most common of long-term complications (5). After 5–7 years the incidence may rise to 30–35% (6). Vock et al. reported that 42% of the eyes needed Nd:YAG laser capsulotomy due to the PCO 10 years after surgery (7).
It develops from remaining lens epithelial cells, which proliferate and migrate over the posterior lens capsule (8). Other postoperative late complications include cystoid macular edema, retinal detachment, endophthalmitis, lens dislocation, and IOL opacification (5). The IOL’s biomaterial is one of the most critical factors leading to possible post-surgery complications, such as posterior and anterior capsule or IOL opacification formation (3). The main IOLs materials are hydrophobic, or hydrophilic acrylate, polymethylmethacrylate (PMMA), and silicone (9). Different types of material are associated with different types of IOLs opacifications, which include photochemical material alterations, precipitations and depositions, glistenings, and discoloration (1). Snowflake degeneration occurred as intraoptic spherical lesions of PMMA material lenses in the central and midperipheral portion of the optic (10). Silicone IOLs were the first foldable lenses and were known to undergo brownish discoloration and central haze within the first 6 weeks postoperatively (11). The hydrophobic acrylic lenses have been known to show glistenings, while calcifications develop more often in hydrophilic acrylic lenses (11).
Acrylic foldable IOLs have become the most popular type of IOLs that are implanted during cataract surgery (9). It was reported that Alcon hydrophobic acrylic IOLs are one of most commonly implanted, and since 1955, over 40 million such IOLs have been implanted (12,13). However, hydrophilic IOLs have different water content, which makes them more flexible and implantable through smaller incisions than hydrophobic lenses (11,14,15). Hydrophilic IOLs have better tissue compatibility, though it encourages lens epithelial cell proliferation and migration, leading to posterior capsule opacifications (4). The incidence of postoperative complications, including posterior capsule opacifications, was noticed to be lower with acrylic IOLs than with other materials, and with the lowest incidence in hydrophobic lenses (16,17). Leydolt et al. performed a randomized controlled study and found that new hydrophobic acrylic Vininex XY1 IOLs had significantly lower PCO rates than hydrophobic AcrySof SN60WF IOLs, which were considered to have one of the lowest PCO rates (18). It was reported that anterior capsule opacification and phimosis were significantly less observed in the Tecnis IOLs with a continuous edge than in AcrySof IOLs with an interrupted sharp optic edge (8,19). However, neither of the acrylic lenses is free of material opacification and degradations, which are infrequent but can reduce visual performance (1).
Data about IOL opacification impact on visual acuity is controversial. While the majority of the peer-reviewed studies did not show a significant impact of glistenings and subsurface nanoglistenings on visual acuity, there are recent data about its significant effect on straylight (1,12,20). Increased straylight can result in disability glare, hazy vision, and loss of contrast (21). The main sources of light scattering in the eye are the crystalline lens, cornea, fundus reflectance, and light transmittance by sclera and iris (22). Due to the aging processes, including cataract formation, the straylight increases to an average of 1.20 log(s) at 65 years of age, while in youth, the straylight value is on average 0.90 log(s) (22). Although straylight decreases significantly after cataract surgery, some of the studies results show high straylight values in pseudophakic eyes (21). This can be determined by the changes in the IOL material such as calcification, glistenings, subsurface nanoglistenings, which can increase the light scattering, therefore having an impact on visual quality (21,23).
The aim of this review is to discuss the recent literature on IOL opacifications and their impact on vision quality.
We present the following article in accordance with the narrative review reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4207).
Methods
PubMed was used for the medical literature search, which was conducted up to Apr 7, 2020. The following keywords were used in various combinations: cataract surgery, phacoemulsification, intraocular lens, opacification, glistening, subsurface nanoglistenings, calcification, snowflake degeneration. Only articles having English abstracts were reviewed. The reference lists of identified publications were also considered as a potential source of relevant articled. Studies were critically reviewed to create an overview and guidance for further search, and no attempts to discover unpublished data were made. Emphasis was placed on articles published since 2010, however, we focused mainly on the risk factors for IOL opacification in particular IOL types. In addition to the literature search, selected chapters from relevant textbooks were included if necessary. Due to the large number of studies, in the tables we have presented only original studies and case series, but not single case reports.
Glistenings
Glistenings are small fluid-filled vacuoles in the IOL material (Figure 1), which size usually varies between 1 and 20 µm (24). Microvacuoles refractive indices differ from IOL material, so when the light redirects and a portion of the light is scattered backward to the observer, it is seen as refractive particles that glisten on a slit lamp examination (1,20).
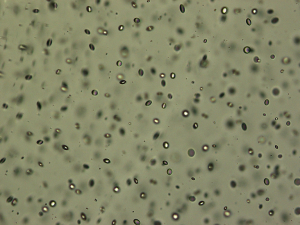
One of the main theories of glistenings formation was described by Kato et al. in 2001 (25). They concluded that small changes in temperature could cause the decompensation of the IOL swollen polymer network, initiating the formation of microvacuoles, which consists of water and loosely packed network chains (25). Saylor and colleagues reported that the water-filled cavities could develop due to osmotic pressure differences between the aqueous solution within the cavity and the external liquid in which the lens is immersed (26). As a result of these mechanisms, water permeates micro-channels within the IOL material and forms small inclusions (1). They can be distributed throughout the entire lens optic, but most often are seen in the anterior and posterior IOL surfaces (27). In addition to these proposed mechanisms, IOLs manufacturing methods and packaging might also have an impact on glistening formation as well as its material itself (13). It is known that osmotic and temperature changes are important components in the mechanism of glistening formation (1,25,26). However, the breakdown of the blood-aqueous barrier (BAB) and intraocular inflammatory factors might also have a significant impact on the development of glistening (1). BAB is known to be damaged in diabetes mellitus, uveitis, postoperative inflammation, glaucoma, so that these pathologies may induce the formation of glistening (28,29).
Subsurface nanoglistening can be referred to as whitening (Figure 2) of the hydrophobic acrylic IOL affecting the surface or subsurface of the IOL (differently from glistening, which occur within the substance of IOL) (30,31). The formation of these vacuoles is caused by an infiltration of water molecules that can form aggregates within the subsurface of the lens optic (1,12,30,31). Nanovacuoles diameter is less than one µm (between 140 and 185 nm) (1,32). Ong et al. reported that the main source of hydrophobic acrylic IOL surface light scattering was subsurface nanoglistening (32).
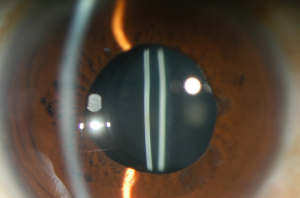
Glistening can be found in all IOL materials, including PMMA, silicone, and hydrophilic acrylic IOLs, but they are observed predominantly in hydrophobic acrylic lenses, which are one of the most commonly used (24,27,28,33). It was reported that glistening in hydrophobic acrylic lenses have higher density (16,33). However, Łabuz et al. reported that different types of hydrophobic acrylic IOLs also differ in their resistance to glistening formation (20). The majority of studies showed a tendency of glistening formation in hydrophobic acrylic lenses that were manufactured by Alcon company (1). The incidence of glistening in AcrySof IOLs increases with time, and it varies from 66% to 100% between published studies results (1). Colin et al. reported the formation of glistening in 86.5% of implanted AcrySof SN60WF IOLs, despite manufacturing changes (34). However, Miyata et al. reported that the improved manufacturing process of AcrySof IOLs suppressed the development of surface light scattering up to 3 years postoperatively (35). It was proposed that other types of hydrophobic acrylic IOLs like enVista (manufactured by Bausch and Lomb) are glistening free clinically (36). Glistening were not noticed to occur in iMics1 NY-60 when compared with the AcrySof SN60WF 3 years after surgery (37). It was found that recently developed hydrophobic acrylic materials that have a higher water content than the standard (less than 0.5%), which include the enVista MX60, the Eternity W-60, PodEye IOLs are glistenings-free in vitro and in vivo (23). Werner et al. compared new Clareon CNA0T0 IOLs, which have a water content of 1.5% with 5 other hydrophobic IOLs (23). The authors found that the Clareon showed the lowest levels of surface haze, surface roughness, subsurface nanoglistening, and glistening (23).
Although hydrophobicity is an important factor in glistening formation, however visual quality may be influenced by glistening properties and their impact on different optical parameters.
Philippaki et al. compared glistening formation, their size, and its effect on straylight between the Alcon AcrySof SN60WF and Santen Eternity Natural Uni NW-60 IOLs (13). However, the authors suggested to evaluate their results carefully, as they had not had the information if the used IOLs were manufactured before or after the changes of manufacturing process that were announced by Alcon in 2011 (13). Nevertheless, they found a statistically significantly higher number of glistening produced in Alcon AcrySof SN60WF while Eternity Natural Uni NW-60 IOLs developed larger glistening (13). Forward light scattering for the AcrySof lenses, which produced smaller, but greater density glistening, was higher than for the Eternity Natural Uni lenses (13). These results were similar to Labuz et al. study results, which showed that there is a proportional relationship between the number and the surface portion of glistening and their effect on straylight (38). More studies found similar results, concluding that glistening of the smaller size and higher density increased the light scattering more (39,40).
Matsushima et al. reported that glistening and subsurface nanoglistenings caused decreased vision in 5 patients, leading to IOL explantation, which followed the improvement of visual acuity (12). However, the majority of the studies did not find a significant reduction of visual acuity caused by glistening or subsurface nanoglistening (1,16,34,35,41-43). Xi with colleagues, reported that more severe glistening caused the reduction of contrast sensitivity at a high spatial frequency and visual field. They suggested that the mean deviation (MD) can be considered to be used as an indicator for the visual performance of glistening in IOLs (44). The significant decrease of contrast sensitivity at high spatial frequencies with a higher grade of glistening severity was reported by Schweitzer et al. (41). The study results also showed a significant association between the incidence of glistening (61.2% including grades 1 and 2) and the number of topical glaucoma drugs. The disruption of BAB can be caused by inflammation, chronic use of glaucoma eye drops (41). These were suggested as possible factors that may impact the development of a higher number of glistening associated with the daily use of glaucoma drops (41). Godlewska et al. performed a study with 252 patients undergoing phacoemulsification with AcrySof IQ IOLs implantation, to evaluate the effect of selected perioperative factors and concomitant diseases to glistening formation. They reported a significantly higher severity of glistening in patients with diabetes, which may influence the breakdown of physiological intraocular barriers (45). A higher refractive power of the intraocular lens and the use of bigger diameter cartridge during phacoemulsification were significantly related to the higher severity of glistening (45). The higher refractive power of the IOL can be associated with the increase in glistening severity due to the thicker IOL matrix and the higher amount of material (24,42). However, other studies did not find a correlation between the IOLs power and the number of glistening in hydrophobic AcrySof IOLs (44,46).
Nevertheless, more studies showed that glistening could degrade vision by inducing glare symptoms more than lowering visual acuity or contrast sensitivity (20,38). The optical performance of IOL is usually evaluated by using the modulation transfer function (MTF), which describes the ability of an IOL to project light from an object onto the retina for different spatial frequencies (47,48). Light scattering from an IOL is quantified as straylight and is used to measure a patient’s glare symptoms (46,47). This parameter is becoming an essential aspect of vision quality evaluation (47-49). In laboratory studies, straylight can be quantified using a clinical device (50). It was reported that straylight is a susceptible measurement to detect the presence of IOL pathology, including opacifications. However, MTF as the optical quality of IOL deteriorates if the opacification is severe (51). The correlation between IOL structural changes, including opacification, and straylight was found in Łabuz et al. study, where IOLs were randomly extracted from donor’s eyes (21). The results were compared with another study, conducted by the same author, where glistening was induced in vitro (19,21). The authors indicated that scattering effects of in vitro induced glistening could be compared to glistening that form in vivo (20).
The straylight increases with age as the crystalline lens ages, and decreases after cataract surgery (20). However, Łabuz et al. reported that 20% of studied IOLs had an increased level of straylight (an average straylight parameter of 18.1 deg2/sr), which could be compared with a 70 to 80-year-old crystalline lens induced straylight (20). The results of this study were affected by glistening formation in hydrophobic acrylic lenses (20). An important conclusion from this study was made that the straylight proportionally depends on the glistening number despite the differences of IOL material. (20). DeHoog et al. reported similar findings that a significant decrease of MTF values depended on the size and density of glistening, not on IOLs material (40).
As more studies used light scattering and straylight measurements, straylight became a reliable indicator for assessing the quality of vision (52). Miyata et al. found that light scattering on the anterior and posterior surfaces of the AcrySof IOL increased during the years (53). They did not find the significant correlation between the increased surface light scattering and changes in visual acuity, however, there were more cases with decreased visual acuity when the light scattering exceeded higher values (53).
The majority of the studies did not find significant subsurface nanoglistening impact on visual acuity, although it can increase light scattering significantly (43,54,55). Werner et al. found that nanoglistening increased the light scatter and straylight, however, straylight levels were below the value of straylight hindrance [1.47 log(s)], leading to the conclusion that they were not able to cause significant and noticeable visual impairment (54).
Although recent studies showed that glistening could cause a significant increase of straylight and glare, straylight measurements cannot be compared to visual acuity or contrast sensitivity as these were identified as independent metrics (52,56). Contrary to that, Alarcon et al. found that the MTF, which is used for the preclinical optical performance of IOL, correlated well with clinical data, including visual acuity and contrast sensitivity (57). Weindler et al. performed a study to evaluate the optical quality of hydrophobic acrylic IOLs with glistening (52). They used a classification based on the glistening number per mm2: grade 0 (none), grade 1 (1 to 100), grade 2 (101 to 200), grade 3 (201 to 500), and grade 4 (more than 500) (52). The authors reported that a low number of glistening (<500 microvacuoles/mm2) did not affect the optical quality of IOLs, but grade 4 glistening had significantly deteriorated MTF and Strehl ratio. However, they were evaluated as small (52). The reported results showed that even severe glistening had minimal impact on MTF values, suggesting that the visual acuity remains unaffected (52).
Son et al. performed a study to analyze different types of IOLs by assessing ray propagation while using their proposed visualization technique (58). The authors confirmed that the ray propagation could be visualized qualitatively and assessed quantitatively in different IOL models by using the proposed imaging technique (58). Moreover, they found that the image quality of IOLs also depends on light energy distribution (58).
Calcifications
Calcification occurs as the deposits of calcium phosphate accumulate in various bioprosthetic or biomaterial implants, including IOL, in the human body (1). Neuhann et al. suggested three main groups of calcifications: primary—related to IOL itself (properties of the polymer, it’s surface or IOL packaging), the secondary calcification can occur as a result of diseases or pathologies that causes the disruption of BAB and pseudocalcification when false positive staining of calcium occurs (59). However, usually, calcification is a multifactorial problem (60). It appears as the surface irregularity in the central part of the optic or distributes over different parts of the IOL (61) (Figures 3,4). Postoperative IOL’s calcification can look similar to the posterior capsule opacification (14). Nd:YAG laser treatment or mechanical scraping is usually ineffective, because most often deposits lie within the IOL material (62). IOL exchange due to the calcification can be the only possible method to restore reduced visual acuity, however, IOL explantation is associated with higher intraoperative complication rate (62). Dagres et al. reported such complications as zonular dehiscence, posterior capsular rupture, and corneal decompensation, which were related with IOL exchange surgery in 48% of cases with opacified IOLs (63). Unnecessary Nd:YAG laser capsulotomy in eyes with opacified IOLs may also increase the complication rate during the IOL’s exchange procedure (64). It was reported that 33% of IOL exchanges require an anterior vitrectomy, this can increase to 48% with a previously performed Nd:YAG capsulotomy (65).

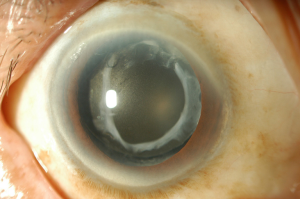
The anterior segment optical coherence tomography (AS-OCT) was reported to be a reliable method to assess the presence, location and density of IOL’s changes, including calcification, however, very superficial changes could not be detected (10,66).
The majority of the studies reported IOL calcification more commonly in hydrophilic acrylic IOLs (1,62,67). Choudhry et al. described two types of hydrophilic acrylic IOLs calcification: first consists of calcium precipitates on the IOL surfaces. In contrast, the second type includes granular calcium deposits within the substance of the IOL optic, beneath the anterior surface and in front of the posterior surface of the lens optic, the haptics and the edge of the optic (66).
Several studies evaluated secondary calcifications, i.e. calcifications related with other diseases, pathologies or specific intraoperative procedures. Although Ma et al. reported calcification of hydrophobic acrylic IOL after pars plana vitrectomy (PPV) with silicone oil tamponade, the patient had diabetes mellitus, that could cause the breakdown of BAB as mentioned above, and it was a single case (68). Few studies showed that the hydrophobic surface of hydrophilic acrylic IOLs does not protect them from the development of calcification because it initiates from the hydrophilic subsurface (14,15).
Studies performed during the last decade indicated that there is a tendency of centrally localized IOL calcification, that is restricted to the pupillary or capsulorhexis area. This type of calcification occurred after posterior lamellar keratoplasty: Descemet-stripping automated endothelial keratoplasty (DSAEK), Descemet membrane endothelial keratoplasty (DMEK), and pars plana vitrectomy (PPV), with the intraocular injection of gas or air (51,62). It was reported that direct air or gas contact with the IOL’s surface increases the risk of hydrophilic IOLs opacification (51). However, the incidence of calcification is very low, after DMEK and DSAEK vary between 2.5–5% (51,69,70).
Werner et al. proposed possible causes of calcification after surgeries that require exogenous gas or other substances injection into the eye (60). Injected gas, air, tissue plasminogen activator, silicone oil can have direct contact to IOL surface. Secondly, it can be related to a metabolic change in the anterior chamber due to the presence of the exogenous substance and lastly—exacerbated inflammatory reaction with the breakdown of BAB caused by the surgical procedure itself (1,60).
The authors also reported that there is no association between this distinctive calcification localization and IOL design or manufacturer (60). A similar conclusion was made by Giers et al., who conducted a study of 11 cases of explanted hydrophilic IOLs with calcification from 4 different manufacturers after DMEK or DSAEK. The authors suggested that calcification occurs irrespective of the manufacturer or the exact composition of the hydrophilic lens material (71).
Most of the single case reports presented calcification of hydrophilic IOLs after DMEK, DSAEK (72-75). It is important to note that all these cases, except the report by Lee, had a somehow complicated postoperative course that led to repeated or different types of surgeries, suggesting that breakdown of BAB during surgeries might also influence the calcification process (68). Lee reported a case when the patient needed the rebulbing after DSAEK (74). It was reported in two studies that rebubbling after DSAEK and DMEK significantly elevates the risk of the development of IOL’s calcification (69,76). It was proposed that elevated IOP after the injection of intracameral air might also be a risk factor of the calcification process (76,77). Although Ahad et al. observed the reduction of the opacification rate after reducing the time of high-pressure (IOP higher than 40 mmHg) air tamponade from 1 h to 10 min, they did not get any statistically significant data (76).
Similar cases of centrally localized IOLs calcification after PPV with intraocular gas/air injection were more commonly observed in recent years. It was reported by Werner et al. that the possible cause of centrally localized calcification of anterior IOLs surface can be the migration of gas or silicone oil into the anterior chamber via zonular fiber defects (60). Khurana et al. reported a single case of anterior surface calcification of a hydrophilic acrylic IOL when the exposure of air in the anterior chamber was observed the next day after PPV (78). The authors proposed a possible mechanism (78). The exposure of gas to the IOL surface results in an increased hydrolyzation of the polyacrylate, forming free carboxylic acid groups that accumulate at the IOL surface triggering biomineralization, thereby covering the IOL optic with calcium phosphate deposits (78). These mechanisms could explain the fact that calcification was not seen in the IOL parts that were covered with the capsule (62,78). However, some of the studies reported cases with no noticeable gas migration to the anterior chamber (61,79). Likewise, the opacification of the posterior capsule was reported by Marcovich et al. (61). The authors performed a study with detected opacification in 11 hydrophilic acrylic IOLs produced by six different manufacturers, 1 month to 6 years after PPV involving the intravitreal gas injection (61). The authors hypothesized that the calcification might be caused by dehydration of the IOL due to slowly dissolving gas (61). The dehydration may induce chemical alterations on the IOL surface, causing deposition of calcium and phosphate from the aqueous humor in the exposed areas (61). Yildirim et al. performed a study with one of the most extensive series of confirmed calcification in 10 explanted hydrophilic acrylic IOLs after PPV with intraocular gas injection (62). They found that calcification was not present only on the surface of the IOL but up to 100 µm within the material (62). In most of the cases, calcification was found in the anterior central pupillary area (62). The authors reported that there is a strong correlation between the density and size of the calcium deposits and the decrease in the IOL’s optical quality (47,62).
We found one study by Fung et al., who reported 7 cases of hydrophilic IOLs opacification after treatment with intracameral recombinant tissue plasminogen activator (rtPA) and hypothesized that rtPA possibly disrupts the BAB (80).
It is known that calcification in silicone IOLs is associated with the coexistence of asteroid hyalosis, as more than 85% of patients with calcification had clinically detectable ipsilateral asteroid hyalosis (10,65,81,82). Calcification is thought to be caused by the same process as asteroid hyalosis because asteroid bodies are rich of calcium and phosphate, and it can occur despite the intact posterior capsule (10,81). Espandar et al. reported three cases of silicone IOLs opacification in patients with asteroid hyalosis, who underwent Nd:YAG capsulotomy and that led to more chalengin IOLs exchange (81). Platt et al. proposed a possible method to remove calcified deposits from the posterior surface of IOL as the exchange of IOLs is associated with intraoperative and postoperative complications (65). The authors presented a surgical technique that included PPV, a lighted pick, and a modified silicone-tipped cannula with successful removal of late calcium deposition. However, it was a single case, and the follow-up was limited to 6 months (65).
Primary calcification is associated with the problems of IOL itself (59). It was reported that some of the different models of Oculentis IOLs, implanted between 2009 and 2012, were affected by primary calcification because no other significant causes were found (11,47). After the efforts to discover possible causes, the company concluded that its origin might be multifactorial and published few safety notifications (11,47). Barra et al. performed a study with the same design hydrophilic Ioflex IOLs with calcification (67). They found the difference of light transmittance at a certain region in explanted and control IOLs, which had different expiration dates (67). The authors noted the importance of the manufacturing process evaluation because they found that different materials can be used or manufacturing processes can vary at a different time while manufacturing the same type of IOLs (67).
Yildirim et al. performed a study with nine segmented refractive bifocal Lentis MF IOLs, which were affected by primary calcification, as the granular deposits were found underneath the anterior and posterior surfaces distributed throughout the whole IOL, including the haptics (47). The authors reported that the density of calcium phosphate granules affected straylight significantly because the highest values were found in most severe cases (47). They did not find the correlation between light scattering and the MTF or visual acuity, suggesting that these are independent factors, and visual acuity may not be sufficient parameter to quantify the effect of IOLs’ calcification (47). The similar results were reported by Łabuz et al. (51). A severe increase of straylight was caused by IOLs’ calcification, which occurrence was associated with intraocular gas injection (51). However, the MTF values decreased just in two IOLs. These results were similar to other studies results, where MTF values did not show a significant effect too (51). The authors found a proportional relationship between the straylight parameter and the size and number of calcium deposits, suggesting that there is variability in the optical quality of affected IOLs (51). Tandogan et al. reported that MTF values deteriorated significantly in explanted Euromaxx ALI313Y and ALI313 IOLs with the calcification of the entire optic (64).
Discussion
We presented above main studies on IOL opacifications based on the review of recent literature. The summary of major recent original papers on glistenings is presented in Table 1 and on calcifications in Table 2. In this section we discuss two important and controversial issues related to IOL opacifications, which are risk factors and impact on visual quality.

Full table

Full table
It is known that the material of IOL plays an important role in opacification formation. Glistenings occurred more in hydrophobic IOLs and some of the studies observed it despite manufacturing changes (34,82). However, Łabuz et al. showed that different hydrophobic materials differ in their resistance to the glistening formation (20). One of the possible causes is the difference of water content, as it was reported that certain hydrophobic IOLs with higher than ordinary water content was glistening free or its amount was showed to be very low (23). It was proposed that the higher refractive power of IOL can be associated with higher severity of glistenings. However, we found three studies (44,46,83) that did not find the significant association between the IOLs power and the severity of glistenings and previously reported findings could be caused by the higher thickness of the material (24,42,45).
Two long follow up studies showed that a higher amount of glistenings formed in AcrySof IOLs than in ZCBOO IOLs, likewise the anterior capsule opacification and fibrosis was more seen in AcrySof IOLs (8,19). It was suggested that glistenings formation might be associated with the occurrence of anterior capsule opacification and tightness of the capsular bag (1). However, the authors did not perform analysis to evaluate this possible association, and they reported it as an observation. It is also known that glistenings tend to occur more often in AcrySof IOLs than in other hydrophobic IOLs.
One of the most important factors in the development of opacifications: glistenings and calcification, despite the properties of IOL, is the breakdown of BAB, which modifies the aqueous humor composition (3). It is known to be disrupted in diabetes mellitus, and it was the main systemic disease associated with opacification formation (12,45).
The significant association between glistenings formation and glaucoma was reported, as the daily use of topical glaucoma medications may lead to the rupture of BAB (41). Although Godlewska et al. did not find a statistically significant difference, they observed that glistenings formation was more frequent in glaucoma patients when compared to subjects without glaucoma (45). The breakdown of BAB can result in uveitis, and it was linked to glistenings formation. We found only one study which assessed patients with uveitis, however, the authors reported that they received high intensity steroid therapy, and the results showed statistically lower severity of glistenings in these patients (45).
The majority of the IOLs with confirmed calcification were hydrophilic in the reviewed studies. However, few cases of calcification in hydrophobic acrylic IOLs were reported, likewise, calcification was observed in silicone IOLs in association with the asteroid hyalosis, suggesting that calcification is not the problem only of hydrophilic IOLs (10,65,68,69,81).
Complex or prolonged surgery and postoperative inflammation can lead to the breakdown of BAB, and that may induce glistening and calcification formation in the IOLs (1,68,73,75). Although Gurabardhi et al. did not find a statistical correlation between ocular or systemic comorbidities and primary calcification, they found that diabetes, uveitis, and glaucoma were the most frequent pathologies (11). Although the incidence of IOLs calcification is low and studies evaluated not more than fifteen IOLs explanted due to the secondary calcification, it showed a significant effect of intraocular injection of exogenous air or gas on calcification formation as well as on the tendency of central localization. The main surgeries that required intraocular gas, air or silicone (86) injection were DSEK, DSAEK, DMEK, and PPV. The rebubbling after DSAEK and DMEK was reported as a significant risk factor for the development of IOL’s calcification. The summary of risk factors for IOL glistenings and calcifications is presented in Table 3.

Full table
The early controversy related with the influence of IOL glistenings on visual function was focused on visual acuity. The majority of the reviewed studies that evaluated visual acuity did not find a significant glistening effect on visual acuity. However, one study, conducted by Matsushima et al. found that glistenings caused significantly decreased visual acuity in five patients, leading to IOL explantation (12). However, they did not perform statistical analysis to evaluate the changes in visual acuity (12). As well as all patients had ocular comorbidities: one had a history of uveitis, one had glaucoma, two had diabetic retinopathy, one macular hole, these last three cases had a history of PPV (12). It was reported that a 5-fold increase in light scattering resulted only in a small decrease of 0.1 log units in contrast sensitivity and had no effect on visual acuity (38,56). This finding is similar to ours, as most of the studies did not find effect on visual acuity, some of the studies showed decreased contrast sensitivity (16,39,44). The reduction of contrast sensitivity can be associated with the increase of straylight and disability glare. As more studies showed decreased contrast sensitivity than visual acuity, it could confirm that disability glare and straylight are more appropriate in evaluating IOLs opacification effect on visual quality.
That was seen in recent years studies that used the straylight measurement more commonly to evaluate the visual quality. All of the reviewed studies that measured straylight values showed a significant increase of straylight when opacification, including both glistenings and calcifications, were detected. The majority of the studies reported increased straylight values with a higher amount of glistenings (20,38). However, few studies highlighted the importance of its size. Henriksen et al. performed an in vivo study with 79 patients and found the correlation between smaller size glistenings and increased light scattering (39). They also found that the age of the IOL had a negative correlation with the contrast visual acuity with glare and corrected distance visual acuity in patients group with smaller glistening size, suggesting that smaller glistenings had a more significant impact on visual function (39). However, they did not include and evaluate IOL opacification resulting in surface light scattering in their study, which could also have an impact on the reported results (39). It was reported by Philippaki and colleagues that the higher amount and smaller glistenings produced higher levels of straylight (13). The authors reported that the straylight of two out of five AcrySof IOLs exceeded that of the 70-year-old CIE standard glare observer, however, it was proposed that these high values could be caused by subsurface nanoglistenings which were not evaluated by the glistenings detection program (13). Although this study presented an important finding of glistenings size effect, the sample of the study is too small, also they did not report the threshold of glistening size and density which could have a significant impact on visual performance.
The majority of the studies did not present the exact levels of glistenings parameters or straylight values that would have a significant impact on certain visual performances. However, straylight values could be compared to the data found in the literature. The straylight level of a 20-year-old was reported to be s = 2.5 deg2/steradian (sr), 70-year-old s = 11.2 deg2/sr, and cataractous s = 33.1 deg2/sr crystalline lens (51). It was reported that the hindrance of straylight could be 1.47 log(s) (54). van der Mooren et al. defined that a lens with significant glistenings would be the lens that caused straylight levels above those of a healthy 20-year-old crystalline lens (85). Increased straylight by 19.0 deg2/sr was reported to be associated with a 76% increase in halo size and a severe loss in luminance detection threshold (79).
Łabuz et al. found a proportional relationship between straylight, amount of glistenings, and the surface portion (38). They concluded that a large number of glistenings were needed to cause significant straylight increase (38). The same group of the authors conducted the other study and reported that 60% of thirty hydrophobic IOLs with in vitro induced glistenings had straylight values below that for the young lens (20). Only 20% reached light scattering levels with an average of 18.1 deg2/sr. that could have the potential to hinder visual performance (20). Łabuz et al. evaluated the light scattering in IOLs extracted from donors’ eyes (21). They reported that the scattering intensity was higher than in the 70-year-old lens in 14% of the IOLs, and none showed straylight values that could correspond to the cataractous lens (21). The median straylight values in donor IOLs were approximately 0.3 to 1.0 log(s) (s = 2 to 10 deg2/sr) (21).
The majority of the studies were performed in vitro. Although studies used protocols for glistening induction by the thermal accelerated aging process with varying temperature and time setting, it was speculated that there is lack of evidence, if this method produces clinically relevant results. However, Łabuz et al. compared the highest straylight values of 3-piece IOLs from two studies: a study with glistenings induced in vitro and another study with IOLs extracted from donors’ eyes. They also compared the glistening size, and the results were similar. Thus, the authors indicated that the scattering effect of in vitro induced glistenings can be compared to glistenings that form in vivo (20). The reviewed studies results showed that only a small part of IOLs with glistenings could have a significant effect on visual quality. Similar to that, subsurface nanoglistenings did not show a significant impact on visual performance as well. The two studies which evaluated subsurface nanoglistenings in vivo did not find a significant effect on visual acuity, although it increased light scattering (43,57). However, Hiraoka et al. evaluated the backward light scattering, and it was reported that the relationship between forward scatter and backward scatter is weak (43,87). Though, Werner et al. performed a study with seventeen IOLs with subsurface nanoglistenings which were removed from cadaver eyes and evaluated the forward light scattering (54). They found that straylight values in removed IOLs were higher than controls. However, none reached the value of 1.47 log(s) as it was reported as a straylight hindrance level, which could have a severe impact on visual function (54).
Contrary to the straylight values of glistenings, calcified IOLs showed much higher straylight values, suggesting that it is a significant parameter to detect and evaluate opacification occurrence and its severity. The majority of the studies assessed backward scattering of calcified explanted IOLs, and only a few evaluated forward light scatterings. Łabuz et al. performed a study with four explanted calcified IOLs after intravitreal gas and air injection (51). They found the mean straylight parameter of 68.1 deg2/sr, however IOLs had a high variance of straylight values, which were associated with differences in opacification morphology (51). The similar straylight values with the mean straylight parameter of those IOLs at a 5- to 10-degree angle was 47.9 deg2/sr for the explanted hydrophilic IOLs were found in Werner et al. study (51,88).
Yildirim et al. reported the mean straylight value of 170.1±71.5 deg2/sr of eight explanted hydrophilic acrylic segmented refractive bifocal IOLs due to primary calcification (47). What is interesting that despite these high straylight values, most of the patients reported foggy and blurred vision, and the visual acuity was reported as good. However, that symptoms could be caused by bifocal IOLs, as they can induce these unwanted symptoms more often than monofocal IOLs. In a different study performed by the same researchers’ group, two patients reported foggy vision, while half of them reported decreased vision before IOL explantation (62). Nevertheless, the majority of the reviewed studies reported a significant decrease in visual acuity that led to IOL explantation. Only one study and few single case reports did not show considerable calcification impact on visual acuity to that date of the study and the IOLs were not explanted (61,68,75,76). In some of the cases, IOLs exchange were not performed due to the patients refusal or pore expectations of visual acuity improvement caused by concomitant ocular pathologies.
We believe that because it is clear from the past that calcification in hydrophilic materials affects visual function easily, it is not preferable to use hydrophilic materials for IOL until the mechanism of calcification and the preventive method are elucidated.
There is a strong association between light scattering, straylight and glare (89). The scattered light excites the photoreceptors in retina and degrade vision (89). The straylight is the visual effect seen as radiation around the point source of the light (89). It can cause disability glare which is associated with reduced retinal contrast leading to reduction of vision (90). Also, straylight effect can occur as hazy vision or decreased contrast sensitivity (91). Likewise, the straylight can decrease visual quality by inducing difficulties in face recognition, spatial orientation problems, contrast and color loss, however it hardly affects visual acuity (89). It was suggested that lenses opacities effect on vision should be evaluated by the assessment of disability glare rather than visual acuity (92). van den Berg et al. reported that glare have a limited impact only for the young eyes or low beams. For healthy older eyes, the problem was already significant with dimmed beams (90). This could suggest that glare could have a much higher impact on visual quality in eyes with ocular pathologies or abnormalities, as for example IOLs opacification, which induce straylight significantly. This could be confirmed by the fact that the majority of the calcified IOLs were explanted due to reduced visual performance. Although the majority of the reviewed studies showed high straylight values in explanted IOLs, however, patients reported not only decreased visual acuity, but also hazy, foggy vision. Glistenings effect on straylight was mostly evaluated during in vitro studies, the results and values of straylight were more likely considered to have a significant impact on visual quality at a certain level. However, most of the studies showed low percentage of affected IOLs by glistenings that could reduce visual performance.
The summary of studies results evaluating the impact of IOL glistenings and calcifications on visual quality is presented in Table 4.

Full table
Although many studies have focused on visual acuity and contrast sensitivity, relatively little effects were found. van den Berg et al. reported that visual acuity and straylight are independent factors with their impact on visual quality with no significant correlation between them (93). They also concluded that aberrations and micro-aberrations are responsible for the loss of visual acuity due to cataract or other opacities, but not straylight (93). However, straylight induces other problems, which we discussed previously while making it an important part of the quality of vision. It is insufficiently realized that visual acuity or contrast sensitivity are measured using highly artificial tests, so the proper way to assess the visual function effects could be through straylight or light scattering.
The limitation of the majority of reviewed studies based on calcification is the low number of cases and the lack of prospective studies. Contrary to that researches based on glistenings included a higher number of cases in retrospective studies, however a significant part of the studies was performed in vitro, which are considered to produce low reliable evidence.
Conclusions
Glistening occur more often than calcification, but usually, it does not cause a significant decrease in visual acuity that would lead to IOL exchange. The incidence of IOLs calcification is low, however, it significantly reduces vision quality, leading to IOLs explantation. Results of recently reported studies show that particular surgeries, such as DSEK, DSAEK, DMEK and PPV with intraocular gas or air injection, might predispose the calcification process. The authors suggest surgeons being aware of the fact that calcification is more common in hydrophilic acrylic IOLs. However, it was reported that both types of opacification significantly increase straylight, which might result in hazy, foggy vision, or difficulties in dynamic light conditions, leading to persistent visual complaints. Straylight levels depend on opacification size and density, which might be determined by different factors, including IOLs material, manufacturing and packaging processes, concomitant ocular pathologies, inflammatory factors.
The identification and better understanding of possible risk factors and how does opacifications affect visual quality could be useful for future investigations as well as for clinical practice. This could lead to the solutions of avoidance or elimination of opacifications formation while evaluating the most significant and specific factors of visual quality.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Dr. Andrzej Grzybowski) for the series “Recent developments in cataract surgery” published in Annals of Translational Medicine. The article was sent for external peer review organized by the editorial office.
Reporting Checklist: The authors have completed the Narrative Review Reporting Checklist. Available at http://dx.doi.org/10.21037/atm-20-4207
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4207). The series “Recent developments in cataract surgery” was commissioned by the editorial office without any funding or sponsorship. AG served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stanojcic N, Hull C, O'Brart DP. Clinical and material degradations of intraocular lenses: A review. Eur J Ophthalmol 2020;30:823-39. [PubMed]
- Almost 5 million cataract surgeries in the EU in 2017 2019. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/DDN-20191204-1
- Pérez-Vives Cari. Biomaterial Influence on Intraocular Lens Performance: An Overview. J Ophthalmol 2018;2018:2687385. [Crossref] [PubMed]
- Özyol P, Özyol E, Karel F. Biocompatibility of Intraocular Lenses. Turk J Ophthalmol 2017;47:221-5. [Crossref] [PubMed]
- Astbury N, Nyamai LA. Detecting and managing complications in cataract patients. Community Eye Health 2016;29:27-9. [PubMed]
- Apple DJ, Escobar-Gomez M, Zaugg B, et al. Modern Cataract Surgery: Unfinished Business and Unanswered Questions. Surv Ophthalmol 2011;56:S3-S53. [Crossref] [PubMed]
- Vock L, Menapace R, Stifter E, et al. Posterior capsule opacification and neodymium:YAG laser capsulotomy rates with a round-edged silicone and a sharp-edged hydrophobic acrylic intraocular lens 10 years after surgery. J Cataract Refract Surg 2009;35:459-65. [Crossref] [PubMed]
- Johansson B. Glistenings, anterior/posterior capsular opacification and incidence of Nd:YAG laser treatments with two aspheric hydrophobic acrylic intraocular lenses - a long‐term intra‐individual study. Acta Ophthalmol 2017;95:671-7. [Crossref] [PubMed]
- Bellucci R. An Introduction to Intraocular Lenses: Material, Optics, Haptics, Design and Aberration. ESASO Course Series. Basel, Karger: Cataract 2013;3:38-55.
- Werner L, Michelson J, Ollerton A, et al. Anterior segment optical coherence tomography in the assessment of postoperative intraocular lens optic changes. J Cataract Refract Surg 2012;38:1077-85. [Crossref] [PubMed]
- Gurabardhi M, Häberle H, Aurich H, et al. Serial intraocular lens opacifications of different designs from the same manufacturer: clinical and light microscopic results of 71 explant cases. J Cataract Refract Surg 2018;44:1326-32. [Crossref] [PubMed]
- Matsushima H, Nagata M, Katsuki Y, et al. Decreased visual acuity resulting from glistening and sub-surface nano-glistening formation in intraocular lenses: A retrospective analysis of 5 cases. Saudi J Ophthalmol 2015;29:259-63. [Crossref] [PubMed]
- Philippaki E, O'Brart DP, Hull CC. Comparison of glistenings formation and their effect on forward light scatter between the AcrySof SN60WF and Eternity Natural Uni NW-60 intraocular lenses. BMJ Open Ophthalmol 2020;5:e000399. [Crossref] [PubMed]
- Gartaganis SP, Prahs P, Lazari ED, et al. Calcification of Hydrophilic Acrylic Intraocular Lenses With a Hydrophobic Surface: Laboratory Analysis of 6 Cases. Am J Ophthalmol 2016;168:68-77. [Crossref] [PubMed]
- Bompastor-Ramos P, Póvoa J, Lobo C, et al. Late postoperative opacification of a hydrophilic-hydrophobic acrylic intraocular lens. J Cataract Refract Surg 2016;42:1324-31. [Crossref] [PubMed]
- Hayashi K, Hirata A, Yoshida M, et al. Long-term effect of surface light scattering and glistenings of intraocular lenses on visual function. Am J Ophthalmol 2012;154:240-51.e2. [Crossref] [PubMed]
- Khurana AK, Raj A, Bahadur H. Comparison of Posterior Capsular Opacification with Hydrophilic and Hydrophobic Acrylic Posterior Chamber Intraocular Lens after Cataract Surgery. J Clin Diagn Res 2017;11:13-6.
- Leydolt C, Schartmueller D, Schwarzenbacher L, et al. Posterior capsule opacification with two hydrophobic acrylic intraocular lenses: 3-year results of a randomized trial. Am J Ophthalmol 2020. [Crossref] [PubMed]
- Kahraman G, Ferdinaro C, Wetzel B, et al. Intraindividual comparison of capsule behavior of 2 hydrophobic acrylic intraocular lenses during a 5-year follow-up. J Cataract Refract Surg 2017;43:228-33. [Crossref] [PubMed]
- Łabuz G, Knebel D, Auffarth GU, et al. Glistening Formation and Light Scattering in Six Hydrophobic-Acrylic Intraocular Lenses. Am J Ophthalmol 2018;196:112-20. [Crossref] [PubMed]
- Łabuz G, Reus NJ, van den Berg TJ. Light scattering levels from intraocular lenses extracted from donor eyes. J Cataract Refract Surg 2017;43:1207-12. [Crossref] [PubMed]
- Van Den Berg TJ, Van Rijn LJ, Michael R, et al. Straylight effects with aging and lens extraction. Am J Ophthalmol 2007;144:358-63. [Crossref] [PubMed]
- Werner L, Thatthamla I, Ong M, et al. Evaluation of clarity characteristics in a new hydrophobic acrylic IOL in comparison to commercially available IOLs. J Cataract Refract Surg 2019;45:1490-7. [Crossref] [PubMed]
- Grzybowski A, Kanclerz P, Beiko GHH. IOLs glistenings and quality of vision. Graefes Arch Clin Exp Ophthalmol 2019;257:2795-6. [Crossref] [PubMed]
- Kato K, Nishida M, Yamane H, et al. Glistening formation in an AcrySof lens initiated by spinodal decomposition of the polymer network by temperature change. J Cataract Refract Surg 2001;27:1493-8. [Crossref] [PubMed]
- Saylor DM, Coleman RD, Dair BJ, et al. Osmotic cavitation of elastomeric intraocular lenses. Acta Biomater 2010;6:1090-8. [Crossref] [PubMed]
- Rønbeck M, Behndig A, Taube M, et al. Comparison of glistenings in intraocular lenses with three different materials: 12-year follow-up. Acta Ophthalmol 2013;91:66-70. [Crossref] [PubMed]
- Werner L. Glistenings and surface light scattering in intraocular lenses. J Cataract Refract Surg 2010;36:1398-420. [Crossref] [PubMed]
- Manuchehri K, Mohamed S, Cheung D, et al. Brown deposits in the optic of foldable intraocular lenses in patients with uveitis. Eye 2004;18:54-8. [Crossref] [PubMed]
- Nishihara H, Yaguchi S, Onishi T, et al. Surface scattering in implanted hydrophobic intraocular lenses. J Cataract Refract Surg 2003;29:1385-8. [Crossref] [PubMed]
- Matsushima H, Mukai K, Nagata M, et al. Analysis of surface whitening of extracted hydrophobic acrylic intraocular lenses. J Cataract Refract Surg 2009;35:1927-34. [Crossref] [PubMed]
- Ong MD, Callaghan TA, Pei R, et al. Etiology of surface light scattering on hydrophobic acrylic intraocular lenses. J Cataract Refract Surg 2012;38:1833-44. [Crossref] [PubMed]
- Tognetto D, Toto L, Sanguinetti G, et al. Glistenings in foldable intraocular lenses. J Cataract Refract Surg 2002;28:1211-6. [Crossref] [PubMed]
- Colin J, Praud D, Touboul D, et al. Incidence of glistenings with the latest generation of yellow-tinted hydrophobic acrylic intraocular lenses. J Cataract Refract Surg 2012;38:1140-6. [Crossref] [PubMed]
- Miyata K, Ogata M, Honbo M, et al. Suppression of surface light scattering in intraocular lenses manufactured using an improved production process. J Cataract Refract Surg 2016;42:1716-20. [Crossref] [PubMed]
- Packer M, Rajan M, Ligabue E, et al. Clinical properties of a novel, glistening-free,single-piece, hydrophobic acrylic IOL. Clin Ophthalmol 2014;8:421-7. [Crossref] [PubMed]
- Leydolt C, Schriefl S, Stifter E, et al. Posterior capsule opacification with the imics1 ny-60 and ACRYSOF sn60wf 1-piece hydrophobic acrylic intraocular lenses: 3-year results of a randomized trial. Am J Ophthalmol 2013;156:375-81.e2. [Crossref] [PubMed]
- Łabuz G, Reus NJ, van den Berg TJ. Straylight from glistenings in intraocular lenses: In vitro study. J Cataract Refract Surg 2017;43:102-8. [Crossref] [PubMed]
- Henriksen BS, Kinard K, Olson RJ. Effect of intraocular lens glistening size on visual quality. J Cataract Refract Surg 2015;41:1190-8. [Crossref] [PubMed]
- DeHoog E, Doraiswamy A. Evaluation of the impact of light scatter from glistenings in pseudophakic eyes. J Cataract Refract Surg 2014;40:95-103. [Crossref] [PubMed]
- Schweitzer C, Orignac I, Praud D, et al. Glistening in glaucomatous eyes: visual performances and risk factors. Acta Ophthalmol 2014;92:529-34. [Crossref] [PubMed]
- Mönestam E, Behndig A. Impact on visual function from light scattering and glistenings in intraocular lenses, a long-term study. Acta Ophthalmol 2011;89:724-8. [Crossref] [PubMed]
- Hiraoka T, Miyata K, Hayashidera T, et al. Influence of intraocular lens subsurface nanoglistenings on functional visual acuity. PLoS One 2017;12:e0173574. [Crossref] [PubMed]
- Xi L, Liu Y, Zhao F, et al. Analysis of glistenings in hydrophobic acrylic intraocular lenses on visual performance. Int J Ophthalmol 2014;7:446-51. [PubMed]
- Godlewska A, Owczarek G, Jurowski P. Glistening phenomenon in acrylic hydrophobic intraocular lenses - how do perioperative factors and concomitant diseases effect it’s incidence and severity. Klin Oczna 2016;118:191-6. [PubMed]
- Chang A, Kugelberg M. Glistenings 9 years after phacoemulsification in hydrophobic and hydrophilic acrylic intraocular lenses. J Cataract Refract Surg 2015;41:1199-204. [Crossref] [PubMed]
- Yildirim TM, Labuz G, Khoramnia R, et al. Impact of Primary Calcification in Segmented Refractive Bifocal Intraocular Lenses on Optical Performance Including Straylight. J Refract Surg 2020;36:20-7. [Crossref] [PubMed]
- Łabuz G, Vargas-Martín F, van den Berg TJ, et al. Method for in vitro assessment of straylight from intraocular lenses. Biomed Opt Express 2015;6:4457-64. [Crossref] [PubMed]
- van den Berg TJ, Franssen L, Kruijt B, et al. History of ocular straylight measurement: A review. Z Med Phys 2013;23:6-20. [Crossref] [PubMed]
- van den Berg TJ. Intraocular light scatter, reflections, fluorescence and 365 absorption: what we see in the slit lamp. Ophthalmic Physiol Opt 2018;38:6-25. [Crossref] [PubMed]
- Łabuz G, Yildirim TM, van den Berg TJTP, et al. Assessment of straylight and the modulation transfer function of intraocular lenses with centrally localized opacification associated with the intraocular injection of gas. J Cataract Refract Surg 2018;44:615-22. [Crossref] [PubMed]
- Weindler JN, Łabuz G, Yildirim TM, et al. The impact of glistenings on the optical quality of a hydrophobic acrylic intraocular lens. J Cataract Refract Surg 2019;45:1020-5. [Crossref] [PubMed]
- Miyata K, Honbo M, Otani S, et al. Effect on visual acuity of increased surface light scattering in intraocular lenses. J Cataract Refract Surg 2012;38:221-6. [Crossref] [PubMed]
- Werner L, Stover JC, Schwiegerling J, et al. Light scattering, straylight, and optical quality in hydrophobic acrylic intraocular lenses with subsurface nanoglistenings. J Cataract Refract Surg 2016;42:148-56. [Crossref] [PubMed]
- Beheregaray S, Yamamoto T, Hiraoka T, et al. Influence on visual function of forward light scattering associated with subsurface nanoglistenings in intraocular lenses. J Cataract Refract Surg 2014;40:1147-54. [Crossref] [PubMed]
- van den Berg T, Franssen L, Coppens J. Ocular media clarity and straylight. Oxford, Academic Press: Encyclopedia of the Eye 2010:173-83.
- Alarcon A, Canovas C, Rosen R, et al. Preclinical metrics to predict through-focus visual acuity for pseudophakic patients. Biomed Opt Express 2016;7:1877-88. [Crossref] [PubMed]
- Son HS, Labuz G, Khoramnia R, et al. Ray propagation imaging and optical quality evaluation of different intraocular lens models. PLoS One 2020;15:e0228342. [Crossref] [PubMed]
- Neuhann IM, Kleinmann G, Apple DJ. A new classification of calcification of intraocular lenses. Ophthalmology 2008;115:73-9. [Crossref] [PubMed]
- Werner L, Wilbanks G, Nieuwendaal CP, et al. Localized opacification of hydrophilic acrylic intraocular lenses after procedures using intracameral injection of air or gas. J Cataract Refract Surg 2015;41:199-207. [Crossref] [PubMed]
- Marcovich AL, Tandogan T, Bareket M, et al. Opacification of hydrophilic intraocular lenses associated with vitrectomy and injection of intraocular gas. BMJ Open Ophthalmol 2018;3:e000157. [Crossref] [PubMed]
- Yildirim TM, Auffarth GU, Łabuz G, et al. Material Analysis and Optical Quality Assessment of Opacified Hydrophilic Acrylic Intraocular Lenses After Pars Plana Vitrectomy. Am J Ophthalmol 2018;193:10-9. [Crossref] [PubMed]
- Dagres E, Khan MA, Kyle GM, et al. Perioperative complications of intraocular lens exchange in patients with opacified Aqua-Sense lenses. J Cataract Refract Surg 2004;30:2569-73. [Crossref] [PubMed]
- Tandogan T, Khoramnia R, Choi CY, et al. Optical and material analysis of opacified hydrophilic intraocular lenses after explantation: a laboratory study. BMC Ophthalmol 2015;15:170. [Crossref] [PubMed]
- Platt SM, Iezzi R, Mahr MA, et al. Surgical removal of dystrophic calcification on a silicone intraocular lens in association with asteroid hyalosis. J Cataract Refract Surg 2017;43:1608-10. [Crossref] [PubMed]
- Choudhry S, Goel N, Mehta A, et al. Anterior segment optical coherence tomography of intraocular lens opacification. Indian J Ophthalmol 2018;66:858-60. [Crossref] [PubMed]
- Barra D, Werner L, Costa JL, et al. Light scattering and light transmittance in a series of calcified single-piece hydrophilic acrylic intraocular lenses of the same design. J Cataract Refract Surg 2014;40:121-8. [Crossref] [PubMed]
- Ma ST, Yang CM, Hou YC. Postoperative intraocular lens opacification. Taiwan J Ophthalmol 2018;8:49-51. [Crossref] [PubMed]
- Schrittenlocher S, Penier M, Schaub F, et al. Intraocular lens calcifications after (triple-) Descemet membrane endothelial keratoplasty. Am J Ophthalmol 2017;179:129-36. [Crossref] [PubMed]
- Nieuwendaal CP, van der Meulen IJE, Patryn EK, et al. Opacification of the intraocular lens after Descemet stripping endothelial keratoplasty. Cornea 2015;34:1375-7. [Crossref] [PubMed]
- Giers BC, Tandogan T, Auffarth GU, et al. Hydrophilic intraocular lens opacification after posterior lamellar keratoplasty - a material analysis with special reference to optical quality assessment. BMC Ophthalmol 2017;17:150. [Crossref] [PubMed]
- Milojcic C, Latz C, Tandogan T, et al. Opacification of a hydrophilic acrylic intraocular lens after DMEK: A material analysis. Ophthalmologe 2017;114:832-7. [Crossref] [PubMed]
- Fellman MA, Werner L, Liu ET, et al. Calcification of a hydrophilic acrylic intraocular lens after Descemet-stripping endothelial keratoplasty: case report and laboratory analyses. J Cataract Refract Surg 2013;39:799-803. [Crossref] [PubMed]
- Lee MS, Tsai IL, Tsai CY, et al. Intraocular lens opacification after Descemet’s stripping automated endothelial keratoplasty. Taiwan J Ophthalmol 2017;7:160-3. [Crossref] [PubMed]
- Ní Mhéalóid Á, Fulcher T, O’Keefe M. Anterior surface opacification of intraocular lenses after Descemet’s stripping automated endothelial keratoplasty. BMJ Case Rep 2015;2015:bcr2015213216. [Crossref] [PubMed]
- Ahad MA, Darcy K, Cook SD, et al. Intraocular lens opacification after descemet stripping automated endothelial keratoplasty. Cornea 2014;33:1307-11. [Crossref] [PubMed]
- Morgan-Warren PJ, Andreatta W, Patel AK. Opacification of hydrophilic intraocular lenses after descemet stripping automated endothelial keratoplasty. Clin Ophthalmol 2015;9:277-83. [Crossref] [PubMed]
- Khurana RN, Werner L. Calcification of a Hydrophilic Acrylic Intraocular Lens after Pars Plana Vitrectomy. Retin Cases Brief Rep 2018;12:204-6. [Crossref] [PubMed]
- van der Mooren M, Rosen R, Franssen L, et al. Degradation of Visual Performance With Increasing Levels of Retinal Stray Light. Invest Ophthalmol Vis Sci 2016;57:5443-8. [Crossref] [PubMed]
- Fung SS, Sykakis E, Islam NM, et al. Intraocular Lens Opacification following Intracameral Injection of Recombinant Tissue Plasminogen Activator to Treat Inflammatory Membranes after Cataract Surgery. J Ophthalmol 2015;2015:975075. [Crossref] [PubMed]
- Espandar L, Mukherjee N, Werner L, et al. Diagnosis and management of opacified silicone intraocular lenses in patients with asteroid hyalosis. J Cataract Refract Surg 2015;41:222-5. [Crossref] [PubMed]
- Thomes BE, Callaghan TA. Evaluation of in vitro glistening formation in hydrophobic acrylic intraocular lenses. Clin Ophthalmol 2013;7:1529-34. [Crossref] [PubMed]
- Chang A, Behndig A, Rønbeck M, et al. Comparison of posterior capsule opacification and glistenings with 2 hydrophobic acrylic intraocular lenses: 5- to 7-year follow-up. J Cataract Refract Surg 2013;39:694-8. [Crossref] [PubMed]
- van der Mooren M, Franssen L, Piers P. Effects of glistenings in intraocular lenses. Biomed Opt Express 2013;4:1294-304. [Crossref] [PubMed]
- Stringham J, Werner L, Monson B, et al. Calcification of different designs of silicone intraocular lenses in eyes with asteroid hyalosis. Ophthalmology 2010;117:1486-92. [Crossref] [PubMed]
- Patel NA, Fan KC, Yannuzzi NA, et al. Akreos AO60 Intraocular Lens Opacification Following Retinal Detachment Repair. Ophthalmology Retina 2020. Available online: https://doi.org/ [Crossref]
- Łabuz G, Reus NJ, van den Berg TJ. Ocular straylight in the normal pseudophakic eye. J Cataract Refract Surg 2015;41:1406-15. [Crossref] [PubMed]
- Werner L, Stover JC, Schwiegerling J, et al. Effects of Intraocular Lens Opacification on Light Scatter, Stray Light, and Overall Optical Quality/Performance. Invest Ophthalmol Vis Sci 2016;57:3239-47. [Crossref] [PubMed]
- Artal P. Handbook of Visual Optics, Two-Volume Set. Boca Raton: CRC Press, 2017.
- van den Berg TJ, René van Rijn LJ, Kaper-Bongers R, et al. Disability Glare in the Aging Eye. Assessment and Impact on Driving. J Optom 2009;2:112-8. [Crossref]
- Mueller-Schotte S, van der Schouw YT, Schuurmans MJ. Ocular Straylight: A Determinant of Quality of Life in the Elderly? Gerontol Geriatr Med 2015. [Crossref] [PubMed]
- Bailey IL, Bullimore MA. A new test for the evaluation of disability glare. Optom Vis Sci 1991;68:911-7. [Crossref] [PubMed]
- van den Berg TJ. The (lack of) relation between straylight and visual acuity. Two domains of the point-spread-function. Ophthalmic Physiol Opt 2017;37:333-41. [Crossref] [PubMed]


