CTC enumeration and characterization: moving toward personalized medicine
Introduction
Breast cancer (BC) remains the most common type of cancer diagnosed among women and is responsible for 15% of all cancer-related deaths with 40,000 estimated deaths in 2014 (1). Although the improvements in BC detection and adjuvant treatments led to a significant decrease in BC-related deaths in the last two decades, about 30% of women initially diagnosed with early-stage cancer eventually develop metastatic disease. Moreover, despite advances in the treatment of metastatic BC, at this point it remains practically incurable, and the aims of therapy are the prolongation of overall survival (OS) time and the improvement of quality of life (2).
The leading causes of tumor-related death in BC remain the complications from distant metastasization. Unfortunately, the spread of tumor cells through haematogenous dissemination from the primary tumor to distant sites cannot be detected by standard imaging methods. Therefore, there is an urgent need to find novel biomarkers, which could monitor efficacy of adjuvant therapies, detect early development of (micro)metastases and at last, assess therapeutic responses of advanced disease. In the last decade, the detection of disseminated tumor cells (DTCs) in the bone marrow and circulating tumor cells (CTCs) in the blood have demonstrated to provide useful information for the clinical management of BC by predicting treatment benefit earlier than traditional imaging methods.
More recently, other potential blood-based markers are emerging as independent parameters for prediction development and outcome in metastatic disease, including circulating tumor microemboli and circulating tumor materials (CTMat). The apoptosis and necrosis processes of CTCs cause the leakage of intracellular components in the bloodstream, such as electrolytes, cellular debris, DNA, and chromatin. Since CTCs are continuously released and destroyed, such CTMat accumulate and could represent an independent biomarker for the prognostication and monitoring of metastatic disease.
The aim of this review is to describe the current state of the art on CTCs detection and clinical use, the evidence to demonstrate their clinical validity, and their potential impact for both future clinical trial design and, decision-making process in our daily practice.
Strategies for CTC analysis
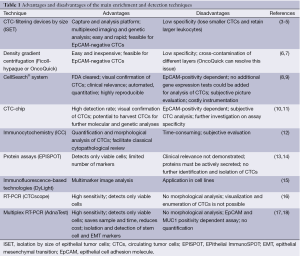
CTCs are present in the bloodstream at a very low concentration, thus their detection and characterization require highly sensitive and specific methods, which consist of a combination of enrichment (isolation) and detection (identification) strategies (Table 1) and both steps are essential components of the identification process (19).
Full table
CTC enrichment
CTC enrichment strategies are based on technologies that can distinguish CTCs among the surrounding hematopoietic cells, according to their physical (size, density, electric charges, deformability) and biological (cell surface protein expression, viability) characteristics. Therefore, enrichment techniques are based on two different strategies: the selection according to morphological features or according to immunologic profile (12).
Several membrane filter devices are available for CTC enrichment based on the differential cellular size, including isolation by size of epithelial tumor cells (ISET) (3-5,20,21), micro electro-mechanical system (MEMS)-opticbased microfilter (3,22), ScreenCell® (23), CellSieve™ (24) and CellOptics® (3). Size-based enrichment techniques are jeopardized by the heterogeneity of size and the shape of CTCs. Filtration by size consents to enrich CTCs from a wide range of tumors, but sometimes results in loss of smaller CTCs or clotting of filter pores by leukocytes. Another morphology-based enrichment strategy is based on density gradient centrifugation using Ficoll-hypaque solution (6). Ficoll density-gradient dependent approaches are easy to handle, even if real losses of tumor cells have still been observed (25). Subsequently, the OncoQuick™ device was developed to avoid the cross-contamination of different layers by using a porous membrane, which keeps them separate (7). To date, several other devices based on physical properties of CTC are available, including a photoacoustic flow cytometer, a label-free biochip that exploits the differences in size and deformability of CTCs, a micro-fluidics device that combines multiorifice flow fractionation (MOFF) and the dielectrophoresis (DEP) cell separation techniques, and a DEP field-flow fractionation device that allows isolation of viable CTCs by their different response to DEP (26-30). Particularly, Gupta et al. have recently described the ApoStream device. This technique exploits differences in the biophysical characteristics between normal blood cells and cancer cells in order to capture CTCs using dielectrophoretic technology in a microfluidic flow chamber (31).
Most of CTC enrichment procedures involve immunomagnetic isolation (32-36). CTCs are subjected to positive or negative selection, utilizing either tumor cell antigens such as epithelial cell adhesion molecule (EpCAM), or hematopoietic cell antigens such as the common leukocyte antigen CD45 for purified cell suspensions (37-39). Antibodies are coupled to magnetic beads, thus the antigen–antibody complex is subsequently isolated from the solution with a magnetic field (8). Unfortunately, the lack of reliable target antigens for cellular capture still represents a significant limitation to the procedure. EpCAM, for example, is the by far most used capture antigen (i.e., CellSearch® system, CTC-Chip, MACS, Dynabeads, RosetteSep, affinity-based microchips) due to its expression across numerous tumor entities (40,41), but several pitfalls exist, including epithelial mesenchymal transition (EMT) and differential antigen expression. Particularly, EMT is a morphogenetic process in which cells lose their epithelial characteristics and acquire a mesenchymal-like migratory phenotype, endowing cells with invasive properties, thereby contributing to the apparition of CTCs and to the formation of metastases. It was further suggested that induction of EMT might generate cells that exhibit molecular and functional stem-like characteristics, leading to the under-expression of epithelial antigens like EpCAM (42). Thus, in order to capture this crucial biological subset of EpCAM low/negative CTCs, which have been suggested to confer aggressive tumor progression (43), future positive separation strategies should take this phenotype into account.
Recently, a structured medical Seldinger guidewire (FSMW), used to obtain safe access to blood vessels, bound with EpCAM antibodies, has been developed. This device has the potential to enrich CTCs in vivo and has been able to enrich EpCAM-positive CTCs from 22 of 24 BC or non-small cell lung cancer (NSCLC) patients (44). Finally, a novel technique using surface-enhanced Raman spectroscopy (SERS) has been described. This method is able to enumerate targeted CTCs in the presence of whole blood, using magnetic beads and SERS tags respectively conjugated to EpCAM and HER2 antibodies (45,46). SERS nanoparticles, with epidermal growth factor peptide as a target, successfully identified CTCs in the peripheral blood of 19 patients with squamous cell carcinoma of the head and neck (47).
More recently, novel methods combining physical (size) and biologic (immunomagnetic) features of CTCs have been developed. Particularly, the CTC-iChip is capable of sorting rare CTCs from whole blood at a rate of 10 million cells per second in both epithelial and non-epithelial cancers by using tumor antigen–independent microfluidic technology (48,49).
CTC detection
After enrichment, the solution usually still contains several leukocytes, thus CTCs need to be identified at the single-cell level and separated from normal blood cells. CTCs detection can be done through cytometric strategies or nucleic acid-based techniques (12).
Among cytometric strategies, classic immunocytochemistry (ICC) is the most widely used immunological approach, and has the advantage to facilitate classical cytopathological review. Furthermore, monoclonal antibodies against various epithelium-specific antigens, surface adhesion molecules, and growth factor receptors as well as diverse other upstream analyses (transcriptome/genome analyses) have been developed.
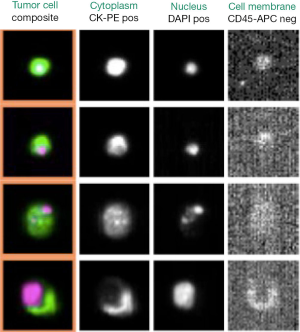
Among the current EpCAM-based technologies, the FDA cleared the CellSearch™ platform and the Ariol system (36), but the CellSearch™ remains the “gold standard” for all the CTC-detection strategies (8). The previously enriched EpCAM-positive cell fraction is additionally treated with a nucleic acid dye, a leukocyte-specific anti-CD45 monoclonal antibody and epithelial-specific anti-cytokeratin 8, 18, and 19 antibodies. Subsequently, a semi-automated fluorescence-based microscopy system (CellSpotter Analyzer) consents a computer-generated reconstruction of cellular images. CTCs express EpCAM and are CD45-negative, exhibit cytoplasmic expression of cytokeratin and contain a nucleus that binds to the nucleic acid dye 4’, 6-doamidino-2-phenylindole (DAPI). The absence of one of these characteristics disqualifies a cell image as a CTC (Figure 1).
In 2007, Nagrath et al. introduced the “CTC-Chip”, a microchip technology on a microfluidic platform that separates CTCs from whole blood using microposts coated with an antibody against EpCAM under precisely controlled laminar-flow conditions. In the pilot study, the CTC-chip successfully identified CTCs in the peripheral blood of 99% patients with metastatic lung, prostate, pancreatic, breast and colon cancer (10). In a first clinical and promising approach, the chip had been tested on the samples of NSCLC patients, demonstrating that changes in tumor genotypes (EGFR mutational analysis on DNA of CTCs) may correlate with response to treatments (50,51). More recently, Stott et al. improved the technique in patients with localized and metastatic prostate cancer. The authors associated the detection of the PSA with the EpCAM-based method and with morphologic criteria and integrated multiple signals in the same 3D microfluidic device, improving the efficacy of the CTC-chip to detect CTCs. The pilot study showed that CTCs rapidly decreased after surgical tumor removal or after the initiation of an effective treatment, while the persistence of CTCs after 3 months from the surgery suggested that CTCs could be released from the localized disease before metastases development (11). Other microfluidic chips used for the identification and isolation of CTCs include the IsoFlux system (based on immunomagnetic capture) (52,53) and the Herringbone-Chip (based on microvortices that increase the number of interactions between CTCs and the antibody) (54).
Nevertheless, EpCAM-based technologies do not consent to recognize whether the detected CTCs are viable or apoptotic cells. A new functional test that allows the detection of only viable cells after CD45-cell depletion has been developed for the CTC and DTC analyses. Avoiding direct contact with the target cells, this method assesses the presence of CTCs on the basis of proteins secreted or released during a 24-48 hours of short-term culture (i.e., CK19 and MUC1). This technique, named EPithelial ImmunoSPOT (EPISPOT) assay, has been applied to blood and bone marrow samples of breast, prostate and colon cancer patients providing first clinical data (13,14).
In 2011, Balic et al. developed a multi-marker imaging approach using DyLight technology (15). This technique requires the use of multiple antibodies (i.e., against CK, HER2, ALDH1, CD44, and CD24) labeled with fluorochromes of different colors and spectral image analysis to separate different color spectra. Interestingly, by the addition of specific markers, this method may help to identify subpopulations that express particular therapeutic targets. Furthermore, the advent of quantum dots (QDs) with narrow emission spectra provided a new tool for multi-marker analysis. Compared to immunofluorescent dyes, QDs are brighter, not prone to photo bleaching, available in a number of colors, and their emission can be tuned to any desired wavelength by modulating the size of the particle (41,55,56). Other available immunofluorescence-based technologies for CTC-detection include automated scanning devices such as the fiber-optic array scanning technology (FAST) (57), the laser scanning cytometer [i.e., Maintrac® (58)] and a dedicated image cytometer [CellTracks® (59)].
Nucleic acid-based techniques have become the most widely used alternative to immunocytochemical assays. Particularly, PCR-based assay evaluates the amount of DNA from CTCs. The main disadvantage of this technique is the inability to distinguish the DNA free in the blood from apoptotic cells, creating false-positive results. For this reason, most groups prefer RT-PCR assays to target specific mRNA, since only viable CTCs produce mRNA (12,60,61). RT-PCR is based on the utilization of several cancer-related genes or epithelial antigens, including CK19, CK7, HER2, and mammaglobin A (62,63). To date, the mRNA encoding CK19 has been the most widely studied in clinical trials (64). Particularly, Stathopoulou et al. developed an RT-qPCR assay for KRT19 mRNA that showed to be highly sensitive and specific for the molecular detection of occult carcinoma cells in peripheral blood of BC patients (65-69). Nevertheless, the principal limitations to these techniques are related to the mRNA markers used, since they may be also present at low concentrations in normal blood, bone marrow cells and in other non-tumor cells (70). Moreover, cancer cells express high genetic instability and, especially in the course of the EMT, gene transcription may be downregulated. Quantitative real-time PCR provides interesting prospects for better quantification of the tumor cell load, provided that the specificity of the applied markers is well controlled.
AdnaTest (Alere) is a commercially available RNA-based CTC assay. This RT-PCR based assay utilizes nonquantitative RT-PCR to identify putative transcripts of genes after immunomagnetic separation of MUC1/HER2/EpCAM-positive cells (17). The principal limitation, along with the others related to EpCAM-based methods, is that MUC1 expression has been found on activated T lymphocytes (18). Another aspect to be considered is the fact that the RT-PCR is unable to quantify the tumor cell load, since the observed bands may be the result of one single cell as well as a thousand. Another promising nucleic acid-based technique is the RNAscope technology used by CTCscope. This assay, recently described by Payne et al. (16), measures single RNA molecules for the detection of single CTCs in metastatic BC patients. This is a method that requires minimal enrichment and that can exclude apoptotic cells, since these do not produce mRNA.
Concluding, in the last two decades several promising CTC detection methods have been developed. These strategies should be validated in appropriately sized clinical trials in order to evaluate their quality and validity.
The rationale for the use of CTCs
The presence of tumor cells in the peripheral circulation was reported for the first time in 1869 by Thomas Ashworth (71). Since then, the existence, origin, and clinical significance of CTCs have been widely discussed. In the late 1970s, the introduction of sensitive and specific immunohistochemical techniques led to renewed interest in the detection of CTCs and their possible association with early metastasization in solid malignancies. However, the lack of sensitivity of the early detection methods on circulating blood and the analogy between tissue metastasis and single cell precursors of solid metastasis, especially in bone, shifted the focus on the detection of DTCs in bone marrow. Bone marrow is accessible by needle aspiration through the iliac crest, and represents the most common homing organ for DTCs derived from different tumors and thus, the most prominent indicator organ for minimal residual disease (72,73).
Initially, DTCs have been detected in the bone marrow of 30-40% of primary BC patients and their presence has been strongly associated with poor prognosis (74). In more recent studies, the DTC detection rate appears to be far lower (about 3%), likely due to the earlier detection of BC ubsequent to increased use of screening mammography (75). Although the presence of DTCs is a significant prognostic factor in the prediction of outcome, the low rate of DTCs questioned the value of its routine use in this highly selective group of patients. Furthermore, bone marrow biopsy is an invasive procedure, with higher morbidity and costs than a simple blood draw, thus subsequent research was directed to the evaluation of CTCs in peripheral blood. Nevertheless, it should be noticed that with the choice of focusing research on the blood compartment, epidemiologic, prospective and biologic evidence regarding CTCs must not simply be extrapolated from the extensive body of evidence on DTCs. Interestingly, several studies compared both compartments in the same patients and reported higher prevalence of DTCs in bone marrow than CTCs in the blood, and rates of CTC-DTC concordance ranging from 63% to 94% (76-82).
A higher presence of DTCs, together with its prognostic significance, favors the notion that bone marrow may be a ‘perfect niche’ in which tumor cells resist host defense mechanisms and survive. Several researchers shared the theory that bone marrow may represent a functional reservoir for cancer cells with the capability of recirculating through the bloodstream and colonizing other distant organs (83,84). As proof of that, a number of studies demonstrated that DTCs are able to persist in bone marrow even after completion of adjuvant therapy. Most of initially DTC-positive tumors turned negative during adjuvant treatment, but those with persistence of DTCs had worse disease-free and OS, suggesting that evaluation of DTCs can help in the selection of patients that will benefit from additional or a switch of adjuvant treatments (85). Even in neoadjuvant setting, the presence of DTCs in locally advanced BC was found to be a significant prognostic factor for cancer-related death, as well as a surrogate predictor of response to neoadjuvant chemotherapy and of disease recurrence (86). However, despite the presence of DTCs in bone marrow, according to the theory of “metastatic inefficiency” (87,88), only a part of tumor cells will be able to survive at the secondary sites and determine tumor mass, therefore only 40-60% of patients will eventually develop a relapse (74,89).
Clinical application of CTCs in BC
CTCs in metastatic BC
In 2004, the seminal work by Cristofanilli et al. demonstrated that CTC count detected using the CellSearch® was an independent prognostic factor for progression-free survival (PFS) and OS in metastatic BC. The cut-off of 5 CTCs/7.5 mL has been identified to classified patients with good or poor clinical outcome (9) and subsequent studies have confirmed the prognostic value of CTCs with the same cut-off (90-92). The data of the pivotal study resulted in FDA-approval of the CellSearch® for prognosis and monitoring of patients with MBC. Interestingly, several authors have then shown that monitoring CTC levels enable prediction of treatment efficacy (93,94). Particularly, Cristofanilli et al. demonstrated that detection of CTCs before initiation of first-line therapy is highly predictive of PFS and OS, even more than traditional imaging techniques (based on RECIST criteria) (95,96), and that detection of elevated CTCs at any time during treatment, since the first cycle of therapy, is an accurate indication of subsequent rapid disease progression and mortality (90). A recent pooled analysis of 1,944 patients across 17 European centers has confirmed the independent prognostic role of CTC level on PFS and OS in metastatic BC patients. Patients with a CTC count of 5/7.5 mL or higher at baseline were associated with decreased PFS (HR 1.92, P<0.0001) and OS (HR 2.78, P<0.0001) compared with patients with a CTC count of less than 5/7.5 mL. Moreover, 3-5 and 6-8 weeks after start of treatment, increased CTC counts were associated with decreased PFS and OS. The authors concluded that survival prediction was significantly improved by addition of CTC count to the clinic-pathological models, while the carcinoembryonic antigen (CEA) and cancer antigen 15-3 (Ca 15.3) levels at baseline and during treatment did not add significant information to the model (92).
Since changes in CTC levels proved to reflect treatment responses as early as after the first cycle of chemotherapy, several other studies evaluated the predictive potential of CTCs, monitoring their dynamic in peripheral blood during specific treatments. Particularly, Smerage et al. have recently published the final results of the SWOG S0500 trial. The aim of this trial was to determine whether switching chemotherapy after 21 days of first-line chemotherapy, in patients with persistent increase in CTCs, could improve their OS. This study showed that early changing to another therapy improved neither OS nor PFS. Nevertheless, the authors concluded that for this population, there would be a need for a wider participation in trials of novel therapeutic agents at the time of progression, rather than moving on to further lines of standard chemotherapy (97). Furthermore, the CirCe01 trial aims to investigate the value of early CTC count-based switch in chemotherapy regimen in third-line or later settings.
With regard to molecular subtypes, in hormone receptor positive BC, detection of higher levels of CTCs may guide the selection of patients who would more likely benefit from chemotherapy rather than endocrine treatment. On the other hand, CTCs seem to lose their prognostic value in patients with metastatic BC treated with targeted therapies, particularly in HER2 positive tumors. This effect could be due to a selective action of bevacizumab (Avastin®) and HER2-targeted therapy against circulating epithelial cells, reducing the prognostic value of CTCs enumeration (98-100).
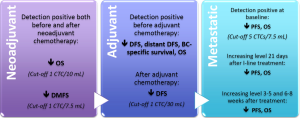
Figure 2 reassumes the evidence for the prognostic and predictive value of CTC count in metastatic BC. CTCs enumeration could guide therapeutic decision-making and the development of tailored treatments, improving the management of metastatic patients.
CTCs in early-stage BC
In a large study involving 2,026 primary BC patients, CTCs have been detected in the peripheral blood of approximately 22% of patients after surgery and before adjuvant therapy (101). This amount appeared to be even higher in a smaller study that detected CTCs in approximately 31% of early stage BC patients (36% ER positive, 32% PR positive and 30% HER2 positive tumors). The authors reported that only 7% of all patients remained CTCs positive after adjuvant therapy, and no association between CTCs and tumor size, tumor grade, histological grade and receptor status was found (102). In adjuvant setting, CTC detection before chemotherapy has shown to be an independent predictor of disease-free survival (DFS) and OS and, not only the presence but also the quantity of CTCs has proven to be associated with worse outcome. Moreover, the persistence of CTCs after adjuvant treatment significantly correlates with a decreased DFS (101,103). These data have been recently updated and confirmed by Rack et al. in the success trial (104). In this large prospective trial, CTCs were detected in 21.5% of 2,026 patients before adjuvant chemotherapy. Particularly, CTCs were detected significantly more frequently in node-positive patients (22.4%) than in node-negative (19.6%) (P<0.001) while, no association was found with tumor size, grading, or hormone receptor status. Patients with at least 5 CTCs/30 mL blood before adjuvant treatment showed the worst prognosis. This trial provided strong evidence that CTC level represents a prognostic marker for reduced DFS, distant DFS, BC-specific survival, and OS before adjuvant chemotherapy and for DFS after completion of the treatment.
Therefore, CTCs evaluation in patients with early-stage BC could provide useful information for adjuvant treatment decision-making. However, in this particular context, CTCs are observed with low frequency thus, CTC detection methods with higher sensitivity could be necessary for their clinical use. Moreover, further studies are needed to better define its efficacy in both the prediction of outcome and monitoring the effect of therapy.
Concerning neoadjuvant chemotherapy, systemic response to treatment seems to be independent from the clinic-pathological features and the local response of the primary BC. Therefore, monitoring CTC and DTC levels during neoadjuvant treatment consents to better define the effectiveness of systemic treatment on tumor cell diffusion and could guide treatment strategies (105). In the neoadjuvant setting, CTCs have been detected in 22-23% of patients before and in 10-17% after systemic treatment. Interestingly, the persistence of CTCs after neoadjuvant chemotherapy was not correlated to the primary tumor response but it identifies a subpopulation of patients with an increased risk for early relapse and worse OS (106,107). Figure 2 reassumes the evidence for the prognostic and predictive value of CTC count in neoadjuvant and adjuvant settings.
Clinical application of CTCs in other cancers
Prostate cancer
In 2005, Moreno et al. demonstrated that in patients with metastatic prostate cancer, CTC detection with the cut-off of 5 CTCs was a predictive factor superior to other clinical variables (108). Moreover, Okegawa et al. confirmed that CTC count represent an independent predictor for OS (109). Nevertheless, the optimal cut-off point to distinguish patients with favorable prognosis from those with poor prognosis still remains widely discussed (110-112). Subsequently, several large cohorts of castration-resistant prostate cancer (CRPC) patients clinically defined the prognostic significance of pre- and post-treatment CTC counts and the superior predictive ability of CTC enumeration in comparison with PSA level at all-time points (113-115). Therefore, changes in CTC counts in response to treatment have been established as indicators of response to treatment (116-118).
Patients with high-risk non-metastatic prostate cancer infrequently present with small number of CTCs in peripheral blood therefore, CTC may not be the optimal marker to predict prognosis or detect residual disease after radical prostatectomy (119,120). Interestingly, in these patients with localized prostate cancer, CTC count did not correlate with tumor volume, pathological stage, and Gleason score, suggesting that CTCs are more likely to originate from metastatic sites instead of primary lesions (121,122).
Colorectal cancer
In 2008, Cohen et al. demonstrated that metastatic colorectal cancer (mCRC) patients with ≥3 CTCs had significantly shorter median PFS and OS and worse treatment outcome compared with those with <3 CTCs (123). More recently, also the presence of at least 1 CTC at baseline count was found to be predictive for poor prognosis. Therefore, patients with 1-2 CTC should be switched from the favorable prognostic group (conventionally defined by the presence of <3 CTCs) to the unfavorable (124). Interestingly, the prevalence of CTCs in colorectal cancer patients are lower than in other cancer types, due to the capture of viable CTCs in the liver as first filter organ (125). Particularly, unfavorable baseline CTC was associated with worse PFS in patients receiving first- or second-line therapy, irinotecan, having liver involvement, ≥65 years, and ECOG PS of zero (126). Moreover, CTC count turned out to be a reliable surrogate biomarker in assessing Japanese patients responsive to oxaliplatin-based chemotherapy (127). More recently, high CTC count predicted reduced OS in patients treated with cetuximab-combination chemotherapy as third-line treatment (128).
In non-metastatic setting, molecular assessment for micrometastasis in sentinel lymph node along with CTC count may help to identify patients at high risk for recurrence and thus who could benefit from adjuvant therapy (129,130). Furthermore, even after curative resection, patients with persistence of CTCs exhibited higher incidence of relapse and worse relapse-free survival rate (131). In a multi-institutional study, a panel of genes (CEA/CK/CD133) investigated in peripheral blood from 753 colorectal cancer patients, turned out to be a superior prognostic factor over other existing clinicopathologic features in patients with Dukes’ stages B and C (132). Particularly, higher CD133 expression was significantly associated with poorer clinical outcome and some clinicopathological factors such as T category, N category and vascular invasion in colorectal cancer patients (133).
Lung cancer
In lung cancer, it was demonstrated that CTC detection had the potential to distinguish malignant from benign lung disease and to predict the presence of distant metastasis. Moreover, CTC status was proportional to both clinical and pathological status and was associated with radiographic response at the end of two cycles of chemotherapy. CTC detection also possessed a significant prognostic value in both small and NSCLC patients who were treated with standard chemotherapy and also in resectable NSCLC independently of disease staging. Patients with a reduction in CTC number after one cycle of chemotherapy have longer PFS and OS (134-145).
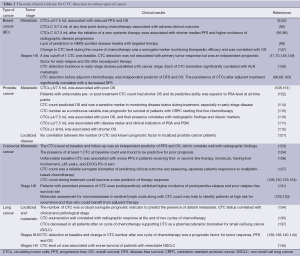
Furthermore, the prognostic and predictive value of CTC level has been investigated in bladder, renal, ovarian, gastric and liver cancer (146). Table 2 reassumes the main clinical evidence for CTC detection in breast, prostate, colorectal and lung cancer.
Full table
Liquid biopsy
As previously reported in literature, the immunehistochemical profile of BC could change in the course of the disease, determining a substantial discordance in receptor status between primary and recurrent BC (155). Particularly, the ER and HER2 status of the primary tumor could be discordant with the status of the metastatic tumor sites and the profile of CTCs (156). Since repeated and approachable tumor biopsies are invasive, costly and not always possible, the assessment of tumor characteristics on CTCs by a peripheral blood sample as a ‘liquid biopsy’ represents an attractive alternative.
Several studies analyzed the genetic aberrations carried by CTCs and compared their genetic profile to that of primary tumor, trying to correlate some mutations to disease aggressiveness and treatment response. Therefore, several clinical trials are currently investigating novel targeted strategies based on expression profiles of CTCs. For instance, Stebbing et al. studied the efficacy of lapatinib in metastatic BC with HER2-negative primary tumors and EGFR-positive CTCs, but the attempt to expand the pool of patients eligible for a targeted therapy in this study was unsuccessful (157). Moreover, the DETECT III trial and the CirCe T-DM1 trial are currently investigating the efficacy of HER2-targeted therapy in HER2-negative MBC with HER2-positive CTC.
Further interventional controlled phase III trials are needed to investigate and define the role of CTCs evaluation in the improvement of patient outcome and in the reduction of medical costs (158). It is likely that the future implementation of molecular and genomic characterization of CTCs will contribute to improve the treatment selection and thus to move toward precision medicine.
Conclusions
DTCs in bone marrow and CTCs in peripheral blood have a wide range of potential applications, including prognostication at diagnosis, assessment of treatment response, detection of early metastasization and evaluation of novel agents, allowing a personalized choice of treatment modalities and timing. Moreover, the capability of detecting and eradicating metastatic cells at an early phase of metastatic process likely has the potential to improve cancer outcomes. These interesting findings provide ample room for well-designed clinical trials in order to further investigate the significance of CTCs in human cancer.
Acknowledgements
We wish to thank Dr. Paolo Fortina and Dr. Carmela Paolillo for their helpful critical comments and revision of the manuscript.
Disclosure: The authors declare no conflict of interest.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [PubMed]
- O’Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist 2005;10 Suppl 3:20-9. [PubMed]
- Vona G, Sabile A, Louha M, et al. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulating tumor cells. Am J Pathol 2000;156:57-63. [PubMed]
- Lin HK, Zheng S, Williams AJ, et al. Portable filter-based microdevice for detection and characterization of circulating tumor cells. Clin Cancer Res 2010;16:5011-8. [PubMed]
- Zheng S, Lin HK, Lu B, et al. 3D microfilter device for viable circulating tumor cell (CTC) enrichment from blood. Biomed Microdevices 2011;13:203-13. [PubMed]
- Rosenberg R, Gertler R, Friederichs J, et al. Comparison of two density gradient centrifugation systems for the enrichment of disseminated tumor cells in blood. Cytometry 2002;49:150-8. [PubMed]
- Gertler R, Rosenberg R, Fuehrer K, et al. Detection of circulating tumor cells in blood using an optimized density gradient centrifugation. Recent Results Cancer Res 2003;162:149-55. [PubMed]
- Riethdorf S, Fritsche H, Müller V, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res 2007;13:920-8. [PubMed]
- Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004;351:781-91. [PubMed]
- Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007;450:1235-9. [PubMed]
- Stott SL, Lee RJ, Nagrath S, et al. Isolation and characterization of circulating tumor cells from patients with localized and metastatic prostate cancer. Sci Transl Med 2010;2:25ra23. [PubMed]
- Broersen LH, van Pelt GW, Tollenaar RA, et al. Clinical application of circulating tumor cells in breast cancer. Cell Oncol (Dordr) 2014;37:9-15. [PubMed]
- Alix-Panabières C. EPISPOT assay: detection of viable DTCs/CTCs in solid tumor patients. Recent Results Cancer Res 2012;195:69-76. [PubMed]
- Alix-Panabières C, Vendrell JP, Slijper M, et al. Full-length cytokeratin-19 is released by human tumor cells: a potential role in metastatic progression of breast cancer. Breast Cancer Res 2009;11:R39. [PubMed]
- Balic M, Rapp N, Stanzer S, et al. Novel immunofluorescence protocol for multimarker assessment of putative disseminating breast cancer stem cells. Appl Immunohistochem Mol Morphol 2011;19:33-40. [PubMed]
- Payne RE, Wang F, Su N, et al. Viable circulating tumour cell detection using multiplex RNA in situ hybridisation predicts progression-free survival in metastatic breast cancer patients. Br J Cancer 2012;106:1790-7. [PubMed]
- Andreopoulou E, Yang LY, Rangel KM, et al. Comparison of assay methods for detection of circulating tumor cells in metastatic breast cancer: AdnaGen AdnaTest BreastCancer Select/Detect™ versus Veridex CellSearch™ system. Int J Cancer 2012;130:1590-7. [PubMed]
- Agrawal B, Krantz MJ, Parker J, et al. Expression of MUC1 mucin on activated human T cells: implications for a role of MUC1 in normal immune regulation. Cancer Res 1998;58:4079-81. [PubMed]
- Sun YF, Yang XR, Zhou J, et al. Circulating tumor cells: advances in detection methods, biological issues, and clinical relevance. J Cancer Res Clin Oncol 2011;137:1151-73. [PubMed]
- Pinzani P, Salvadori B, Simi L, et al. Isolation by size of epithelial tumor cells in peripheral blood of patients with breast cancer: correlation with real-time reverse transcriptase-polymerase chain reaction results and feasibility of molecular analysis by laser microdissection. Hum Pathol 2006;37:711-8. [PubMed]
- Wong NS, Kahn HJ, Zhang L, et al. Prognostic significance of circulating tumour cells enumerated after filtration enrichment in early and metastatic breast cancer patients. Breast Cancer Res Treat 2006;99:63-9. [PubMed]
- Zheng S, Lin H, Liu JQ, et al. Membrane microfilter device for selective capture, electrolysis and genomic analysis of human circulating tumor cells. J Chromatogr A 2007;1162:154-61. [PubMed]
- Desitter I, Guerrouahen BS, Benali-Furet N, et al. A new device for rapid isolation by size and characterization of rare circulating tumor cells. Anticancer Res 2011;31:427-41. [PubMed]
- Adams D, Zhu P, Makarova O, et al. HER-2 FISH analysis and H & E staining of circulating tumor cells pre-isolated using high porosity precision microfilters. Cancer Res 2012;72:2395.
- Woelfle U, Breit E, Zafrakas K, et al. Bi-specific immunomagnetic enrichment of micrometastatic tumour cell clusters from bone marrow of cancer patients. J Immunol Methods 2005;300:136-45. [PubMed]
- Alix-Panabières C, Pantel K. Circulating tumor cells: liquid biopsy of cancer. Clin Chem 2013;59:110-8. [PubMed]
- Moon HS, Kwon K, Kim SI, et al. Continuous separation of breast cancer cells from blood samples using multi-orifice flow fractionation (MOFF) and dielectrophoresis (DEP). Lab Chip 2011;11:1118-25. [PubMed]
- Alazzam A, Stiharu I, Bhat R, et al. Interdigitated comb-like electrodes for continuous separation of malignant cells from blood using dielectrophoresis. Electrophoresis 2011;32:1327-36. [PubMed]
- Wang XB, Yang J, Huang Y, et al. Cell separation by dielectrophoretic field-flow-fractionation. Anal Chem 2000;72:832-9. [PubMed]
- Balic M, Lin H, Williams A, et al. Progress in circulating tumor cell capture and analysis: implications for cancer management. Expert Rev Mol Diagn 2012;12:303-12. [PubMed]
- Gupta V, Jafferji I, Garza M, et al. ApoStream(™), a new dielectrophoretic device for antibody independent isolation and recovery of viable cancer cells from blood. Biomicrofluidics 2012;6:24133. [PubMed]
- Fehm T, Solomayer EF, Meng S, et al. Methods for isolating circulating epithelial cells and criteria for their classification as carcinoma cells. Cytotherapy 2005;7:171-85. [PubMed]
- Königsberg R, Obermayr E, Bises G, et al. Detection of EpCAM positive and negative circulating tumor cells in metastatic breast cancer patients. Acta Oncol 2011;50:700-10. [PubMed]
- Mostert B, Kraan J, Bolt-de Vries J, et al. Detection of circulating tumor cells in breast cancer may improve through enrichment with anti-CD146. Breast Cancer Res Treat 2011;127:33-41. [PubMed]
- Schindlbeck C, Stellwagen J, Jeschke U, et al. Immunomagnetic enrichment of disseminated tumor cells in bone marrow and blood of breast cancer patients by the Thomsen-Friedenreich-Antigen. Clin Exp Metastasis 2008;25:233-40. [PubMed]
- Deng G, Herrler M, Burgess D, et al. Enrichment with anti-cytokeratin alone or combined with anti-EpCAM antibodies significantly increases the sensitivity for circulating tumor cell detection in metastatic breast cancer patients. Breast Cancer Res 2008;10:R69. [PubMed]
- Bilkenroth U, Taubert H, Riemann D, et al. Detection and enrichment of disseminated renal carcinoma cells from peripheral blood by immunomagnetic cell separation. Int J Cancer 2001;92:577-82. [PubMed]
- Flatmark K, Bjørnland K, Johannessen HO, et al. Immunomagnetic detection of micrometastatic cells in bone marrow of colorectal cancer patients. Clin Cancer Res 2002;8:444-9. [PubMed]
- Naume B, Borgen E, Beiske K, et al. Immunomagnetic techniques for the enrichment and detection of isolated breast carcinoma cells in bone marrow and peripheral blood. J Hematother 1997;6:103-14. [PubMed]
- Went PT, Lugli A, Meier S, et al. Frequent EpCam protein expression in human carcinomas. Hum Pathol 2004;35:122-8. [PubMed]
- Lin H, Balic M, Zheng S, et al. Disseminated and circulating tumor cells: Role in effective cancer management. Crit Rev Oncol Hematol 2011;77:1-11. [PubMed]
- Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008;133:704-15. [PubMed]
- Sieuwerts AM, Kraan J, Bolt J, et al. Anti-epithelial cell adhesion molecule antibodies and the detection of circulating normal-like breast tumor cells. J Natl Cancer Inst 2009;101:61-6. [PubMed]
- Saucedo-Zeni N, Mewes S, Niestroj R, et al. A novel method for the in vivo isolation of circulating tumor cells from peripheral blood of cancer patients using a functionalized and structured medical wire. Int J Oncol 2012;41:1241-50. [PubMed]
- Sha MY, Xu H, Natan MJ, et al. Surface-enhanced Raman scattering tags for rapid and homogeneous detection of circulating tumor cells in the presence of human whole blood. J Am Chem Soc 2008;130:17214-5. [PubMed]
- Shi W, Paproski RJ, Moore R, et al. Detection of circulating tumor cells using targeted surface-enhanced Raman scattering nanoparticles and magnetic enrichment. J Biomed Opt 2014;19:056014. [PubMed]
- Kroes R, Williams GM, Weisburger JH. Early appearance of serum -fetoprotein during hepatocarcinogenesis as a function of age of rats and extent of treatment with 3'-methyl-4-dimethylaminoazobenzene. Cancer Res 1972;32:1526-32. [PubMed]
- Ozkumur E, Shah AM, Ciciliano JC, et al. Inertial focusing for tumor antigen-dependent and -independent sorting of rare circulating tumor cells. Sci Transl Med 2013;5:179ra47.
- Karabacak NM, Spuhler PS, Fachin F, et al. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nat Protoc 2014;9:694-710. [PubMed]
- Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med 2008;359:366-77. [PubMed]
- Sequist LV, Nagrath S, Toner M, et al. The CTC-chip: an exciting new tool to detect circulating tumor cells in lung cancer patients. J Thorac Oncol 2009;4:281-3. [PubMed]
- Harb W, Fan A, Tran T, et al. Mutational Analysis of Circulating Tumor Cells Using a Novel Microfluidic Collection Device and qPCR Assay. Transl Oncol 2013;6:528-38. eCollection 2013.
- Wynne JF, Modlin LA, Fan A, et al. Analysis of Circulating Tumor Cells in Early Stage Non-Small Cell Lung Cancer Patients Treated with Stereotactic Ablative Radiotherapy. Int J Radiat Oncol Biol Phys 2013;87:S200-1.
- Stott SL, Hsu CH, Tsukrov DI, et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc Natl Acad Sci U S A 2010;107:18392-7. [PubMed]
- Gao X, Yang L, Petros JA, et al. In vivo molecular and cellular imaging with quantum dots. Curr Opin Biotechnol 2005;16:63-72. [PubMed]
- Watson A, Wu X, Bruchez M. Lighting up cells with quantum dots. Biotechniques 2003;34:296-300, 302-3. [PubMed]
- Hsieh HB, Marrinucci D, Bethel K, et al. High speed detection of circulating tumor cells. Biosens Bioelectron 2006;21:1893-9. [PubMed]
- Pachmann K, Clement JH, Schneider CP, et al. Standardized quantification of circulating peripheral tumor cells from lung and breast cancer. Clin Chem Lab Med 2005;43:617-27. [PubMed]
- Scholtens TM, Schreuder F, Ligthart ST, et al. CellTracks TDI: an image cytometer for cell characterization. Cytometry A 2011;79:203-13. [PubMed]
- Smith B, Selby P, Southgate J, et al. Detection of melanoma cells in peripheral blood by means of reverse transcriptase and polymerase chain reaction. Lancet 1991;338:1227-9. [PubMed]
- Sergeant G, Penninckx F, Topal B. Quantitative RT-PCR detection of colorectal tumor cells in peripheral blood--a systematic review. J Surg Res 2008;150:144-52. [PubMed]
- Xenidis N, Ignatiadis M, Apostolaki S, et al. Cytokeratin-19 mRNA-positive circulating tumor cells after adjuvant chemotherapy in patients with early breast cancer. J Clin Oncol 2009;27:2177-84. [PubMed]
- Ignatiadis M, Kallergi G, Ntoulia M, et al. Prognostic value of the molecular detection of circulating tumor cells using a multimarker reverse transcription-PCR assay for cytokeratin 19, mammaglobin A, and HER2 in early breast cancer. Clin Cancer Res 2008;14:2593-600. [PubMed]
- Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer 2008;8:329-40. [PubMed]
- Stathopoulou A, Gizi A, Perraki M, et al. Real-time quantification of CK-19 mRNA-positive cells in peripheral blood of breast cancer patients using the lightcycler system. Clin Cancer Res 2003;9:5145-51. [PubMed]
- Stathopoulou A, Ntoulia M, Perraki M, et al. A highly specific real-time RT-PCR method for the quantitative determination of CK-19 mRNA positive cells in peripheral blood of patients with operable breast cancer. Int J Cancer 2006;119:1654-9. [PubMed]
- Stathopoulou A, Vlachonikolis I, Mavroudis D, et al. Molecular detection of cytokeratin-19-positive cells in the peripheral blood of patients with operable breast cancer: evaluation of their prognostic significance. J Clin Oncol 2002;20:3404-12. [PubMed]
- Xenidis N, Vlachonikolis I, Mavroudis D, et al. Peripheral blood circulating cytokeratin-19 mRNA-positive cells after the completion of adjuvant chemotherapy in patients with operable breast cancer. Ann Oncol 2003;14:849-55. [PubMed]
- Xenidis N, Perraki M, Kafousi M, et al. Predictive and prognostic value of peripheral blood cytokeratin-19 mRNA-positive cells detected by real-time polymerase chain reaction in node-negative breast cancer patients. J Clin Oncol 2006;24:3756-62. [PubMed]
- Panteleakou Z, Lembessis P, Sourla A, et al. Detection of circulating tumor cells in prostate cancer patients: methodological pitfalls and clinical relevance. Mol Med 2009;15:101-14. [PubMed]
- Ashworth TR. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aus Med J 1869;14:146-9.
- Cote RJ, Rosen PP, Lesser ML, et al. Prediction of early relapse in patients with operable breast cancer by detection of occult bone marrow micrometastases. J Clin Oncol 1991;9:1749-56. [PubMed]
- Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer 2004;4:448-56. [PubMed]
- Braun S, Vogl FD, Naume B, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med 2005;353:793-802. [PubMed]
- Giuliano AE, Hawes D, Ballman KV, et al. Association of occult metastases in sentinel lymph nodes and bone marrow with survival among women with early-stage invasive breast cancer. JAMA 2011;306:385-93. [PubMed]
- Pierga JY, Bonneton C, Vincent-Salomon A, et al. Clinical significance of immunocytochemical detection of tumor cells using digital microscopy in peripheral blood and bone marrow of breast cancer patients. Clin Cancer Res 2004;10:1392-400. [PubMed]
- Müller V, Stahmann N, Riethdorf S, et al. Circulating tumor cells in breast cancer: correlation to bone marrow micrometastases, heterogeneous response to systemic therapy and low proliferative activity. Clin Cancer Res 2005;11:3678-85. [PubMed]
- Benoy IH, Elst H, Philips M, et al. Real-time RT-PCR detection of disseminated tumour cells in bone marrow has superior prognostic significance in comparison with circulating tumour cells in patients with breast cancer. Br J Cancer 2006;94:672-80. [PubMed]
- Daskalaki A, Agelaki S, Perraki M, et al. Detection of cytokeratin-19 mRNA-positive cells in the peripheral blood and bone marrow of patients with operable breast cancer. Br J Cancer 2009;101:589-97. [PubMed]
- Wiedswang G, Borgen E, Schirmer C, et al. Comparison of the clinical significance of occult tumor cells in blood and bone marrow in breast cancer. Int J Cancer 2006;118:2013-9. [PubMed]
- Naume B, Borgen E, Tøssvik S, et al. Detection of isolated tumor cells in peripheral blood and in BM: evaluation of a new enrichment method. Cytotherapy 2004;6:244-52. [PubMed]
- Schindlbeck C, Andergassen U, Hofmann S, et al. Comparison of circulating tumor cells (CTC) in peripheral blood and disseminated tumor cells in the bone marrow (DTC-BM) of breast cancer patients. J Cancer Res Clin Oncol 2013;139:1055-62. [PubMed]
- Pantel K, Alix-Panabières C, Riethdorf S. Cancer micrometastases. Nat Rev Clin Oncol 2009;6:339-51. [PubMed]
- Norton L, Massagué J. Is cancer a disease of self-seeding? Nat Med 2006;12:875-8. [PubMed]
- Gruber I, Fehm T, Taran FA, et al. Disseminated tumor cells as a monitoring tool for adjuvant therapy in patients with primary breast cancer. Breast Cancer Res Treat 2014;144:353-60. [PubMed]
- Solá M, Margelí M, Castellá E, et al. Detection of disseminated tumor cells in locally advanced breast cancer patients before primary systemic therapy. Breast 2013;22:908-13. [PubMed]
- Luzzi KJ, MacDonald IC, Schmidt EE, et al. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol 1998;153:865-73. [PubMed]
- Méhes G, Witt A, Kubista E, et al. Circulating breast cancer cells are frequently apoptotic. Am J Pathol 2001;159:17-20. [PubMed]
- Mansi JL, Gogas H, Bliss JM, et al. Outcome of primary-breast-cancer patients with micrometastases: a long-term follow-up study. Lancet 1999;354:197-202. [PubMed]
- Hayes DF, Cristofanilli M, Budd GT, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res 2006;12:4218-24. [PubMed]
- Nolé F, Munzone E, Zorzino L, et al. Variation of circulating tumor cell levels during treatment of metastatic breast cancer: prognostic and therapeutic implications. Ann Oncol 2008;19:891-7. [PubMed]
- Bidard FC, Peeters DJ, Fehm T, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol 2014;15:406-14. [PubMed]
- Smith BM, Slade MJ, English J, et al. Response of circulating tumor cells to systemic therapy in patients with metastatic breast cancer: comparison of quantitative polymerase chain reaction and immunocytochemical techniques. J Clin Oncol 2000;18:1432-9. [PubMed]
- Liu MC, Shields PG, Warren RD, et al. Circulating tumor cells: a useful predictor of treatment efficacy in metastatic breast cancer. J Clin Oncol 2009;27:5153-9. [PubMed]
- Cristofanilli M, Hayes DF, Budd GT, et al. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol 2005;23:1420-30. [PubMed]
- Budd GT, Cristofanilli M, Ellis MJ, et al. Circulating tumor cells versus imaging--predicting overall survival in metastatic breast cancer. Clin Cancer Res 2006;12:6403-9. [PubMed]
- Smerage JB, Barlow WE, Hortobagyi GN, et al. Circulating Tumor Cells and Response to Chemotherapy in Metastatic Breast Cancer: SWOG S0500. J Clin Oncol 2014;32:3483-9. [PubMed]
- Bidard FC, Mathiot C, Degeorges A, et al. Clinical value of circulating endothelial cells and circulating tumor cells in metastatic breast cancer patients treated first line with bevacizumab and chemotherapy. Ann Oncol 2010;21:1765-71. [PubMed]
- Giordano A, Giuliano M, De Laurentiis M, et al. Circulating tumor cells in immunohistochemical subtypes of metastatic breast cancer: lack of prediction in HER2-positive disease treated with targeted therapy. Ann Oncol 2012;23:1144-50. [PubMed]
- Giuliano M, Giordano A, Jackson S, et al. Circulating tumor cells as prognostic and predictive markers in metastatic breast cancer patients receiving first-line systemic treatment. Breast Cancer Res 2011;13:R67. [PubMed]
- Rack B, Andergassen U, Janni W, et al. CTCs in primary breast cancer (I). Recent Results Cancer Res 2012;195:179-85. [PubMed]
- Mikulová V, Cabiňaková M, Janatková I, et al. Detection of circulating tumor cells during follow-up of patients with early breast cancer: Clinical utility for monitoring of therapy efficacy. Scand J Clin Lab Invest 2014;74:132-42. [PubMed]
- Lucci A, Hall CS, Lodhi AK, et al. Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol 2012;13:688-95. [PubMed]
- Rack B, Schindlbeck C, Jückstock J, et al. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst 2014;106: [PubMed]
- Riethdorf S, Müller V, Zhang L, et al. Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin Cancer Res 2010;16:2634-45. [PubMed]
- Serrano MJ, Rovira PS, Martínez-Zubiaurre I, et al. Dynamics of circulating tumor cells in early breast cancer under neoadjuvant therapy. Exp Ther Med 2012;4:43-8. [PubMed]
- Pierga JY, Bidard FC, Mathiot C, et al. Circulating tumor cell detection predicts early metastatic relapse after neoadjuvant chemotherapy in large operable and locally advanced breast cancer in a phase II randomized trial. Clin Cancer Res 2008;14:7004-10. [PubMed]
- Moreno JG, Miller MC, Gross S, et al. Circulating tumor cells predict survival in patients with metastatic prostate cancer. Urology 2005;65:713-8. [PubMed]
- Okegawa T, Nutahara K, Higashihara E. Prognostic significance of circulating tumor cells in patients with hormone refractory prostate cancer. J Urol 2009;181:1091-7. [PubMed]
- Goodman OB Jr, Fink LM, Symanowski JT, et al. Circulating tumor cells in patients with castration-resistant prostate cancer baseline values and correlation with prognostic factors. Cancer Epidemiol Biomarkers Prev 2009;18:1904-13. [PubMed]
- Chen BT, Loberg RD, Neeley CK, et al. Preliminary study of immunomagnetic quantification of circulating tumor cells in patients with advanced disease. Urology 2005;65:616-21. [PubMed]
- Garcia JA, Rosenberg JE, Weinberg V, et al. Evaluation and significance of circulating epithelial cells in patients with hormone-refractory prostate cancer. BJU Int 2007;99:519-24. [PubMed]
- de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 2008;14:6302-9. [PubMed]
- Olmos D, Arkenau HT, Ang JE, et al. Circulating tumour cell (CTC) counts as intermediate end points in castration-resistant prostate cancer (CRPC): a single-centre experience. Ann Oncol 2009;20:27-33. [PubMed]
- Scher HI, Jia X, de Bono JS, et al. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol 2009;10:233-9. [PubMed]
- Goldkorn A, Ely B, Quinn DI, et al. Circulating tumor cell counts are prognostic of overall survival in SWOG S0421: a phase III trial of docetaxel with or without atrasentan for metastatic castration-resistant prostate cancer. J Clin Oncol 2014;32:1136-42. [PubMed]
- Lee RJ, Saylor PJ, Michaelson MD, et al. A dose-ranging study of cabozantinib in men with castration-resistant prostate cancer and bone metastases. Clin Cancer Res 2013;19:3088-94. [PubMed]
- Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet 2010;375:1437-46. [PubMed]
- Loh J, Jovanovic L, Lehman M, et al. Circulating tumor cell detection in high-risk non-metastatic prostate cancer. J Cancer Res Clin Oncol 2014;140:2157-62. [PubMed]
- Khurana KK, Grane R, Borden EC, et al. Prevalence of circulating tumor cells in localized prostate cancer. Curr Urol 2013;7:65-9. [PubMed]
- Davis JW, Nakanishi H, Kumar VS, et al. Circulating tumor cells in peripheral blood samples from patients with increased serum prostate specific antigen: initial results in early prostate cancer. J Urol 2008;179:2187-91; discussion 2191. [PubMed]
- Kolostova K, Broul M, Schraml J, et al. Circulating tumor cells in localized prostate cancer: isolation, cultivation in vitro and relationship to T-stage and Gleason score. Anticancer Res 2014;34:3641-6. [PubMed]
- Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:3213-21. [PubMed]
- Gazzaniga P, Raimondi C, Gradilone A, et al. Circulating tumor cells in metastatic colorectal cancer: do we need an alternative cutoff? J Cancer Res Clin Oncol 2013;139:1411-6. [PubMed]
- Denève E, Riethdorf S, Ramos J, et al. Capture of viable circulating tumor cells in the liver of colorectal cancer patients. Clin Chem 2013;59:1384-92. [PubMed]
- Cohen SJ, Punt CJ, Iannotti N, et al. Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann Oncol 2009;20:1223-9. [PubMed]
- Matsusaka S, Suenaga M, Mishima Y, et al. Circulating tumor cells as a surrogate marker for determining response to chemotherapy in Japanese patients with metastatic colorectal cancer. Cancer Sci 2011;102:1188-92. [PubMed]
- Kuboki Y, Matsusaka S, Minowa S, et al. Circulating tumor cell (CTC) count and epithelial growth factor receptor expression on CTCs as biomarkers for cetuximab efficacy in advanced colorectal cancer. Anticancer Res 2013;33:3905-10. [PubMed]
- Koyanagi K, Bilchik AJ, Saha S, et al. Prognostic relevance of occult nodal micrometastases and circulating tumor cells in colorectal cancer in a prospective multicenter trial. Clin Cancer Res 2008;14:7391-6. [PubMed]
- Lankiewicz S, Zimmermann S, Hollmann C, et al. Circulating tumour cells as a predictive factor for response to systemic chemotherapy in patients with advanced colorectal cancer. Mol Oncol 2008;2:349-55. [PubMed]
- Uen YH, Lu CY, Tsai HL, et al. Persistent presence of postoperative circulating tumor cells is a poor prognostic factor for patients with stage I-III colorectal cancer after curative resection. Ann Surg Oncol 2008;15:2120-8. [PubMed]
- Iinuma H, Watanabe T, Mimori K, et al. Clinical significance of circulating tumor cells, including cancer stem-like cells, in peripheral blood for recurrence and prognosis in patients with Dukes’ stage B and C colorectal cancer. J Clin Oncol 2011;29:1547-55. [PubMed]
- Chen S, Song X, Chen Z, et al. CD133 expression and the prognosis of colorectal cancer: a systematic review and meta-analysis. PLoS One 2013;8:e56380. [PubMed]
- Tanaka F, Yoneda K, Kondo N, et al. Circulating tumor cell as a diagnostic marker in primary lung cancer. Clin Cancer Res 2009;15:6980-6. [PubMed]
- Blümke K, Bilkenroth U, Schmidt U, et al. Detection of circulating tumor cells from renal carcinoma patients: experiences of a two-center study. Oncol Rep 2005;14:895-9. [PubMed]
- Krebs MG, Sloane R, Priest L, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol 2011;29:1556-63. [PubMed]
- Hou JM, Greystoke A, Lancashire L, et al. Evaluation of circulating tumor cells and serological cell death biomarkers in small cell lung cancer patients undergoing chemotherapy. Am J Pathol 2009;175:808-16. [PubMed]
- Najjar F, Alammar M, Bachour M, et al. Predictive and prognostic value of circulating endothelial cells in non-small cell lung cancer patients treated with standard chemotherapy. J Cancer Res Clin Oncol 2014. [Epub ahead of print]. [PubMed]
- Hofman V, Bonnetaud C, Ilie MI, et al. Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin Cancer Res 2011;17:827-35. [PubMed]
- Hiltermann TJ, Pore MM, van den Berg A, et al. Circulating tumor cells in small-cell lung cancer: a predictive and prognostic factor. Ann Oncol 2012;23:2937-42. [PubMed]
- Naito T, Tanaka F, Ono A, et al. Prognostic impact of circulating tumor cells in patients with small cell lung cancer. J Thorac Oncol 2012;7:512-9. [PubMed]
- Hou JM, Krebs MG, Lancashire L, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol 2012;30:525-32. [PubMed]
- Muinelo-Romay L, Vieito M, Abalo A, et al. Evaluation of Circulating Tumor Cells and Related Events as Prognostic Factors and Surrogate Biomarkers in Advanced NSCLC Patients Receiving First-Line Systemic Treatment. Cancers (Basel) 2014;6:153-65. [PubMed]
- Wang J, Wang K, Xu J, et al. Prognostic significance of circulating tumor cells in non-small-cell lung cancer patients: a meta-analysis. PLoS One 2013;8:e78070. [PubMed]
- Hofman V, Ilie MI, Long E, et al. Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-small-cell lung carcinoma: comparison of the efficacy of the CellSearch Assay™ and the isolation by size of epithelial tumor cell method. Int J Cancer 2011;129:1651-60. [PubMed]
- Liberko M, Kolostova K, Bobek V. Essentials of circulating tumor cells for clinical research and practice. Crit Rev Oncol Hematol 2013;88:338-56. [PubMed]
- Hartkopf AD, Wagner P, Wallwiener D, et al. Changing levels of circulating tumor cells in monitoring chemotherapy response in patients with metastatic breast cancer. Anticancer Res 2011;31:979-84. [PubMed]
- Tewes M, Aktas B, Welt A, et al. Molecular profiling and predictive value of circulating tumor cells in patients with metastatic breast cancer: an option for monitoring response to breast cancer related therapies. Breast Cancer Res Treat 2009;115:581-90. [PubMed]
- Mathiesen RR, Borgen E, Renolen A, et al. Persistence of disseminated tumor cells after neoadjuvant treatment for locally advanced breast cancer predicts poor survival. Breast Cancer Res 2012;14:R117. [PubMed]
- Nakagawa T, Martinez SR, Goto Y, et al. Detection of circulating tumor cells in early-stage breast cancer metastasis to axillary lymph nodes. Clin Cancer Res 2007;13:4105-10. [PubMed]
- Danila DC, Heller G, Gignac GA, et al. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res 2007;13:7053-8. [PubMed]
- de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 2008;14:6302-9. [PubMed]
- Tol J, Koopman M, Miller MC, et al. Circulating tumour cells early predict progression-free and overall survival in advanced colorectal cancer patients treated with chemotherapy and targeted agents. Ann Oncol 2010;21:1006-12. [PubMed]
- Sastre J, Maestro ML, Gómez-España A, et al. Circulating tumor cell count is a prognostic factor in metastatic colorectal cancer patients receiving first-line chemotherapy plus bevacizumab: a Spanish Cooperative Group for the Treatment of Digestive Tumors study. Oncologist 2012;17:947-55. [PubMed]
- Amir E, Clemons M, Purdie CA, et al. Tissue confirmation of disease recurrence in breast cancer patients: pooled analysis of multi-centre, multi-disciplinary prospective studies. Cancer Treat Rev 2012;38:708-14. [PubMed]
- Somlo G, Lau SK, Frankel P, et al. Multiple biomarker expression on circulating tumor cells in comparison to tumor tissues from primary and metastatic sites in patients with locally advanced/inflammatory, and stage IV breast cancer, using a novel detection technology. Breast Cancer Res Treat 2011;128:155-63. [PubMed]
- Stebbing J, Payne R, Reise J, et al. The efficacy of lapatinib in metastatic breast cancer with HER2 non-amplified primary tumors and EGFR positive circulating tumor cells: a proof-of-concept study. PLoS One 2013;8:e62543. [PubMed]
- Bidard FC, Fehm T, Ignatiadis M, et al. Clinical application of circulating tumor cells in breast cancer: overview of the current interventional trials. Cancer Metastasis Rev 2013;32:179-88. [PubMed]


