Role of galectins in re-epithelialization of wounds
Re-epithelialization of wounds
Relevant in a variety of clinical scenarios, impaired wound healing remains among medicine’s most frustrating therapeutic challenges. Healing defects may occur in organ systems as different as cornea, skin and gastrointestinal (GI) tract (1-6). These are but a representative three of the number of human organ systems which may be threatened by impaired or delayed re-epithelialization which results in persistent epithelial defects. This defines a condition with serious medical implications. However, scientific effort has yet to comprehensively explain the failure of some, and not other, wounds to heal within a reasonable course of time. Meanwhile, patients with debilitations caused by a range of wounds, from relatively obscure to commonplace causes, disease-associated, accidental, surgical or inflicted (for example of combat), rely on what we know to support their treatment. Resolution of chronic wounds of various etiologies can be frustrating and may not always be successful. Around the world, millions of individuals are affected and in the United States alone, combat-related and other traumatic wounds cause over 300,000 hospitalizations each year (7,8).
Persistent corneal epithelial defects may undermine the integrity of the anterior stroma, produce ulceration and in the direst cases cause perforation of the stromal tissue with significant visual loss. The insidious damage of delayed re-epithelialization and resultant persistent epithelial defects are also evident in the chronic wounds of the elderly, decubitus ulcer, and venous statis ulcer of the skin. Impairment of the intestinal surface barrier and related damage are frequently observed in a number of GI ailments including inflammatory bowel diseases (IBDs). In these conditions, the treatment goal is prompt re-epithelialization of the wound, essential for rapid resealing of the epithelial surface barrier to control inflammation and to restore intestinal homeostasis. Delayed re-epithelialization of intestinal wounds in IBDs gives rise to uncontrolled intestinal inflammation and general immune responses (9,10).
In general, failure to re-epithelialize is caused more by a reduced potential of the epithelium to migrate across the wound bed than inadequate cell proliferation (11-13). Cell migration requires sequential adhesion to and release from the substrate, representing a complex process of cell-matrix interactions (14-17). Recent studies indicate that members of the galectin class of β-galactoside-binding proteins play a critical role in modulating cell-matrix interactions and re-epithelialization of wounds by novel carbohydrate-based recognition systems (18-26).
Galectins
Galectins are a family of widely distributed carbohydrate-binding proteins defined by their affinity for the β-galactoside-containing glycans which are present on various cell surface and extracellular matrix (ECM) glycoproteins (27,28). There are 15 presently identified members of the galectin family in mammals, ranging in subunit size from 14 to 39 kDa. Each galectin contains a canonical carbohydrate recognition domain (CRD) of ~130 amino acids. Galectins can be expressed both intracellularly and extracellularly. Galectins do not contain a classical signal sequence or a transmembrane domain and are secreted from the cell via nonclassical pathways. Some galectins such as galectins-1, -3, -8 and -9 have wide tissue distribution, whereas others, such as galectins-4, -5 and -6, exhibit tissue specificity. The current interest in delineating the function of galectins is explained by studies demonstrating that many critical cellular response including cell adhesion (29-31), migration (18,32), immune response (33,34) and angiogenesis (35-41) are modulated by this class of lectins.
Carbohydrate-binding specificity of galectins
All galectins specifically recognize galactose-containing glycans, yet each galectin has unique, fine specificity for more complex galactose-containing oligosaccharides, a consequence of variability in the CRD sequence. Each galectin associates with certain types of glycans for signaling based on differences in the carbohydrate-binding specificities (42,43). The sugar-binding specificity of different members of the galectin family can differ greatly, e.g., galectin-1 (Gal1) recognizes α2,3 sialylated, but not α2-6 sialylated, glycans; Gal2 does not bind glycans that are sialylated with either linkage; Gal3 binds internal N-acetyllactosamine (LacNAc) within polyLacNAc (42); and depending on cellular microenvironment, sialylation may also affect Gal3 binding and signaling (44). Thus, on the basis of fine distinctions in carbohydrate-binding specificities, each galectin may interact with a discrete spectrum of glycoprotein receptors, with resulting specific downstream effects. For example, the affinity of Gal1 for the blood group A tetrasaccharide is approximately 100-fold lower than that for Gal3 (45), and only Gal8, but not Gal1, Gal2, Gal3, or Gal7, interact with the glycans of podoplanin, a lymphatic vessel glycoprotein (46).
Galectin-glycan lattices
All lectins are either dimers or oligomers, and this multivalency enables formation of lectin-carbohydrate lattices to cross-link and clusterize cell surface receptors including growth factor receptors and integrins. The diverse functions of galectins are thought to result from the formation of galectin-glycan lattice (47-49), by which the glycoprotein receptors are trapped, and as a result, prevented from undergoing endocytosis (50). By this mechanism, the interactions between galectins and N-glycans of the cell surface receptors regulate the density and distribution of cell surface receptors as well as cell responsiveness to the receptor ligand (47-50). Thus, Gal3 interacts, in a carbohydrate-dependent manner with the N-glycans of the epidermal growth factor (EGF) receptor which defers its constitutive endocytic removal and promotes EGF signaling (50). Likewise, studies in our laboratory have shown that Gal3 stimulates epithelial cell migration and formation of lamellipodia by activating α3β1-integrin-Rac1 signaling, and carbohydrate-mediated interaction between Gal3 and complex N-glycans on the α3β1 integrin is inherent in Gal3-induced lamellipodia formation and cell migration (18). It should be noted that cytoplasmic Gal3 also promotes re-epithelialization of wounds, however, by mechanisms that are independent of galectin-glycan lattices (51).
Role of galectins in wound healing
Galectin-3 (Gal3)
Gal3 expression occurs in inflammatory cells, epithelia, and fibroblasts of a variety of tissues (27). It is present on the cell surface, within ECM, and in the cytoplasm. Gal3 influences cell-matrix interaction by binding to the ECM and cell surface glycosylated counter receptors (e.g., growth factor receptors, integrins, certain isoforms of laminin, fibronectin and vitronectin). Furthermore, Gal3 in the nucleus of cells may influence cell-matrix interactions indirectly by its effect on the expression of well-known cell adhesion molecules (e.g., α6β1 and α4β7 integrins) and cytokines (e.g., IL-1).
Role of Gal3 in corneal wound healing
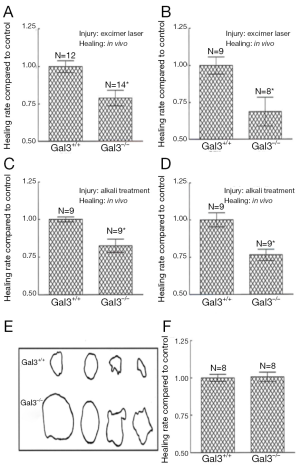
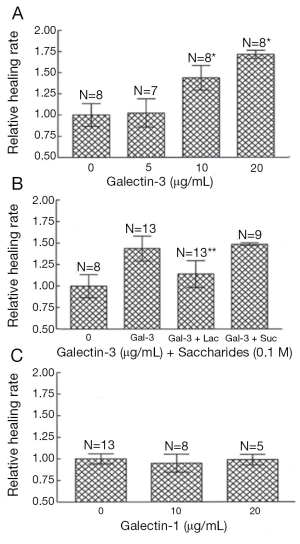
Gal3 is present in high density at sites of corneal epithelial cell-matrix adhesion (25), an ideal placement for influence on cell-matrix interactions and cell migration. To examine whether Gal3 plays a role in re-epithelialization of corneal wounds and to determine whether the rate of wound closure rate is impaired in Gal3-deficient mice, we utilized two different models of corneal wound healing. In this study, corneas with either excimer laser ablations or alkali-burns were allowed to partially heal in vivo or in vitro for up to 22 h, at which time remaining wound areas were quantitated and compared among the study groups. Whether the corneas were injured by excimer laser or by alkali treatment and whether the corneas healed in vivo or in vitro, epithelial wound closure rate (mm2/h) was significantly slower in Gal3−/−mice compared with Gal3+/+ mice (Figure 1A-E) (25). However, no differences were found in the wound closure rates between Gal1+/+ and Gal1−/− groups (Figure 1F). Whether delayed re-epithelialization of corneal wounds in Gal3−/− mice is due to a deficiency in the rate of corneal epithelial cell proliferation is the question addressed by the next experiment. In order to identify cells undergoing DNA synthesis, normal and healing Gal3+/+ and Gal3−/− corneas were labeled with BrdUrd. This study found no significant difference in the number BrdUrd-labeled cells between Gal3+/+ and Gal3−/− corneas (25). Thus the rate of corneal epithelial cell proliferation seemed not to be perturbed in Gal3−/− mice. It follows that delayed re-epithelialization of corneal wounds found in Gal3−/− mice is more likely caused by impairment in the cell migration process. The next experiments set out to learn whether exogenous Gal3 would stimulate re-epithelialization of corneal wounds. In this study, Gal3+/+ mouse corneas with alkali-burn wounds were incubated in serum-free media with varying amounts of recombinant Gal3. The remaining wound areas were quantified following the healing period of 22-24 h. The rate of wound closure was stimulated by exogenous Gal3 in a concentration-dependent manner in Gal3+/+ mice (Figure 2). In the presence of 10 and 20 μg/mL Gal3, the acceleration rate of re-epithelialization of wounds was 43% and 71%, respectively over control corneas incubated in media alone without Gal3. It was further shown that a competing disaccharide, β-lactose, but not an irrelevant disaccharide, sucrose, can nearly completely undermine the stimulatory effect of Gal3 on the rate of corneal epithelial wound closure, indicating that the lectin CRD is directly involved in the positive effect of the exogenous lectin on the wound closure. Parallel experiments demonstrated that recombinant Gal1 did not increase the healing rate of corneal epithelial wound. Other studies have subsequently revealed that exogenous Gal3 advances re-epithelialization of wounds in rat corneas (52), monkey corneas (53) as well as in a rat dry eye model (54).
Role of Gal3 in intestinal wound healing
Gal3 is expressed to a high degree in enterocytes and subepithelial macrophages of the GI react (55,56), and is thought to have a wound healing function. Scratch wound-healing assays in which colonic epithelial cells (T84 cells) were treated with Gal3 for 24 hours demonstrated improved healing with a 60.4%±4.4% reduction in wound width (20). The Gal3-induced reduction in wound width was inhibited by a pan-inhibitor of galectins, β-lactose, and an anti-Gal3 neutralizing antibody (–9.8%±24.8%). It is of interest to note that epithelia derived from IBD tissues (57-59) have reduced levels of Gal-3, but it is yet to be shown whether this is a causative factor in the wound healing related complications of patients with IBD. However, from in vitro experiments, it was learned that matrix metalloproteinase-7 (MMP7), highly expressed in IBD tissues (57,58), cleaves Gal3, and the addition of MMP7 to Gal3 abrogates the wound healing and cell migration induced by Gal3 (20). Based on these findings, Puthenedam and colleagues (20) proposed that cleavage of Gal3 may be one mechanism by which MMP7 inhibits wound healing. This study is important to our understanding of delayed wound healing in chronic intestinal diseases such as intestinal ulcers and IBD, in which MMP7 protein expression is elevated, with an accompanying decrease in Gal3 protein expression.
Role of Gal3 in skin wound healing
In a recent study, using Gal3−/− mice and cells isolated from these mice, Liu et al. (51) demonstrated that the absence of Gal3 impairs keratinocyte migration and skin wound re-epithelialization. Interestingly, in this study, the promigratory function of the lectin was attributed to cytosolic Gal3, and, therefore, is likely to be carbohydrate independent.
Galectin-7 (Gal7)
As a prototype galectin that forms homodimers (60), Gal7 can cross-link cell surface receptors. Gal7 is expressed preferentially in stratified epithelia including epidermis, oral cavity, cornea, esophagus and anorectal epithelium (61). During re-epithelialization of corneal wounds, and in some cancers such as skin tumors (62), significant changes in the levels of Gal7 expression have been detected. Gal7 is considered a marker for stratified epithelia (61). Nevertheless, this lectin has been found to be present in cilia isolated from cultured human airway, and in most of the cilia of multiciliated cells in human airway epithelia primary cultures (61,63,64). Gal7 expression is evident as well in the primary cilia of Madin-Darby canine kidney (MDCK) cells (65), LLC-PK1 porcine kidney, and mpkCCDc14 mouse kidney cells and on cilia in the rat renal proximal tubule (19). Gal7 plays a role in wound healing of not only stratified epithelium which lack cilia such as that of cornea and skin, but also of simple epithelia such as that of kidney epithelium (19).
Role in corneal wound healing
Gal7 expression is upregulated substantially in mouse corneas upon injury and exogenous Gal7 was shown to stimulate corneal wound re-epithelialization in organ culture specimens (24). The stimulation of wound closure by Gal7 is partly undermined by β-lactose, a competing disaccharide, but not by sucrose, an irrelevant disaccharide, again suggesting that the Gal7 CRD is directly involved in stimulatory effect of the exogenous lectin in promoting wound closure.
Role in skin wound healing
Gendronneau et al. (23) have used Gal7 knockout mice to assess the role of this lectin in skin wound healing. Superficial scratches were made along the sagittal axis of the tail of Gal7+/+ and Gal7−/− adult mice. Tissue sections of healing tails at 24 and 48 h after experimental injury were stained with hematoxylin and eosin and distance between the two wound margins was measured. The process of wound closure was judged to be less efficient in the Gal7-/- mice compared to the Gal7+/+ mice. Additionally, according to an ex vivo wound healing assay, outgrowth of keratinocyte from Gal7−/− skin explants was also reduced in comparison with the Gal7+/+ controls. It was further shown that Gal7 accumulates in podosomes, which are specialized cell-matrix adhesion complexes connecting the ECM to the microfilament network, and that distribution of cortactin, an actin-binding protein implicated in membrane ruffle formation, is severely affected in migrating keratinocytes lacking Gal7, suggesting that that the formation and/or stabilization of actin-based lamellipodia is abnormal in Gal7 null keratinocytes. In the in vivo model, even when proliferation was blocked by mitomycin-C, the rate of wound closure rate was slower in Gal7 null mice, supporting a conclusion that as shown with Gal3, Gal7 also promotes re-epithelialization of skin wounds by influencing cell migration and not cell proliferation.
Role of Gal7 in wound repair of polarized kidney epithelial cells
Rondanino et al. (19) compared the length of cilia and wound closure rate between the Gal7 shRNA knockdown and control kidney epithelial cells. In this study, the control cells exhibited significantly longer cilia than Gal7 knockdown cells. A 33% reduction in wound healing was observed in scratch wound assays for Gal7 knockdown cells compared to control cells (19).
Role of Gal7 in uterine repair
Gal7 is also thought to be important for normal uterine repair following menstruation (66). Gal7 immunoreactivity is detected in the endometrial luminal and glandular epithelium during the late secretory and menstrual phases, and exogenous Gal7 enhances endometrial epithelial wound repair in vitro. Also, Gal7 immunoreactivity is significantly reduced in the endometrium of women with amenorrhoea compared with normally cycling women, suggesting the putative role of Gal7 in uterine repair.
Galectins-2 and -4
Gal2 and Gal4 are of particular interest relative to the GI tract. Both are expressed specifically in GI tissues, but not in various other tissues including brain, kidney, skeletal muscle, liver, or lung tissues (67).
Role of galectins-2 and -4 in intestinal epithelial wound healing
In several models of intestinal inflammation, exogenous Gal2 was demonstrated to ameliorate colitis (68). In an effort to elucidate the function of Gal2 in wound healing, Paclik et al. (21) employed scratch wound assays to assess the influence of exogenous Gal2 on cell migration. Confluent monolayers of Caco-2 cells were injured with a surgical blade and incubated for 24 hours either with or without 50 μg/mL Gal2, after which wound closure rate was quantified. Gal-2 significantly enhanced epithelial cell migration over the wound edge (21). In the study by Paclik et al. (21), exogenous Gal4 also promoted wound closure, whereas Gal1 did not. Both Gal2 and -4 promoted cell migration as well as proliferation of Caco-2 cells suggesting that both processes may be involved in resealing the disrupted epithelial barrier in GI disorders (21). In contrast, as described above, Gal3 and Gal7 promote cell migration, but not cell proliferation of corneal and skin epithelial cells.
Molecular mechanism by which galectins modulate wound healing
Gal3 promotes wound healing by activating α3β1-integrin-Rac1 signaling
Cell migration is complex and requires first, the extension of protrusions, e.g., lamellipodia or filopodia, from the cell; secondly, the interaction of the surface molecules of these protrusions with the permissive ligands in the underlying matrix to create transient cell-matrix adhesions; and thirdly, actomyosin-mediated cell contraction and forward movement with a concurrent detachment of adhesions at the rear end (69). Contributing to regulating the cell migration process are transmembrane integrin receptors that mediate cell-matrix adhesions and intracellular signaling pathways, leading to cytoskeletal reorganization and cell motility (69). Nearly all integrins are glycosylated proteins, and various recent studies have demonstrated modulation of transmembrane signaling by integrin glycans (70,71). That interactions between integrin glycans and carbohydrate-binding proteins, galectins, have an essential function in integrin-dependent cell adhesion and migration has specifically been demonstrated (44,72-78). Lagana et al. (74) demonstrated that Gal3 interactions with N-acetylglucosaminyltransferase V (GnT-V)-modified N-glycans on mammary carcinoma cell surface support α5β1 integrin activation and cell motility. Studies in our laboratory aimed at characterizing the molecular mechanism by which Gal3 promotes epithelial cell migration during corneal wound closure, have demonstrated that Gal3, by interacting with GnT-V-modified complex N-glycans, activates α3β1-integrin-Rac1 signaling to induce formation of lamellipodia in epithelial cells, and, this in turn, promotes cell migration and re-epithelialization of wounds (18).
Galectin-3 promotes wound healing by interacting with N-glycans of laminin-332
Laminin-332 (Lm332; also known as laminin-5), a component of basement membranes in the cornea, skin and other stratified squamous epithelial tissues (79-81), is overexpressed at the leading edge of wounds during healing and promotes cell migration (82-84). It is believed to have a critical role in wound re-epithelialization. A null mutation of Lm332 causes a lethal blistering disease of the skin. Laminins are heavily glycosylated; nevertheless, the role of Lm332 has not been widely studied relative to its glycosylation pattern. The glycosyltransferase GnT-V catalyzes addition of the β1,6-linked GlcNAc branch which serves as a substrate for polylactosamine, the high affinity ligands for Gal3. By contrast, GnT-III adds GlcNAc to the inner β-linked mannose to form bisecting GlcNAc, which suppresses both further processing by branching enzymes, such as GnT-V, and elongation of N-glycans (85-87), resulting in downregulation of interaction with Gal3 with a concomitant reduction in cell migration and cancer metastasis (88). Therefore, it may be inferred that the sugar chains are an on/off switch for galectin binding during wound healing. This is particularly relevant since changes in glycosylation are observed during re-epithelialization of wounds. Kariya et al.’s elegant study (84) has shown that Gal3 binds to Lm332 coated wells, which greatly enhances Lm332-dependent keratinocyte motility. On the other hand, exogenous Gal3 did not induce an increase in cell migration on GnT-III-Lm332 substratum because modifying Lm332 by GnT-III diminishes its ability to bind to Gal3. These results led authors to propose that Gal3 may be a cofactor for Lm332-induced cell motility during wound healing and squamous cell carcinoma tumor progression, conditions that are associated with GnT-V overexpression (84).
Galectin-3 promotes wound healing by interacting with N-glycans of CD147
Subsequent to the injury, epithelial cells are required to change their shape and rearrange their position to assume a migratory phenotype. It is well established that induction of matrix metalloproteinase activity contributes to the disassembly of intercellular junctions and the degradation of the ECM to mitigate the physical constraint to cell movement. CD147 (EMMPRIN) is a widely distributed cell surface glycoprotein highly enriched on the surface of keratinocytes during wound healing. A major function of CD147 is stimulation of MMP synthesis through homophilic interactions involving both heterotypic and homotypic cell-cell interactions. In a recent study, Mauris and colleagues (26) have demonstrated that Gal3 plays a key in destabilizing cell-cell interactions by interacting with and clustering CD147 on the epithelial cell surface. In this study, the authors identified CD147 as a membrane receptor for galectin-3 in human keratinocytes and demonstrated that Gal3 initiates keratinocyte cell-cell disassembly by inducing MMP expression in a CD147-dependent manner. Thus, one of the mechanisms by which Gal3 promotes cell migration and re-epithelialization of wound is by destabilizing cell-cell contacts to promote the epithelial rearrangement and cellular plasticity that are associated with cell motility.
Galectin-3 promotes cell migration by interaction with Alix
Alix, is a protein component of the endosomal sorting complex required for transport (ESCRT) machinery (89) and has been reported to attenuate EGFR endocytosis (90). Galectin-3 is an intracellular partner of Alix (91) and Liu and colleagues (51) have demonstrated that cytoplasmic Gal3 promotes keratinocyte migration and skin wound re-epithelialization by modulating intracellular trafficking of EGFR by interacting with Alix. Unlike Gal3 interactions with the glycans of cell surface receptors, interactions between intracellular Gal3 and Alix are likely to be carbohydrate-independent. Thus, both extracellular and intracellular galectins play a role in wound healing by distinct mechanisms.
Therapeutic implications
As described in the introduction, there is an ongoing and expanding need for effective treatment of chronic wounds in the elderly, decubitus ulcers, and venous stasis ulcers of the skin. Paralleling this need, wound healing related complications in various GI diseases including IBDs remain a major clinical challenge, as does the treatment of persistent epithelial defects of the cornea. In ophthalmology we find an example of a contemporary development that expands the scope of the challenge to find the key to wound healing. It has been estimated that in the United States alone in a given year nearly half a million excimer laser keratectomy procedures are performed to obviate the need for eyeglasses and contact lenses to correct myopia (92). Considering that over 25-30% of the adult population worldwide is myopic, the potential number of myopia surgeries is enormous. In some cases following excimer laser surgery, there is a delay in epithelial healing, which puts the pre-surgically healthy cornea at risk of developing postoperative haze, infectious keratitis, and ulceration.
The quest has led to investigations of EGF, transforming growth factor-α, fibroblast growth factor, keratinocyte growth factor, and hepatocyte growth factor, all of which are known to stimulate cell proliferation, as possible drug targets to promote wound healing. Generally, the results have been disappointing (1,5,93-96). The extent of acceleration of re-epithelialization of wounds was far less in most of these studies using growth factors (92,94) than that observed with galectins in some of the studies discussed above. Additionally, it was found that treating corneas with growth factors such as EGF resulted in hyperplastic epithelium, a clearly undesirable condition (93,97,98). In this respect, the lectins Gal3 and Gal7 do not induce cell mitosis in healing corneas and skin, implying that galectin-based drugs may be more attractive as they do not have the disadvantage of causing epithelial hyperplasticity. In summary, findings that galectins stimulate the re-epithelialization of corneal, dermal, intestinal and kidney wounds provide the basis for developing novel therapeutic strategies for the treatment of nonhealing wounds.
Acknowledgements
I am greatly indebted to my long-time colleague and a senior research associate in the lab, Dr. Zhiyi Cao, who performed many of the studies, described in this review and also trained all postdoctoral fellows and students who undertook studies on corneal wound healing in the author’s laboratory. The work carried out in the author’s laboratory was supported by a National Eye Institute (NIH) Grant EY007088, a core grant for vision research P30EY13078, New England Corneal Transplant Fund, Mass Lions Eye Research fund and an unrestricted grant from Research to Prevent Blindness.
Disclosure: The author declares no conflict of interest.
References
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341:738-46. [PubMed]
- Raja Sivamani K. Wound re-epithelialization: Modulating keratinocyte migration in wound healing. Front Biosci 2007;12:2849-68. [PubMed]
- Reinecke RD. eds. Ophthalmology annual. New York: Raven Press, 1989.
- Fonder MA, Lazarus GS, Cowan DA, et al. Treating the chronic wound: A practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol 2008;58:185-206. [PubMed]
- Eaglstein WH, Falanga V. Chronic wounds. Surg Clin North Am 1997;77:689-700. [PubMed]
- Krachmer JH, Mannis MJ, Holland EJ. eds. Cornea. St. Louis: Mosby-Year Book, 1997.
- Hostetler SG, Xiang H, Gupta S, et al. Discharge patterns of injury-related hospitalizations with an acute wound in the United States. Wounds 2006;18:340-51.
- Belmont PJ, Schoenfeld AJ, Goodman G. Epidemiology of combat wounds in operation iraqi freedom and operation enduring freedom: Orthopaedic burden of disease. J Surg Orthop Adv 2010;19:2-7. [PubMed]
- Dignass AU. Mechanisms and modulation of intestinal epithelial repair. Inflamm Bowel Dis 2001;7:68-77. [PubMed]
- Dignass A, Sturm A, Podolsky DK. eds. Epithelial injury and restitution. Dordrecht: Kluwer Academic, 2002.
- Seiler WO, Stahelin HB, Zolliker R, et al. Impaired migration of epidermal cells from decubitus ulcers in cell cultures. A cause of protracted wound healing? Am J Clin Pathol 1989;92:430-4. [PubMed]
- Hanna C. Effect of idu on DNA synthesis. During corneal wound healing. Am J Ophthalmol 1966;61:279-82. [PubMed]
- Clark RAF. eds. The molecular and cellular biology of wound repair. New York: Plenum Press, 1996.
- Lauffenburger DA, Horwitz AF. Cell migration: A physically integrated molecular process. Cell 1996;84:359-69. [PubMed]
- Hynes RO. The extracellular matrix: Not just pretty fibrils. Science 2009;326:1216-9. [PubMed]
- Gumbiner BM. Cell adhesion: The molecular basis of tissue architecture and morphogenesis. Cell 1996;84:345-57. [PubMed]
- Bornstein P, Sage EH. Matricellular proteins: Extracellular modulators of cell function. Curr Opin Cell Biol 2002;14:608-16. [PubMed]
- Saravanan C, Liu FT, Gipson IK, et al. Galectin-3 promotes lamellipodia formation in epithelial cells by interacting with complex n-glycans on alpha3beta1 integrin. J Cell Sci 2009;122:3684-93. [PubMed]
- Rondanino C, Poland PA, Kinlough CL, et al. Galectin-7 modulates the length of the primary cilia and wound repair in polarized kidney epithelial cells. Am J Physiol Renal Physiol 2011;301:F622-633. [PubMed]
- Puthenedam M, Wu F, Shetye A, et al. Matrilysin-1 (mmp7) cleaves galectin-3 and inhibits wound healing in intestinal epithelial cells. Inflamm Bowel Dis 2011;17:260-7. [PubMed]
- Paclik D, Lohse K, Wiedenmann B, et al. Galectin-2 and -4, but not galectin-1, promote intestinal epithelial wound healing in vitro through a tgf-beta-independent mechanism. Inflamm Bowel Dis 2008;14:1366-72. [PubMed]
- Kariya Y, Kawamura C, Tabei T, et al. Bisecting glcnac residues on laminin-332 down-regulate galectin-3-dependent keratinocyte motility. J Biol Chem 2010;285:3330-40. [PubMed]
- Gendronneau G, Sidhu SS, Delacour D, et al. Galectin-7 in the control of epidermal homeostasis after injury. Mol Biol Cell 2008;19:5541-9. [PubMed]
- Cao Z, Said N, Wu HK, et al. Galectin-7 as a potential mediator of corneal epithelial cell migration. Arch Ophthalmol 2003;121:82-6. [PubMed]
- Cao Z, Said N, Amin S, et al. Galectins-3 and -7, but not galectin-1, play a role in re-epithelialization of wounds. J Biol Chem 2002;277:42299-305. [PubMed]
- Mauris J, Woodward AM, Cao Z, et al. Molecular basis for mmp9 induction and disruption of epithelial cell-cell contacts by galectin-3. J Cell Sci 2014;127:3141-8. [PubMed]
- Yang RY, Rabinovich GA, Liu FT. Galectins: Structure, function and therapeutic potential. Expert Rev Mol Med 2008;10:e17. [PubMed]
- Leffler H, Carlsson S, Hedlund M, et al. Introduction to galectins. Glycoconj J 2004;19:433-40. [PubMed]
- Tadokoro T, Ikekita M, Toda T, et al. Involvement of galectin-3 with vascular cell adhesion molecule-1 in growth regulation of mouse balb/3t3 cells. J Biol Chem 2009;284:35556-63. [PubMed]
- Friedrichs J, Torkko JM, Helenius J, et al. Contributions of galectin-3 and -9 to epithelial cell adhesion analyzed by single cell force spectroscopy. J Biol Chem 2007;282:29375-83. [PubMed]
- Diskin S, Cao Z, Leffler H, et al. The role of integrin glycosylation in galectin-8-mediated trabecular meshwork cell adhesion and spreading. Glycobiology 2009;19:29-37. [PubMed]
- Lagana A, Goetz JG, Cheung P, et al. Galectin binding to mgat5-modified n-glycans regulates fibronectin matrix remodeling in tumor cells. Mol Cell Biol 2006;26:3181-93. [PubMed]
- Rabinovich GA, Liu FT, Hirashima M, et al. An emerging role for galectins in tuning the immune response: Lessons from experimental models of inflammatory disease, autoimmunity and cancer. Scand J Immunol 2007;66:143-58. [PubMed]
- Liu FT, Rabinovich GA. Galectins: Regulators of acute and chronic inflammation. Ann N Y Acad Sci 2010;1183:158-82. [PubMed]
- Thijssen VL, Postel R, Brandwijk RJ, et al. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc Natl Acad Sci U S A 2006;103:15975-80. [PubMed]
- Thijssen VL, Poirier F, Baum LG, et al. Galectins in the tumor endothelium: Opportunities for combined cancer therapy. Blood 2007;110:2819-27. [PubMed]
- Nangia-Makker P, Honjo Y, Sarvis R, et al. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am J Pathol 2000;156:899-909. [PubMed]
- Markowska AI, Liu FT, Panjwani N. Galectin-3 is an important mediator of vegf- and bfgf-mediated angiogenic response. J Exp Med 2010;207:1981-93. [PubMed]
- Markowska AI, Jefferies KC, Panjwani N. Galectin-3 protein modulates cell surface expression and activation of vascular endothelial growth factor receptor 2 in human endothelial cells. J Biol Chem 2011;286:29913-21. [PubMed]
- Hsieh SH, Ying NW, Wu MH, et al. Galectin-1, a novel ligand of neuropilin-1, activates vegfr-2 signaling and modulates the migration of vascular endothelial cells. Oncogene 2008;27:3746-53. [PubMed]
- Delgado VM, Nugnes LG, Colombo LL, et al. Modulation of endothelial cell migration and angiogenesis: A novel function for the “tandem-repeat” lectin galectin-8. FASEB J 2011;25:242-54. [PubMed]
- Stowell SR, Arthur CM, Mehta P, et al. Galectin-1, -2, and -3 exhibit differential recognition of sialylated glycans and blood group antigens. J Biol Chem 2008;283:10109-23. [PubMed]
- Stillman BN, Hsu DK, Pang M, et al. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce t cell death. J Immunol 2006;176:778-89. [PubMed]
- Zhuo Y, Chammas R, Bellis SL. Sialylation of beta1 integrins blocks cell adhesion to galectin-3 and protects cells against galectin-3-induced apoptosis. J Biol Chem 2008;283:22177-85. [PubMed]
- Sparrow CP, Leffler H, Barondes SH. Multiple soluble beta-galactoside-binding lectins from human lung. J Biol Chem 1987;262:7383-90. [PubMed]
- Cueni LN, Detmar M. Galectin-8 interacts with podoplanin and modulates lymphatic endothelial cell functions. Exp Cell Res 2009;315:1715-23. [PubMed]
- Rabinovich GA, Toscano MA, Jackson SS, et al. Functions of cell surface galectin-glycoprotein lattices. Curr Opin Struct Biol 2007;17:513-20. [PubMed]
- Garner OB, Baum LG. Galectin-glycan lattices regulate cell-surface glycoprotein organization and signalling. Biochem Soc Trans 2008;36:1472-7. [PubMed]
- Brewer CF, Miceli MC, Baum LG. Clusters, bundles, arrays and lattices: Novel mechanisms for lectin-saccharide-mediated cellular interactions. Curr Opin Struct Biol 2002;12:616-23. [PubMed]
- Partridge EA, Le Roy C, Di Guglielmo GM, et al. Regulation of cytokine receptors by golgi n-glycan processing and endocytosis. Science 2004;306:120-4. [PubMed]
- Liu W, Hsu DK, Chen HY, et al. Galectin-3 regulates intracellular trafficking of egfr through alix and promotes keratinocyte migration. J Invest Dermatol 2012;132:2828-37. [PubMed]
- Yabuta C, Yano F, Fujii A, et al. Galectin-3 enhances epithelial cell adhesion and wound healing in rat cornea. Ophthalmic Res 2014;51:96-103. [PubMed]
- Fujii A, Shearer TR, Azuma M. Galectin-3 Facilitates Epithelial Wound Healing in Explanted Monkey Corneas. ARVO 2012, Abstract 3504.
- Sasaki A, Tamada Y, Shearer TR, et al. Galection-3 Facilitates Corneal Epithelial Wound Healing In A Rat Model Of Dry Eye. ARVO 2012, Abstract 2359.
- Lotz MM, Andrews CW Jr, Korzelius CA, et al. Decreased expression of mac-2 (carbohydrate binding protein 35) and loss of its nuclear localization are associated with the neoplastic progression of colon carcinoma. Proc Natl Acad Sci U S A 1993;90:3466-70. [PubMed]
- Brazowski E, Dotan I, Tulchinsky H, et al. Galectin-3 expression in pouchitis in patients with ulcerative colitis who underwent ileal pouch-anal anastomosis (ipaa). Pathol Res Pract 2009;205:551-8. [PubMed]
- Muller S, Schaffer T, Flogerzi B, et al. Galectin-3 modulates t cell activity and is reduced in the inflamed intestinal epithelium in ibd. Inflamm Bowel Dis 2006;12:588-97. [PubMed]
- Matsuno K, Adachi Y, Yamamoto H, et al. The expression of matrix metalloproteinase matrilysin indicates the degree of inflammation in ulcerative colitis. J Gastroenterol 2003;38:348-54. [PubMed]
- Jensen-Jarolim E, Gscheidlinger R, Oberhuber G, et al. The constitutive expression of galectin-3 is downregulated in the intestinal epithelia of crohn’s disease patients, and tumour necrosis factor alpha decreases the level of galectin-3-specific mrna in hct-8 cells. Eur J Gastroenterol Hepatol 2002;14:145-52. [PubMed]
- Leonidas DD, Vatzaki EH, Vorum H, et al. Structural basis for the recognition of carbohydrates by human galectin-7. Biochemistry 1998;37:13930-40. [PubMed]
- Magnaldo T, Fowlis D, Darmon M. Galectin-7, a marker of all types of stratified epithelia. Differentiation 1998;63:159-68. [PubMed]
- Saussez S, Kiss R. Galectin-7. Cell Mol Life Sci 2006;63:686-97. [PubMed]
- Timmons PM, Colnot C, Cail I, et al. Expression of galectin-7 during epithelial development coincides with the onset of stratification. Int J Dev Biol 1999;43:229-35. [PubMed]
- Ostrowski LE, Blackburn K, Radde KM, et al. A proteomic analysis of human cilia: Identification of novel components. Mol Cell Proteomics 2002;1:451-65. [PubMed]
- Poland PA, Rondanino C, Kinlough CL, et al. Identification and characterization of endogenous galectins expressed in madin darby canine kidney cells. J Biol Chem 2011;286:6780-90. [PubMed]
- Evans J, Yap J, Gamage T, et al. Galectin-7 is important for normal uterine repair following menstruation. Mol Hum Reprod 2014;20:787-98. [PubMed]
- Huflejt ME, Leffler H. Galectin-4 in normal tissues and cancer. Glycoconj J 2004;20:247-55. [PubMed]
- Paclik D, Berndt U, Guzy C, et al. Galectin-2 induces apoptosis of lamina propria t lymphocytes and ameliorates acute and chronic experimental colitis in mice. J Mol Med (Berl) 2008;86:1395-406. [PubMed]
- Ridley AJ, Schwartz MA, Burridge K, et al. Cell migration: Integrating signals from front to back. Science 2003;302:1704-9. [PubMed]
- Gu J, Taniguchi N. Regulation of integrin functions by n-glycans. Glycoconj J 2004;21:9-15. [PubMed]
- Bellis SL. Variant glycosylation: An underappreciated regulatory mechanism for beta1 integrins. Biochim Biophys Acta 2004;1663:52-60.
- Nishi N, Shoji H, Seki M, et al. Galectin-8 modulates neutrophil function via interaction with integrin alpham. Glycobiology 2003;13:755-63. [PubMed]
- Levy Y, Ronen D, Bershadsky AD, et al. Sustained induction of erk, protein kinase b, and p70 s6 kinase regulates cell spreading and formation of f-actin microspikes upon ligation of integrins by galectin-8, a mammalian lectin. J Biol Chem 2003;278:14533-42. [PubMed]
- Lagana A, Goetz JG, Cheung P, et al. Galectin binding to mgat5-modified n-glycans regulates fibronectin matrix remodeling in tumor cells. Mol Cell Biol 2006;26:3181-93. [PubMed]
- Goetz JG, Joshi B, Lajoie P, et al. Concerted regulation of focal adhesion dynamics by galectin-3 and tyrosine-phosphorylated caveolin-1. J Cell Biol 2008;180:1261-75. [PubMed]
- Friedrichs J, Manninen A, Muller DJ, et al. Galectin-3 regulates integrin alpha2beta1-mediated adhesion to collagen-i and -iv. J Biol Chem 2008;283:32264-72. [PubMed]
- Fischer C, Sanchez-Ruderisch H, Welzel M, et al. Galectin-1 interacts with the {alpha}5{beta}1 fibronectin receptor to restrict carcinoma cell growth via induction of p21 and p27. J Biol Chem 2005;280:37266-77. [PubMed]
- Cárcamo C, Pardo E, Oyanadel C, et al. Galectin-8 binds specific beta1 integrins and induces polarized spreading highlighted by asymmetric lamellipodia in jurkat t cells. Exp Cell Res 2006;312:374-86. [PubMed]
- Rousselle P, Lunstrum GP, Keene DR, et al. Kalinin: An epithelium-specific basement membrane adhesion molecule that is a component of anchoring filaments. J Cell Biol 1991;114:567-76. [PubMed]
- Colognato H, Yurchenco PD. Form and function: The laminin family of heterotrimers. Dev Dyn 2000;218:213-34. [PubMed]
- Carter WG, Ryan MC, Gahr PJ. Epiligrin, a new cell adhesion ligand for integrin alpha 3 beta 1 in epithelial basement membranes. Cell 1991;65:599-610. [PubMed]
- Ryan MC, Tizard R, VanDevanter DR, et al. Cloning of the lama3 gene encoding the alpha 3 chain of the adhesive ligand epiligrin. Expression in wound repair. J Biol Chem 1994;269:22779-87. [PubMed]
- Nguyen BP, Gil SG, Carter WG. Deposition of laminin 5 by keratinocytes regulates integrin adhesion and signaling. J Biol Chem 2000;275:31896-907. [PubMed]
- Kariya Y, Miyazaki K. The basement membrane protein laminin-5 acts as a soluble cell motility factor. Exp Cell Res 2004;297:508-20. [PubMed]
- Schachter H. Biosynthetic controls that determine the branching and microheterogeneity of protein-bound oligosaccharides. Biochem Cell Biol 1986;64:163-81. [PubMed]
- Gu J, Taniguchi N. Potential of n-glycan in cell adhesion and migration as either a positive or negative regulator. Cell Adh Migr 2008;2:243-5. [PubMed]
- Gu J, Sato Y, Kariya Y, et al. A mutual regulation between cell-cell adhesion and n-glycosylation: Implication of the bisecting glcnac for biological functions. J Proteome Res 2009;8:431-5. [PubMed]
- Yoshimura M, Nishikawa A, Ihara Y, et al. Suppression of lung metastasis of b16 mouse melanoma by n-acetylglucosaminyltransferase iii gene transfection. Proc Natl Acad Sci U S A 1995;92:8754-8. [PubMed]
- Katoh K, Shibata H, Suzuki H, et al. The alg-2-interacting protein alix associates with chmp4b, a human homologue of yeast snf7 that is involved in multivesicular body sorting. J Biol Chem 2003;278:39104-13. [PubMed]
- Schmidt MH, Hoeller D, Yu J, et al. Alix/aip1 antagonizes epidermal growth factor receptor downregulation by the cbl-seta/cin85 complex. Mol Cell Biol 2004;24:8981-93. [PubMed]
- Chen HY, Fermin A, Vardhana S, et al. Galectin-3 negatively regulates tcr-mediated cd4+ t-cell activation at the immunological synapse. Proc Natl Acad Sci U S A 2009;106:14496-501. [PubMed]
- Wu H, Steiner R, Slade S, et al. eds. Refractive surgery. New York: Thieme, 1999.
- Watanabe K, Nakagawa S, Nishida T. Stimulatory effects of fibronectin and egf on migration of corneal epithelial cells. Invest Ophthalmol Vis Sci 1987;28:205-11. [PubMed]
- Schultz G, Khaw PT, Oxford K, et al. Growth factors and ocular wound healing. Eye (Lond) 1994;8:184-7. [PubMed]
- Kandarakis AS, Page C, Kaufman HE. The effect of epidermal growth factor on epithelial healing after penetrating keratoplasty in human eyes. Am J Ophthalmol 1984;98:411-5. [PubMed]
- Albert DM, Jakobiec FA. eds. Principle and practice of ophthalmology. Philadelphia: WB Saunders, 2000.
- Singh G, Foster CS. Epidermal growth factor in alkali-burned corneal epithelial wound healing. Am J Ophthalmol 1987;103:802-7. [PubMed]
- Singh G, Foster CS. Gross factors in treatment of nonhealing corneal ulcers and recurrent erosions. Cornea 1989;8:45-53. [PubMed]


