
European Biological Variation Study (EuBIVAS): within- and between-subject biological variation estimates for serum biointact parathyroid hormone based on weekly samplings from 91 healthy participants
Introduction
Parathyroid hormone (PTH) is the secreted product of the parathyroid glands chief cells, and its production and secretion is predominantly regulated by the extracellular calcium concentration. In fact, extracellular calcium binds the Calcium Sensing Receptor (CaSR), situated at the level of parathyroid cell membrane, which activates an intracellular signalling that results in the inhibition of PTH secretion. Also, the active 1,25-dihydroxyvitamin D is able to inhibit PTH production. Once secreted, PTH plays an essential role in regulating extracellular calcium and phosphate homeostasis (1). Reduced extracellular calcium concentration induces PTH production which in turn directly enhances calcium and inhibits phosphate reabsorption by the kidneys. In the kidney, PTH also stimulates renal 1-α hydroxylase, thus inducing the conversion of the inactive 25-hydroxyvitamin D to the active 1,25-dihydroxyvitamin D, which then promotes calcium absorption at the intestinal level. At the same time, PTH acts on bone remodelling: persistent increased PTH levels, caused by reduced extracellular calcium concentration, induce bone resorption resulting in calcium and phosphate release from bone. PTH exerts also an anabolic activity on bone, in fact, it is known that an intermittent PTH administration may also stimulate bone formation in osteoporosis patients (1). In clinical practice, PTH in blood is an essential and routinely used biomarker for the assessment of primary/secondary hyperparathyroidism, hypoparathyroidism, calcium-phosphate metabolism disorders and, as recommended by the Kidney Disease Improving Global Outcomes (KDIGO) guidelines (2), for monitoring patients with chronic kidney disease (CKD) and the associated bone mineral pathologies (MBD) (1,3,4). In the bloodstream, PTH circulates in different molecular forms, as: (I) PTH 1-84, also described as biointact PTH, which is a peptide composed by 84 amino acids and the most bioactive form of the hormone; and as (II) numerous truncated forms, such as PTH 7-84 and other smaller fragments. The available assays for PTH measurement recognise the truncated forms to different extent, thus leading to significant between-method differences in PTH evaluation. The second-generation assays, defined as intact PTH assays, recognise both PTH 1-84 and truncated fragments, especially PTH 7-84, whereas third-generation assays, defined as biointact PTH assays, specifically detect only PTH 1-84 (3). The International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) Committee for Bone Metabolism (C-BM) is presently working on the standardization of PTH measurement in order to obtain reliable decision limits (3,4). The availability of reliable biological variation (BV) data for PTH is essential for defining analytical performance specifications (APS), which are utilized to assess the suitability and the quality of analytical methods (5,6). Additionally, BV data are used to establish reference change value (RCV), which may be applied to assess the significance of change when performing serial measurements in a subject, as a tool for patient monitoring (7). The within-subject (CVI) and between-subject (CVG) BV estimates with the associated APS for the most routinely used analytes have been available in a historical online 2014 BV database (https://www.westgard.com/biodatabase1.htm) (8,9). Now, this database has been superseded by the newly published European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) Biological Variation Database, available at https://biologicalvariation.eu/ (10). As of March 2020, 165 analytes have been published, with the review being finalized or under way (10). In this database, studies are appraised by the Biological Variation Data Critical Appraisal Checklist (BIVAC), which is based on 14 Quality Items (QIs), each of which is associated with a score A, B, C or D (11). For PTH, ten studies have been identified by systematic literature review (12-21), all of which have received a BIVAC grade C. Five of these fulfil the inclusion criteria for meta-analysis (healthy adults, sampling from biweekly to monthly and > two samples included per participant, second or third-generation assay) from which relevant data sets have been derived and used to deliver the global CVI and CVG estimates presented in the EFLM BV Database (13-16,21).
To further facilitate the delivery of updated and reliable BV estimates for all clinically important measurands, the ELFM WG-BV, in agreement to the requirements of the checklist published by Bartlett et al. (22), designed the European Biological Variation Study (EuBIVAS). EuBIVAS is a large-scale, fully BIVAC compliant BV study (all 14 quality items were scored as A), including 91 participants from 5 different European countries, and from which a number of studies have been already published (11,23-29). The aim of the present paper is to provide EuBIVAS-based BV data and the associated APS and RCV for serum PTH in its biointact form (PTH 1-84).
Methods
The EuBIVAS project
Detailed information about involved laboratories, exclusion/inclusion criteria for subjects’ enrolment, and protocols for sample collection, handling, and storage, has been previously published in detail (23). Briefly, 91 healthy subjects (38 men, 43 pre-menopausal women aged 50; overall age range, 21–69 years) were enrolled by 6 different laboratories in 5 European countries (Italy, Spain, Norway, the Netherlands, and Turkey). At the first visit, participants were asked to fill an enrolment questionnaire on lifestyle habits, medical, and family history, in order to be able to confirm their state of well-being. Subjects who fulfilled the inclusion criteria underwent phlebotomy for ten consecutive weeks (April–June 2015). Seventy-seven participants completed all 10 collections, 10 completed 9, 2 completed 8, and 2 completed 7. Serum was obtained from fasting blood collected in serum tubes with clot activator, silicone coated, plastic, 10 mL (16 × 100 mm2) [Becton Dickinson, USA, code 367820] kept for 30 min up to 2 h at room temperature and then centrifuged for 10 min at 3,000 g. Samples were aliquoted and stored at ‒80 °C, before being shipped frozen to San Raffaele Hospital, the coordinating centre.
The study was approved by the Institutional Ethical Review board of San Raffaele Hospital (Milan, Italy) (protocol number: WG-BV project #001, 50/INT 2014) in agreement with the World Medical Association Declaration of Helsinki (as revised in 2013) and by the Ethical board/regional Ethics Committee for each involved centre (protocol number: WG-BV project #001, PI-1993. April 2015 for Spain; WG-BV project #001, 2014-26 for The Netherlands; WG-BV project #001, 3452/AO/15 for PD Italy; 2015-3/17 for Turkey; 2014/1988 for Norway). Informed consent was taken from all the patients.
Analytical method
Quantitative determination of PTH 1-84 was performed in December 2016 at the San Raffaele Hospital (Milan, Italy) on the Roche Cobas e801, using the PTH 1-84 ELECSYS E2G 100 reagent (code 07027745190) and the PTH 1-84 CS ELECSYS calibrator (code 5608554190).
The PTH 1-84 immunoassay is a third-generation test aimed at quantifying the biointact PTH form (PTH 1-84); in fact, the antibodies cross reactions with the 1-34 and 7-84 PTH fragments are ≤0.1%, thus highly specific for the intact bioactive hormone (3). According to the manufacturer, the reference interval of the assay is 14.9–56.9 ng/L (2.5° and 97.5° percentile) with 31.3 ng/L as the mean value. Samples from each subject were analysed in duplicate within a single run. PreciControl Varia ELECSYS 1 and 2 (code 5618860190) were used as internal controls of quality for PTH 1-84 and were evaluated in duplicate for each run.
Data analysis
The CV-ANOVA, an ANOVA method based on the CV-transformation of data (30), was used to analyze PTH 1-84 data and to produce estimates of analytical variation (CVA) and CVI. Assessment for outliers between replicates (for CVA) and for variance homogeneity (for CVI) was performed by Bartlett’s test (31) and by the Cochran test (32), respectively. The steady state of the participants was evaluated using the linear regression of the 180 values mean for each blood drawing 1, 2, .. 10 (pooled mean group sample concentrations) vs. the blood drawing number [1–10]. The CVG estimates were obtained using ANOVA on the natural log-transformed data after outliers between subjects were identified by the Dixon q-test (33) and the verification of the normality assumption by the Shapiro-Wilk test (34).
The BV components were estimated for the overall group as well as separately for women and men; with women being also divided in two groups: women 50 (post-menopausal women). The 95% CI for BV estimates (35) and mean concentrations were calculated. If the 95% CI between the PTH 1-84 mean values, CVG, and CVI estimates of men and women or female subgroups did not overlap, they were considered significantly different. In addition, correlations between mean concentrations and age or body mass index (BMI) were evaluated for women
The APS for the analytical imprecision (CVAPS) and analytical bias (BAPS), and the RCV were calculated as described in (27,36).
Data were analyzed using Excel 2016, XLSTAT (Statistical software for Excel), and IBM SPSS Statistic (version 20).
Results
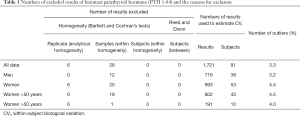
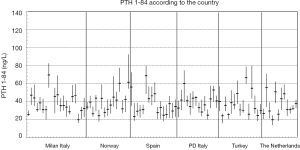
In the subgroups of men and pre-menopausal women, the median age was 35 years and 34 years and median BMI 24.4 and 21.3 kg/m2, respectively (see Table S1 for further details). The 91 participants reported the following level of physical exercise: 18% did 3 h/week. The alcohol intake was moderate and drug consumption was limited. Three of the 91 subjects were heavy smokers (10–20 cigarettes/day), while 17 were moderate smokers (Table S2). To fulfil criteria for variance homogeneity, 3.3% of results were excluded, but no outliers were identified by the Dixon test (Table 1). Based on the Shapiro-Wilk test, PTH 1-84 data for the whole population as well as for men and women were normally distributed only if ln-transformed. No significant trend of the mean PTH 1-84 values during the 10 weeks of collection was identified. Mean PTH 1-84 concentrations among the 5 countries were similar (Figure 1). The PTH 1-84 mean value of the women 50 years (Table 2, Figure 2). Considering separately the mean concentrations of men and women Figure 3). CVG estimates were calculated for the three subgroups; men, women above and below 50 years (Table 2). Pre- and post-menopausal women had similar CVI estimates (Table 2), and thus a common CVI estimate was calculated for all women at 15.2%, (95% CI: 14.3–16.3%), which was significantly higher than the CVI estimate of 13.0% (95% CI: 12.1–14.2%) derived in men. As women G estimate was applied in calculation of APS (Table 2). In the case of RCV calculation (Table 2), the men’s CVI estimate was applied because it was the lowest. Estimates from the women >50 years subgroup were not applied due to the exiguous number of subjects in this group. The EuBIVAS BV estimates for PTH 1-84 were compared to previously published estimates from studies appraised and included in the EFLM BV database (Table 3) (10). In addition, the potential impact of the EuBIVAS CVI estimate in patient monitoring was evaluated creating a probability plot of percentage unidirectional change that showed the percentage increase between two consecutive PTH 1-84 results necessary, at any given probability, to evidence a significant difference (Figure 4).

Full table

Full table
Full table

Full table

Full table

Discussion
PTH is a key biomarker for diagnosing of parathyroid glands’ pathologies, calcium-phosphate metabolism disorders and for monitoring chronic kidney disease mineral and bone disorder (CKD-MBD). In this paper, the use of the most suitable and updated methodology for BV estimation of PTH, in its biointact form (PTH 1-84), allowed us to obtain robust and reliable BV data with implications for the calculation of the APS for bias and imprecision, and RCV. The differences in PTH 1-84 mean values between the women 50 years (Table 2, Figure 2) underline that PTH 1-84 concentrations are affected by sex and pre-menopausal status, as previously described (37,38). In addition, the positive correlation between PTH 1-84 and BMI in men (see Figure 3) is a feature already described in literature (39). In addition, our data indicate that there is a weak correlation between the individual CVI estimates of total calcium and PTH 1-84, which is strengthened when including only subjects with PTH 1-84 mean values higher than the median of the whole population (see Figure S1). CVI estimates were calculated also for subpopulations of smokers vs. non-smokers, and for subjects who had an alcohol intake of 1–2 U/day vs. those who had less alcohol intake; no significant differences in the CVI values were found (data not shown). In the EFLM BV database 10 studies delivering BV estimates for PTH in healthy (12-16,19-21) or diseased populations (15,17,18,20) are included and meta-analysis-derived BV estimate based on BIVAC compliant studies fulfilling the set criteria are published. Considering the studies performed in healthy populations: 2 declared to assess the biointact PTH (PTH 1-84) BV using third-generation assays (15,19); 4 the intact PTH BV using second-generation assays (12,15,19,21), whereas 4 did not specify the assay used for PTH evaluation and thus it is not clear whether the obtained BV data refer to intact or biointact PTH (13,14,16,20).
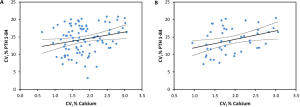
The two studies performed in healthy subjects using a third-generation assay for biointact PTH (PTH 1-84) evaluation (see Table 3), reported the following CVI: 23.8% (95% CI: 21.2–27.1%) (15) and 24.0% (95% CI: 22.0–26.4%) (19), both significantly higher than the EuBIVAS CVI estimates. These two papers have received a BIVAC grade C, typically for the QI related to statistical handling (variance homogeneity and outlier evaluation). This difference in statistical approach may potentially explain the higher CVI results found in these studies compared to the EuBIVAS. Standard statistical approaches used for estimating BV, such as the CV-ANOVA applied in our study, are sensitive to “noisy data” and assume homogeneity of the within-participant CV. Thus, the EuBIVAS data set has been trimmed to achieve this for PTH 1-84, requiring 3–4% of data to be excluded. A recently published Bayesian model for estimating BV is robust to “noisy data” and is an approach for delivering BV estimates without the need for data trimming (40).
The CVI estimates from the 7 studies on healthy subjects that had applied second-generation assays (for intact PTH) or that had not specified the assay employed for PTH evaluation ranged from 16.4% (12) to 25.9% (95% CI: 21.3–32.4%) (14). The very recently published study performed by Ercan and colleagues (21) reported intact PTH BV data for a population of 20 healthy subjects and also BV estimates in subgroups of men and women. The CVI estimates for the entire population and for the female subgroup were significantly higher than the EuBIVAS ones, while the male CVI estimate was slightly higher (Table 3). In this case, the higher CVI estimates reported by the Ercan study could be related to the inclusion of different data as variance homogeneity and outlier evaluation were not performed in the Ercan study. Furthermore, this study applied a second-generation assay for intact PTH analysis. Two studies have analysed their samples in parallel with both second-generation and third-generation assays, where one does not demonstrate any difference in BV estimates delivered by the two methods (15), while the other reports generation-dependent differences in PTH estimates (19).
The two studies performed by Cavalier et al. (17) and Gardham et al. (15) also evaluated the BV components for PTH in haemodialysis patients (Table 3). In these cases, the CVI estimates for the entire diseased population were derived using both the second- and the third-generation assays.
The EuBIVAS CVA estimate, as well as CVA estimates reported in the other two biointact PTH (PTH 1-84) BV studies, are lower than the EuBIVAS APS for imprecision (Tables 2,3). This indicates that, even if the EuBIVAS CVA estimate is based on duplicate analysis of study samples, PTH 1-84 analysis satisfy the analytical quality requirements. The APS for PTH 1-84 obtained from this EuBIVAS study may provide important information about the desirable performance thus being able to select a suitable method for PTH estimations in different laboratories.
It is interesting to evaluate how the EuBIVAS CVI estimates can impact the RCV calculation and the potential consequences when monitoring patients. In fact, RCV may be a helpful tool for the interpretation of the PTH 1-84 concentration changes observed between two consecutive measurements from a subject. An important clinical question (e.g., in CKD-MBD subjects) is when an increase in two consecutive PTH 1-84 evaluations is significant. In this case, the RCV formula should be adapted for obtaining a Z value and thus for the 1-tailed change value calculation, which can be considered as the critical increase between two consecutive results of an analyte, using the following formula: Z = change/[21/2 (CVA2 + CVI2)1/2] (41). The obtained Z value can be converted in a 1-tailed probability allowing the construction of the percentage increase plot between consecutive results against the calculated 1-tailed probability. This type of plot can be useful for evaluating the potential impact of EuBIVAS BV estimates when monitoring patients and thus to understand if a change between two consecutive results can be explained by biological and analytical variations (Figure 4). In this case, using the analytical imprecision calculated in this study, the obtained 1-tailed value for PTH 1-84 percentage increase, set at 95%, was 31.2% (30.4–32.0%). In clinical practice, considering a subject with a PTH 1-84 of 60.7 ng/L, a rise to 83.3 ng/L could be explained by biological and analytical variations (1-sided z-score, α=0.025). It is important to take into account that the EuBIVAS RCV for PTH 1-84 were obtained using the specific EuBIVAS CVA based on analysis of duplicate samples, and, for this reason, cannot be considered as universal values thus underlining that each laboratory has to calculate its own PTH 1-84 RCV using relevant CVA estimates.
In conclusion, this EuBIVAS results for PTH 1-84, provided using the most suitable and updated methodology for BV estimation, led to the availability of reliable and robust CVI estimates, APS for analytical imprecision, and RCV. The EuBIVAS CVI estimates were lower than those delivered by previously published papers on biointact PTH, possibly related to different statistical approaches and to the strict control of the fasting status, thus minimizing possible effects of the ingestion of calcium-containing nutrients (42). These EuBIVAS BV estimates, together with a suitable interpretation of the PTH 1-84 concentration changes, represent a key tool in medical practice for a correct diagnosis and monitoring of bone turnover and parathyroid glands pathologies, for patient management, for creating standardized protocols for the pre-analytical, analytical, and post-analytical stages of PTH evaluation, and for giving information about the analytical quality of the method used for PTH 1-84 evaluation.
Study limitations
Long samples storage before analysis, but the samples were always continuously stored at ‒80 °C and thawed only once prior to analysis. The PTH 1-84 analysis was performed using only one manufacturer’s reagents, but it is unlikely that the BV estimates were affected by this for the same measurand. In addition, the analyses have been performed on serum kept at room temperature for a maximum of two hours before centrifugation, this procedure may have introduced some small degradation of PTH 1-84 that reasonably can be considered acceptable [as reported by Hanon et al. (43) and Dupuy et al. (44)]. In any case, considering that the possible PTH 1-84 degradation would be proportional in each sample, it is improbable that it may have increased the within-subject BV.
Acknowledgments
We thank Roche for allowing us to work on a dedicated COBAS 8000 e801 and for donating all materials used for the measurements. We thank all study participants and other EuBIVAS partners for their essential contribution to the project: Gerhard Barla, Bill Bartlett, Giulia Cajano, Niels Jonker, Mario Plebani, Thomas Røraas, Una Ørvim Sølvik, Marit Sverresdotter Sylte, Mustafa Serteser, Francesca Tosato and Ibrahim Unsal. We would also like thank Cavalier Etienne who has critically read and improved the paper.
Funding: The work was supported by Roche through the donation of reagents, calibrators, and quality control materials and BD that donated all materials used for the blood collections. This paper was supported by the Italian Ministry of Health.
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-19-4498.
Peer Review File: Available at http://dx.doi.org/10.21037/atm-19-4498
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-19-4498). The authors have no conflicts of interest to declare.
Ethical Statement: the authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Institutional Ethical Review board of San Raffaele Hospital (Milan, Italy) (protocol number: WG-BV project #001, 50/INT 2014) in agreement with the World Medical Association Declaration of Helsinki (as revised in 2013) and by the Ethical board/regional Ethics Committee for each involved centre (protocol number: WG-BV project #001, PI-1993. April 2015 for Spain; WG-BV project #001, 2014-26 for The Netherlands; WG-BV project #001, 3452/AO/15 for PD Italy; 2015-3/17 for Turkey; 2014/1988 for Norway). Informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Goltzman D. Physiology of Parathyroid Hormone. Endocrinol Metab Clin North Am 2018;47:743-58. [Crossref] [PubMed]
- KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD). Kidney International Supplements 2017.
- Sturgeon CM, Sprague S, Almond A, et al. Perspective and priorities for improvement of parathyroid hormone (PTH) measurement - A view from the IFCC Working Group for PTH. Clin Chim Acta 2017;467:42-7. [Crossref] [PubMed]
- Souberbielle JC, Brazier F, Piketty ML, et al. How the reference values for serum parathyroid hormone concentration are (or should be) established? J Endocrinol Invest 2017;40:241-56. [Crossref] [PubMed]
- Sandberg S, Fraser CG, Horvath AR, et al. Defining analytical performance specifications: Consensus Statement from the 1st Strategic Conference of the European Federation of Clinical Chemistry and Laboratory Medicine. Clin Chem Lab Med 2015;53:833-5. [Crossref] [PubMed]
- Fraser CG, Petersen PH. Analytical performance characteristics should be judged against objective quality specifications. Clin Chem 1999;45:321-3. [Crossref] [PubMed]
- Fraser CG. Reference change values. Clin Chem Lab Med 2011;50:807-12. [PubMed]
- Perich C, Minchinela J, Ricos C, et al. Biological variation database: structure and criteria used for generation and update. Clin Chem Lab Med 2015;53:299-305. [Crossref] [PubMed]
- Desirable Biological Variation Database specifications. Available online: https://www.westgard.com/biodatabase1.htm
- EFLM Biological Variation Database. Accessed March 2020. Available online: https://biologicalvariation.eu/
- Aarsand AK, Roraas T, Fernandez-Calle P, et al. The Biological Variation Data Critical Appraisal Checklist: A Standard for Evaluating Studies on Biological Variation. Clin Chem 2018;64:501-14. [Crossref] [PubMed]
- Takahashi M, Kawana K, Nagano A. Biological variability of biochemical markers of bone turnover in healthy women. Endocr Res 2002;28:257-64. [Crossref] [PubMed]
- Viljoen A, Singh DK, Twomey PJ, et al. Analytical quality goals for parathyroid hormone based on biological variation. Clin Chem Lab Med 2008;46:1438-42. [Crossref] [PubMed]
- Ankrah-Tetteh T, Wijeratne S, Swaminathan R. Intraindividual variation in serum thyroid hormones, parathyroid hormone and insulin-like growth factor-1. Ann Clin Biochem 2008;45:167-9. [Crossref] [PubMed]
- Gardham C, Stevens PE, Delaney MP, et al. Variability of parathyroid hormone and other markers of bone mineral metabolism in patients receiving hemodialysis. Clin J Am Soc Nephrol 2010;5:1261-7. [Crossref] [PubMed]
- Smith ER, Cai MM, McMahon LP, et al. Biological variability of plasma intact and C-terminal FGF23 measurements. J Clin Endocrinol Metab 2012;97:3357-65. [Crossref] [PubMed]
- Cavalier E, Delanaye P, Moranne O. Variability of new bone mineral metabolism markers in patients treated with maintenance hemodialysis: implications for clinical decision making. Am J Kidney Dis 2013;61:847-8. [Crossref] [PubMed]
- Lutsey PL, Parrinello CM, Misialek JR, et al. Short-term Variability of Vitamin D-Related Biomarkers. Clin Chem 2016;62:1647-53. [Crossref] [PubMed]
- Schleck ML, Souberbielle JC, Delanaye P, et al. Parathormone stability in hemodialyzed patients and healthy subjects: comparison on non-centrifuged EDTA and serum samples with second- and third-generation assays. Clin Chem Lab Med 2017;55:1152-9. [Crossref] [PubMed]
- Meijers WC, van der Velde AR, Muller Kobold AC, et al. Variability of biomarkers in patients with chronic heart failure and healthy controls. Eur J Heart Fail 2017;19:357-65. [Crossref] [PubMed]
- Ercan M, Akbulut ED, Avci E, et al. Determining biological variation of serum parathyroid hormone in healthy adults. Biochem Med (Zagreb) 2019;29:030702. [Crossref] [PubMed]
- Bartlett WA, Braga F, Carobene A, et al. A checklist for critical appraisal of studies of biological variation. Clin Chem Lab Med 2015;53:879-85. [Crossref] [PubMed]
- Carobene A, Strollo M, Jonker N, et al. Sample collections from healthy volunteers for biological variation estimates' update: a new project undertaken by the Working Group on Biological Variation established by the European Federation of Clinical Chemistry and Laboratory Medicine. Clin Chem Lab Med 2016;54:1599-608. [Crossref] [PubMed]
- Carobene A, Roraas T, Solvik UO, et al. Biological Variation Estimates Obtained from 91 Healthy Study Participants for 9 Enzymes in Serum. Clin Chem 2017;63:1141-50. [Crossref] [PubMed]
- Carobene A, Marino I, Coskun A, et al. The EuBIVAS Project: Within- and Between-Subject Biological Variation Data for Serum Creatinine Using Enzymatic and Alkaline Picrate Methods and Implications for Monitoring. Clin Chem 2017;63:1527-36. [Crossref] [PubMed]
- Carobene A, Guerra E, Locatelli M, et al. Biological variation estimates for prostate specific antigen from the European Biological Variation Study; consequences for diagnosis and monitoring of prostate cancer. Clin Chim Acta 2018;486:185-91. [Crossref] [PubMed]
- Aarsand AK, Diaz-Garzon J, Fernandez-Calle P, et al. The EuBIVAS: Within- and Between-Subject Biological Variation Data for Electrolytes, Lipids, Urea, Uric Acid, Total Protein, Total Bilirubin, Direct Bilirubin, and Glucose. Clin Chem 2018;64:1380-93. [Crossref] [PubMed]
- Carobene A, Aarsand AK, Guerra E, et al. European Biological Variation Study (EuBIVAS): Within- and Between-Subject Biological Variation Data for 15 Frequently Measured Proteins. Clin Chem 2019;65:1031-41. [Crossref] [PubMed]
- Carobene A, Guerra E, Locatelli M, et al. Providing Correct Estimates of Biological Variation-Not an Easy Task. The Example of S100-beta Protein and Neuron-Specific Enolase. Clin Chem 2018;64:1537-9. [Crossref] [PubMed]
- Røraas T, Støve B, Petersen PH, et al. Biological Variation: The Effect of Different Distributions on Estimated Within-Person Variation and Reference Change Values. Clin Chem 2016;62:725-36. [Crossref] [PubMed]
- Snedecor GW, Cochran WG. Statistical methods. 8th ed. Press ISU, 1991.
- Cochran WG. The distribution of the largest of a set of estimated variances as a fraction of their total. Ann Eugenics 1941;11:47-52. [Crossref]
- Dixon WJ. Processing data for outliers. Biometrics 1953;9:74-89. [Crossref]
- Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples). Biometrika 1965;52:591-611. [Crossref]
- Sahai H, Ojeda MM. Analysis of Variance for Random Models. Volume 2: Unbalanced Data, 1st ed. Birkhäuser Basel, 2004.
- Fokkema MR, Herrmann Z, Muskiet FA, et al. Reference change values for brain natriuretic peptides revisited. Clin Chem 2006;52:1602-3. [Crossref] [PubMed]
- Zittermann A, Schwarz I, Scheld K, et al. Physiologic fluctuations of serum estradiol levels influence biochemical markers of bone resorption in young women. J Clin Endocrinol Metab 2000;85:95-101. [Crossref] [PubMed]
- Nielsen HK, Brixen K, Bouillon R, et al. Changes in biochemical markers of osteoblastic activity during the menstrual cycle. J Clin Endocrinol Metab 1990;70:1431-7. [Crossref] [PubMed]
- Kamycheva E, Sundsfjord J, Jorde R. Serum parathyroid hormone level is associated with body mass index. The 5th Tromso study. Eur J Endocrinol 2004;151:167-72. [Crossref] [PubMed]
- Røraas T, Sandberg S, Aarsand AK, et al. A Bayesian Approach to Biological Variation Analysis. Clin Chem 2019;65:995-1005. [Crossref] [PubMed]
- Fraser CG. Reference change values: the way forward in monitoring. Ann Clin Biochem 2009;46:264-5. [Crossref] [PubMed]
- Siyam FF, Klachko DM. What is hypercalcemia? The importance of fasting samples. Cardiorenal Med 2013;3:232-8. [Crossref] [PubMed]
- Hanon EA, Sturgeon CM, Lamb JE. Sampling and storage conditions influencing the measuremtn of parathyroid hormone in blood samples: a systematic review. Clin Chem Lab Med 2013;51:1925-41. [Crossref] [PubMed]
- Dupuy AM, Cristol JP, Vincent B, et al. Stability of routine biochemical analytes in whole blood and plasma/serum: focus on potassium stability from lithium heparin. Clin Chem Lab Med 2018;56:413-21. [Crossref] [PubMed]


