
The protective effect and mechanism of lentinan on acute kidney injury in septic rats
Introduction
Sepsis refers to a series of persistent hyperinflammation and immunosuppressive reactions caused by pathogens invading the body; i.e., the imbalance between systemic inflammatory reaction and anti-inflammatory reaction (1). Sepsis is one of the major causes of death for critically ill patients in the intensive care unit (ICU). Sepsis can cause cardiovascular complications (2), shock (3), acute lung injury/acute respiratory distress syndrome (4), stress ulcer (5), and acute kidney injury (6). Severe sepsis patients often die of multiple organ failure, with the kidney being one of the organs to be damaged earlier. Acute kidney injury (AKI) is the most common and severe sepsis complications and is the main cause of death in septic patients, with a morbidity of 50% and a mortality of 70% (6). The causes of AKI are systemic lupus erythematosus (7), the use of contrast media (8), acute pancreatitis (9), leukemia (10), etc. Therefore, kidney protection is particularly important. Changes in renal hemodynamics, endotoxin-induced inflammation, apoptosis of renal tubular cells, and glomerular microthrombosis after sepsis are all risk factors for AKI (11). The treatment of acute kidney injury is particularly important. In clinic, renal replacement therapy can be used for acute kidney injury caused by sepsis (12).
In the pathogenesis of sepsis, due to certain external stimuli, the body disrupts the balance between inflammatory mediators by regulating hemodynamics and apoptosis, and by releasing a large number of inflammatory factors and regulatory proteins (cytokines, chemokines, acute reactive proteins), leading to excessive inflammatory response or immunosuppression and directly contributing to the formation of AKI (13). The marked increase of tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and IL-1β, (14) will eventually lead to kidney damage by inducing glomerular neutrophil infiltration and monocyte metastasis (15). According to the relevant research, TNFs such as TNF-α, an important pro-inflammatory medium, can cause the production of IL and secondary inflammatory medium, which can lead to an immunosuppressive biochemistry and immunohistochemical phenomena in the body, causing death in severe cases (16). Transforming growth factor beta 1 (TGF-β1) may also be elevated during the sepsis process, and participate in the inflammatory reaction process of sepsis, inhibit the production of TNF-α, and play an inflammatory role (17).
Except for protective mechanical ventilation, there is currently no specific and effective drug intervention for acute lung injury (18), so the mortality rate of sepsis is still high. Clinically, septic AKI is often treated by strategies of infusion, intravenous drip of antibiotics, and diuretics, but the high mortality rate of septic AKI patients has not been effectively alleviated (19). Therefore, it is crucial to explore new therapeutic drugs for septic AKI to alleviate kidney injury and save patients’ health and lives .
Lentinan (LNT) is a traditional Chinese medicine extract. Its main chain component is a macromolecule with β-(1→3)-D-glucan residue, which is extracted and separated from Lentinus edodes. Studies have shown that LNT has antiviral, antitumor, anti-oxidative, antidepressant, anti-aging, antiradiation, and antiparasitic effects, and can regulate immune function and stimulate interferon formation (20). Due to its ability to strengthen innate or acquired immune response, LNT has been used extensively in anticancer immune research in recent years, with its effects being widely recognized by countries around the world (16).
LNT is a strong immune enhancer, as it can activate natural killer cells, lymphocytes, dendritic cells, and other immune cells, and is a typical T cell activator (16). Studies have shown that LNT can reduce the secretion of TNF-α by inhibiting IL. Wan et al. (21) found that LNT injection combined with FOLFIRI chemotherapy can reduce the levels of inflammatory factor TGF-β, IL-17, and IL-6, along with TNF-α in patients’ peripheral blood, thus improving the immune function of patients. Wang Wang et al. (22) studied the immunoregulative effect and mechanism of LNT injection on burn sepsis (BS) model mice and found that it could inhibit the extracellular regulatory protein kinase (ERK)-FOXO1 pathway of CD4+CD25+FOXP3+regulatory T cells and promote the polarization of CD4+T cells to Th1 cells. Furthermore, LNT was also shown to inhibit macrophage ERK1/2, stress-activated protein kinase 1/2(JNK1/2), and nuclear factor k light chain enhancer (NF-κB) pathway of activated b cells, and promote macrophage activation, thus enhancing immune cell function and improving prognosis of model mice. Han’s research (16) also confirmed that LNT is effective in the prevention and treatment of sepsis, and has a significant effect on the concentration of TNF-α in rat serum, with the reduction of TNF-α demonstrating a protective effect on the body’s immunity.
The purpose of this study is to build a model of acute kidney injury in sepsis rats, focusing on the protective effect of lentinan on acute kidney injury in sepsis rats.
We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-5158).
Methods
Main drugs and reagents
LNT was purchased from Beijing Soledad Technology Co., Ltd., enzyme-linked immunosorbent assay (ELISA) kits for creatinine (Cr) and blood urea nitrogen (BUN) were purchased from Zhejiang IKON Biotechnology Co., Ltd., and 10% pentobarbital sodium injection and phosphate-buffered saline (PBS) buffer were purchased from Shanghai BioLeaf Biotechnology Co., Ltd.
Animal grouping and modeling
Animal grouping
A total of 72 six week old healthy Male SD rats with an average mass of 200±18 g were provided by Guangdong Medical Laboratory Animal Center. First, 72 rats were numbered from 1 to 72, and then 72 rats were selected by random number table and sorted according to the size. The rats corresponding to each 12 numbers were divided into a group. They were randomly divided into a sham group (Equal volume normal saline), BS group (Equal volume normal saline), positive drug control group, (dexamethasone, 5 mg/kg, PC group), LNT low-concentration group (LNT-L group) (50 mg/kg), LNT medium-concentration group (LNT-M group) (100 mg/kg), and LNT high-concentration group (LNT-H group) (200 mg/kg), each with 12 rats. All animal experiments were performed in accordance with the guidelines for animal care and approved by the ethics committee of the First Affiliated Hospital of Soochow University.
Animal modeling
A sepsis model was established by cecal ligation and perforation. The rats in the BS group and LNT group were adapted to the standard feeding conditions for 1 week before operation and anesthetized by intraperitoneal injection of 2–3 mL of 10% pentobarbital sodium solution. The abdominal wall was cut 2 cm in the middle. After the cecum was exposed, two-thirds of the total length of the cecum was ligated. After the cecum was punctured 4 times at 5 mm below the ligature line with a domestic no. 16 needle, the cecum contents were squeezed out to form the cecum leakage, which was then returned to the cecum, and the abdomen was closed. After cecal ligation and perforation, rat showed listlessness, lethargy, restlessness, chills and bristles. Immediately after the operation, 1 mL of 0.9% sodium chloride injection was injected intraperitoneally. The sham group underwent the same procedure as the BS group except that the cecum was not ligated and punctured. The LNT group received 50 mg/kg, 100 mg/kg and 200 mg/kg of LNT intraperitoneally 1 hour after operation.
Detection index
Observing histopathological changes of rat kidney by hematoxylin and eosin (HE) staining
Renal tissue was taken, fixed with 4% paraformaldehyde, dehydrated conventionally, paraffin-embedded, sectioned, dewaxed with xylene, stained with hematoxylin (H) for 5 min, stained with (E) eosin for 3 min, dehydrated with gradient ethanol, xylene dewaxing, sealed, photographed, and observed under an ordinary light microscope for any pathological morphology.
Detecting the changes of serum BUN and Cr
Blood was taken from the eyeball of the tested rat and left to stand for 30 min, after which the supernatant was extracted. Cr and BUN were detected according to the instructions of the kit’s manufacturer, and 5 mL of blood was collected by a centrifuge tube. The blood sample was left at room temperature for 30 min and then centrifuged at 1,500 rpm for 10 min. After that, serum was collected and frozen at −80 °C for biochemical measurement. The levels of BUN and serum Cr were measured with Bechman Coulter LX-2000 (Bechman Commercial Kits, Bechman Coulter LX-2000, Brea, CA, USA).
Detecting the levels of IL-4, IL-10, IL-6, and TNF-α in serum by ELISA
Rat blood was left at room temperature for 1.5 hours and centrifuged at 1,500 ×g for 10 min. Serum was collected from the upper layer of the centrifuge tube and stored in a refrigerator at 80 °C. The detection of serum IL-4, IL-10, IL-6, and TNF-α was carried out in strict accordance with the manufacturer’s instructions by using a ELISA kit (Shenzhen Dayou Bioengineering Co., Ltd., Shenzhen). The +e concentrations of IL-4, IL-10, IL-6, and TNF-α were calculated according to the standard curve. The absorbance was read at 450 nm (time set at 10 ms), and 570 nm was used as a reference.
Detecting the protein expression levels of iNOS, ICAM-1, and NF-κB in kidney tissue by Western blot
Total proteins were isolated from kidney tissues with cell lysis buffer (Thermo Fisher) and quantified with a bicinchonic acid (BCA) kit (Sigma-Aldrich). The same amount of protein denatured at 95 °C for 5 min was separated by 10% SDS-PAGE gel electrophoresis and transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was blocked overnight with a blocking agent (Thermo Fisher) at 4 °C, and incubated with anti inducible nitric oxide synthase (iNOS) (Ab15323, Abcam), intercellular adhesion molecule 1 (ICAM-1) (ab2213, Abcam), and nuclear factor-κB (NF-κB) (ab220803, Abcam); horseradish peroxidase (HRP)-labeled secondary antibody (Abcam) was then applied at 37 °C for 1.5 h. Protein spots were observed by enhanced chemiluminescence (ECL) (Thermo Fisher), and the relative expression of protein was quantified by Image Lab software (Bio-Rad, Hercules, CA, USA). Protein samples were normalized with β-actin.
Statistical analysis
SPSS 16.0 statistical analysis software was used, and the measurement data were expressed by
Results
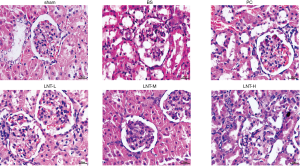
The histopathological changes of rat kidney by HE staining
HE staining (Figure 1) showed that the glomerular and renal tubules in the normal sham group were clear and complete in structure, and there was no congestion and inflammatory cell infiltration in the renal interstitium. In the BS group, the glomerular volume was increased, a large number of renal tubular epithelial cells were swollen and necrotized, the renal interstitium was seriously hyperemic, and extensive inflammatory cell infiltration had occurred. Compared with the BS group, the PC, LNT-L, LNT-M, and LNT-H groups showed reduced renal tubular necrosis, interstitial hyperemia, and renal interstitial inflammatory cell infiltration, with greater LNT concentrations corresponding to a decrease in symptom severity.
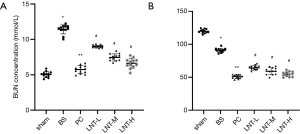
Determination of serum Cr and BUN
The determination results of BUN and Cr levels in serum of rats in each group are shown in Figure 2A,B. Compared with the sham group, the serum BUN and Cr levels in the BS group increased significantly. Compared with the BS group, the BUN and Cr levels in the PC, LNT-L, LNT-M, and LNT-H groups decreased significantly, and the higher the LNT concentration, the more obvious the decrease.
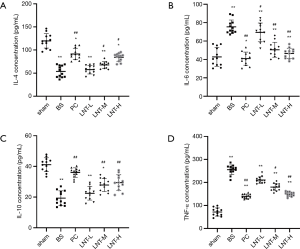
ELISA test results
The levels of IL-4, IL-10, IL-6, and TNF-α in the tissues of rats in each group are shown in Figure 3. Compared with the sham group, the levels of IL-4 and IL-10 of the kidney tissues in the BS group were significantly decreased, while the levels of IL-6 and TNF-α were significantly increased. Compared with the BS group, the levels of IL-4 and IL-10 of kidney tissues in the PC, LNT-L, LNT-M, and LNT-H groups increased significantly in a concentration-dependent manner. Meanwhile, compared with the BS group, the levels of IL-6 and TNF-α of kidney tissues in the PC, LNT-L, LNT-M, and LNT-H groups decreased significantly in a concentration-dependent manner. The higher the LNT concentration, the more obvious the reduction.
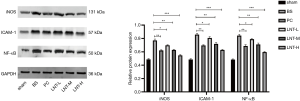
Western blot test results
The protein expressions of iNOS, ICAM-1, and NF-κB in kidney tissues of rats in each group were detected by Western blot, and the results are shown in Figure 4. Compared with the sham group, the expression levels of iNOS, ICAM-1, and NF-κB in kidney tissues of the BS group were significantly increased. Compared with the BS group, the protein expression levels of iNOS, ICAM-1, and NF-κB in kidney tissues of PC group and LNT-L, LNT-M, and LNT-H groups were significantly reduced. The higher the LNT concentration, the more obvious the reduction was.
Discussion
Sepsis is a systemic inflammatory response syndrome accompanied by infection, but sepsis does not refer to infection itself, but the immune response process caused by infection, more specifically, the imbalance between the systemic inflammatory response and the anti-inflammatory response (18,19). Severe sepsis patients often die of multiple organ failure, with the kidney being of one the organs damaged earlier in this process; thus, the protection of kidney is particularly important. In this experiment, the septic AKI model of rats was prepared by cecal ligation and perforation, which is currently recognized as the septic model for experimental rats (20-22). In this experiment, differences in various renal function indexes among the BS group, LNT group, and sham group were checked at 24 hours in order to provide a theoretical basis for clinical application. Cr and BUN levels are the most commonly used indicators for monitoring renal function (23,24). Cr is discharged from the body through glomerular filtration, and renal tubules have minimal reabsorption of Cr. BUN is the main end product of human protein metabolism. Under normal circumstances, After BUN is filtered by the glomerulus, it can be reabsorbed to all sections of tubules, but the faster the flow rate of urine in the tubules, the less reabsorption, that is, the maximum clearance rate is reached. In the early stage of renal damage, blood BUN can be normal range. When the glomerular filtration rate drops below 50% of normal, the blood BUN concentration increases rapidly (25).
TNF-α is a kind of pro-inflammatory medium, which can be produced in large quantities under the stimulation of serum endotoxin, and then can promote the blowout production of the IL-1, -6, and -8 oxygen radicals, along with nitric oxide (NO) and other inflammatory media, thus leading to systemic inflammatory phenomena (16). Therefore, TNF-α plays a critical role in the course of sepsis. In recent years, researchers and medical workers have focused on anti-infection treatment of sepsis and have made great progress. However, the mortality rate of sepsis is still very high, so it is urgent to explore more effective treatment methods. Relevant research has proven that anti-inflammatory treatment without immune reinforcement is insufficient for the treatment process of sepsis, and the immune reinforcement of the body is of great benefit to the treatment of sepsis (17,18). TNF-α is mainly secreted by IL, and LNT has a relatively strong inhibitory effect on IL (19). LNT is also a strong immune enhancer, being able to activate natural killer cells, lymphocytes, dendritic cells, and other immune cells, while being a typical T cell activator.
HE staining was used to observe the histopathological changes of rat kidneys. This revealed that, after LNT treatment, renal tubular necrosis and interstitial hyperemia in renal tissue of BS, along renal interstitial inflammatory cell infiltration, had been alleviated. With the increase of LNT concentration, the symptoms showed a decreasing trend, and the levels of BUN and Cr in serum decreased in a concentration-dependent manner (P<0.05). ELISA showed that, compared with the BS group, the levels of IL-4 and IL-10 of kidney tissue in LNT-treated rats increased in a concentration-dependent manner, while the level of IL-6 and TNF-α of kidney tissues in the LNT-L, LNT-M, and LNT-H groups significantly decreased.
In order to explore the molecular mechanism of LNT’s effect on burn concentration disorder, our research evaluated the gene expression of iNOS, ICAM-1, and NF-κ B. iNOS is an inducible NO synthase. Unlike constitutive NO synthase, iNOS expression is regulated by transcription at the gene level (26). Under different induction conditions, iNOS can be widely and continuously expressed in a variety of cells (27,28). Research shows that iNOS activity is related to tissue damage in a variety of pathological states such as nephritis, septic shock, and arthritis (29-31). ICAM-1 is a member of the immunoglobulin superfamily, and is related to a series of inflammatory diseases and states (32). Its expression is induced and regulated by inflammatory mediators such as TNF-α and IL-1β. Among many eukaryotic transcription factors, NF-κB is an important transcription factor that exists in almost all cell types. Studies have found that NF-κB plays an active role in immune response, cell growth and apoptosis, arthritis, and other diseases (33). Huang et al. (34) found that TNF-α and TNF-Q are cis-binding sites of NF-κB and can activate NF-κB itself, while NF-κB can stimulate the production of IL-1, IL-6, and TNF-o, thus forming an autoregulating negative feedback loop (33). In ICAM-1–induced expression, the NF-κB binding site upstream of a translation initiation is critical in mediating TNF-α and IL-1β to induce ICAM-1 expression (35). The binding site of NF-κB also exists in the 5’ regulatory region of iNOS. NF-κB was first discovered in B cells and participates in the transcription control of T cell activation and growth-related genes, indicating that NF-κB may participate in the function of T cells (33). With regards to the relationship between iNOS, ICAM-1, and NF-κB, the three may be involved in T cell function. Compared with the sham group, the expression levels of iNOS, ICAM-1, and NF-κB in kidney tissues of the BS group were significantly increased by Western blot. Compared with the BS group, the expression levels of iNOS, ICAM-1, and NF-κB protein in kidney tissues of PC, LNT-L, LNT-M, and LNT-H groups were significantly reduced, and the higher the LNT concentration, the more obvious the reduction was. The experimental results show that the increase of LNT sepsis can alleviate the symptoms of BS to some extent. Therefore, LNT can be presumed to be able to treat AKI by inhibiting the expression of iNOS, ICAM-1, and NF-κB genes.
At present, LNT plays a very important role in the prevention and treatment of various diseases and the reduction of tumor necrosis factor, but there are no reports on the prevention and treatment of sepsis. The results of this study confirm that LNT is effective in the prevention and treatment of sepsis, which provides a certain basis for further broadening the drug treatment methods of sepsis and for the application of LNT in the treatment and prevention of sepsis. However, it is unclear whether the sepsis prevention and recovery aided by LNT is related to other factors, and this question needs to resolved by further research and exploration.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-5158
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-5158
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-5158). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal experiments were performed in accordance with the guidelines for animal care and approved by the ethics committee of the First Affiliated Hospital of Soochow University.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Maloney PJ. Sepsis and septic shock. Emerg Med Clin North Am 2013;31:583-600. [Crossref] [PubMed]
- Shahreyar M, Fahhoum R, Akinseye O, et al. Severe sepsis and cardiac arrhythmias. Ann Transl Med 2018;6:6. [Crossref] [PubMed]
- Cecconi M, Evans L, Levy M, et al. Sepsis and septic shock. Lancet 2018;392:75-87. [Crossref] [PubMed]
- Mehta S, Gill SE. Improving clinical outcomes in sepsis and multiple organ dysfunction through precision medicine. J Thorac Dis 2019;11:21-8. [Crossref] [PubMed]
- Sasabuchi Y, Matsui H, Lefor AK, et al. Risks and Benefits of Stress Ulcer Prophylaxis for Patients With Severe Sepsis. Crit Care Med 2016;44:e464-9. [Crossref] [PubMed]
- Holthoff JH, Wang Z, Patil NK, et al. Rolipram improves renal perfusion and function during sepsis in the mouse. J Pharmacol Exp Ther 2013;347:357-64. [Crossref] [PubMed]
- Wong KW. A case of minimal change disease complicated by acute kidney injury in systemic lupus erythematosus. Saudi J Kidney Dis Transpl 2014;25:1308-11. [Crossref] [PubMed]
- Fähling M, Seeliger E, Patzak A, et al. Understanding and preventing contrast-induced acute kidney injury. Nat Rev Nephrol 2017;13:169-80. [Crossref] [PubMed]
- Ye B, Mao W, Chen Y, et al. Aggressive Resuscitation Is Associated with the Development of Acute Kidney Injury in Acute Pancreatitis. Dig Dis Sci 2019;64:544-52. [Crossref] [PubMed]
- Du Plessis L, Rassekh SR, Mammen C. High incidence of acute kidney injury during chemotherapy for childhood acute myeloid leukemia. Pediatr Blood Cancer 2018;65. [Crossref] [PubMed]
- Zhao X, Liu H. Research Progress on Biomarkers Related to Diagnosis and Prognosis Evaluation of Sepsis Acute Kidney Injury. Chinese General Practice 2016;19:19-22.
- Godin M, Murray P, Mehta RL. Clinical approach to the patient with AKI and sepsis. Semin Nephrol 2015;35:12-22. [Crossref] [PubMed]
- Chaudhari D, Khan S, Saleem A, et al. Obstructive jaundice as an initial manifestation of non-hodgkin lymphoma: treatment dilemma and high mortality. Case Rep Med 2013;2013:259642. [Crossref] [PubMed]
- Shi M, Nan C, Xu L, et al. Effect of TRPV1 activation on lipopolysaccharide-induced lung inflammatory injury in mice. Chinese Journal of Pathophysiology 2013;29:2223-8.
- Hu J, Liu J., Licochalcone A. Attenuates Lipopolysaccharide-Induced Acute Kidney Injury by Inhibiting NF-κB Activation. Inflammation 2016;39:569-74. [Crossref] [PubMed]
- Han SB, Park SH, Lee KH, et al. Polysaccharide isolated from the radix of Platycodon grandiflorum selectively activates B cells and macrophages but not T cells. Int Immunopharmacol 2001;1:1969-78. [Crossref] [PubMed]
- Zhang Y, Xing J. Effect of transforming growth factor-β1 on monocyte Toll-like receptor 4 expression in septic rats. World J Emerg Med 2011;2:228-31. [Crossref] [PubMed]
- Fuller BM, Mohr NM, Dettmer M, et al. Mechanical ventilation and acute lung injury in emergency department patients with severe sepsis and septic shock: an observational study. Acad Emerg Med 2013;20:659-69. [Crossref] [PubMed]
- Gaieski DF, Edwards JM, Kallan MJ, et al. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med 2013;41:1167-74. [Crossref] [PubMed]
- Wan Q, Ren Y, Liu S, et al. Advances in pharmacological activities of lentinan. Chinese Pharmacy 2018;(8):1140-4.
- Wan M, Zhu Y, Qiu R, et al. Influence of lentinan on the immune function and the levels of inflammatory factors in peripheral blood of patients with colon cancer. China Medical Herald 2017;14:159-63.
- Wang S. Experimental Study on Immune Regulation Effect and Mechanism of Lentinan on Burn Sepsis, Hebei Medical University, 2016.
- Li XY, Luo GJ, Li SR, et al. Occurrence and Survival Analysis of Acute Kidney Injury in Patients with Normal BUN and SCr in the Early Post-liver Transplantation Period. Journal of Sun Yat-sen University Medical Sciences 2013;34:397-401.
- Wang WW, Jiang Y, Wang W, et al. Effect of adipose-derived stem cells modified with hypoxia-inducible factor-1 alpha gene on acute renal injury induced by cisplatin in mice. Chinese Journal of Tissue Engineering Research 2012;016:7651-7.
- Yu R, Zhang XB, Wen XD, et al. Effect of Fangji Fuling Tang on proliferation and MMP-2 of Glomerular Mesangial Cells. Chinese Journal of Experimental Traditional Medical Formulae 2006;12:25-7.
- Guo A. Transcriptional Regulation of iNOS Gene by p38 MAPK and Its Mechanism, First Military Medical University, 2002.
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J 1992;6:3051-64. [Crossref] [PubMed]
- Vodovotz Y, Kwon NS, Pospischil M, et al. Inactivation of nitric oxide synthase after prolonged incubation of mouse macrophages with IFN-gamma and bacterial lipopolysaccharide. J Immunol 1994;152:4110-8. [PubMed]
- Weinberg JB, Granger DL, Pisetsky DS, et al. The role of nitric oxide in the pathogenesis of spontaneous murine autoimmune disease: increased nitric oxide production and nitric oxide synthase expression in MRL-lpr/lpr mice, and reduction of spontaneous glomerulonephritis and arthritis by orally administered NG-monomethyl-L-arginine. J Exp Med 1994;179:651-60. [Crossref] [PubMed]
- Petros A, Bennett D, Vallance P. Effect of nitric oxide synthase inhibitors on hypotension in patients with septic shock. Lancet 1991;338:1557-8. [PubMed]
- McCartney-Francis N, Allen JB, Mizel DE, et al. Suppression of arthritis by an inhibitor of nitric oxide synthase. J Exp Med 1993;178:749-54. [Crossref] [PubMed]
- Yan W, Jiang Y, Zhao K. Regulation of ICAM-1 Gene Expression. Journal of Clinical and Pathology 2001;21:397-9.
- Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 1998;16:225-60. [Crossref] [PubMed]
- Huang Y, Krein PM, Muruve DA, et al. Complement factor B gene regulation: synergistic effects of TNF-alpha and IFN-gamma in macrophages. J Immunol 2002;169:2627-35. [Crossref] [PubMed]
- Lee SJ, Hou J, Benveniste EN. Transcriptional regulation of intercellular adhesion molecule-1 in astrocytes involves NF-kappaB and C/EBP isoforms. J Neuroimmunol 1998;92:196-207. [Crossref] [PubMed]
(English Language Editor: J. Gray)


