Clinical advances in the development of novel VEGFR2 inhibitors
Introduction: understanding the molecular mechanisms of vascular endothelial growth factor receptor 2 (VEGFR2)-mediated angiogenesis
Cancer angiogenesis is a fundamental process for the tumor growth as it ensures oxygen and nutrients supply to proliferating cells through the development of new blood vessels, potentially causing cancer progression and metastasis (1). The vascular endothelial growth factor (VEGF) family, which includes VEGF-A, VEGF-B, VEGF-C, VEGF-D and placental growth factor (PlGF), is a group of key proteins involved in the angiogenic pathway. VEGF and its receptor are highly expressed in many tumor types, including cancer of the gastrointestinal tract (2). Therefore they may represent potential targets for anticancer therapy. Sustained VEGF expression leads to the development and maintenance of a vascular network that promotes tumor growth and metastases. Environmental factors like hypoxia, inflammatory cytokines, low pH as well as the silencing of specific tumor suppressor genes (PTEN, p53, VHL) or the activation of oncogenes (e.g., RAS, SRC, EGFR, HER2) result in increased VEGF production (3).
The VEGF family members bind three different tyrosine kinase (TK) receptors: VEGFR1 (Flt-1), VEGFR2 (Flk-1-KDR), and VEGFR3, which is expressed on the lymphatic and vascular endothelium (Figure 1).
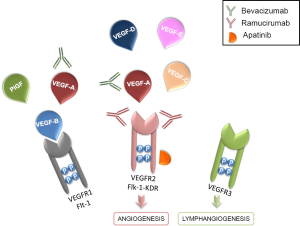
VEGFR2, a type II transmembrane TK receptor expressed on endothelial cells and on circulating bone marrow-derived endothelial progenitor cells, is the principal mediator of the VEGF-induced angiogenic signaling. This receptor contains three different parts, including an Ig-like domain extracellular region, a hydrophobic transmembrane region containing the TK domain and the carboxyl terminal tail. VEGFR2 binds all VEGF-A isoforms, VEGF-C and VEGF-D. Contrarily, VEGFR1 is a selective ligand for VEGF-B and PlGFs peptides (Figure 1). Also the binding affinity of VEGF ligands to their receptors is increased by the presence of the two non-enzymatic co-receptors neuropilin (NRP)-1 and NRP-2 (4). Since NRP receptors expression correlates with tumor aggressiveness and poor prognosis, these molecules are currently studied as potential antiangiogenic targets (5). Compared to VEGFR1, VEGFR2 has a lower affinity for VEGF but at the same time it has a stronger kinase activity.
The binding of VEGF-A to VEGFR2 induces a cascade of different signaling pathways. The dimerization of the receptor and the following autophosphorylation of the intracellular TK domains lead to the simultaneous activation of PLC-γ-Raf kinase-MEK-MAP kinase and PI3K-AKT pathways, causing cellular proliferation and endothelial-cell survival.
Additionally, a soluble circulating form of VEGFR2 may be found in the human plasma (6).
Strategies for blocking these pathways include the use of specific inhibitors (antibodies or small molecules), which may either bind VEGF or interfere with the different domains of VEGFR.
Over the last decade, a number of monoclonal antibodies and small molecules that specifically target the VEGF pathway have been studied as single agents or in combination with chemotherapy. Bevacizumab, for example, is a monoclonal antibody (mAb) that binds VEGF and it has gained worldwide approval for first- or second-line treatment in several different tumor types, mainly in association with standard chemotherapies (1).
VEGFR2 is also a novel target. Biological and preclinical evidence suggests that the blockage of VEGFR2 could be a promising strategy to inhibit tumor-induced angiogenesis (7). In order to proof this hypothesis, a rat mAb against murine VEGFR2 named DC101 was developed. DC101 specifically interferes with the binding of VEGF to VEGFR2, inhibiting VEGF-induced signal, and strongly blocks tumor growth in mice (8). However, since DC101 is not able to bind the human VEGFR2, the hybridoma technology was used to produce molecules for clinical use (9). Among these antibodies, ramucirumab (IMC-1121B) was the only one tested in human subjects.
Ramucirumab is a novel human IgG1 mAb that selectively inhibits the VEGFR2 and blocks the VEGFR2-related signaling and activating pathways (Figure 1) (10). Preclinical models showed that ramucirumab selectively binds to the extracellular domain of human VEGFR2 with half-maximal inhibitory concentration of 0.8 to 1.0 nM (11) and it has a 8-fold higher affinity for VEGFR2 when compared with its natural ligands (12). Pharmacokinetic evaluation has demonstrated a nonlinear pharmacokinetic, with incremental doses of this agent being associated with a decrease in clearance (10). Pharmacodynamic evaluations have confirmed the increase in the VEGF ligand, along with the decrease in VEGFR2, after administration of ramucirumab (13). It has a potential therapeutic role in many different tumor types, including gastric cancer, with a favorable toxicity profile. The results of several trials demonstrated its efficacy not only in association with chemotherapy but also as a single agent (4).
Many small molecules may also inhibit VEGFR2, even if the inhibition is not specific. Among those agents we should mention sorafenib, that is approved for first-line treatment of renal cell carcinoma (RCC) and hepatocarcinoma (HCC), sunitinib, that is approved for RCC and gastrointestinal stromal tumors (GIST), and pazopanib, recently approved for RCC and soft tissue sarcoma (14). Moreover, apatinib is a small molecule that may specifically inhibit VEGFR2 (15). Several ongoing trials are testing the efficacy of VEGFR2 inhibitors. In this review we report on the main studies that investigate the use of VEGFR2 inhibitors.
Phase I/II trials: moving the first steps in the clinical development
As VEGFR2 is the predominant mediator of VEGF-induced angiogenesis, its blockade has been extensively investigated. In murine models (16), DC101 was able to reduce tumor growth, to prevent cancer dissemination and to limit the neoangiogenic sprout. Notably, DC101 produced a 30% increase in median survival in mice with peritoneal metastases (17). Moreover, when added to continuous low-dose doxorubicin, DC101 inhibits angiogenesis and tumor growth in soft tissue sarcoma mouse xenografts (18). Preclinical models evidenced the possibility to combine DC101 with standard antiblastic agents. When combined with gemcitabine and administered to nude mice implanted with human pancreatic cancers, DC101 increased the rate of tumor cell death and decreased tumor cell proliferation (19). Similarly, the combination of DC101 and paclitaxel induced significant regression of transitional cell carcinomas implanted in athymic nude mice (20).
However, since DC101 could only link and block the murine VEGFR2, the clinical development of this antibody in humans was no further possible. In order to overcome this problem, a single chain variable fragment with human VEGFR2 reactivity was isolated from a phage library and named IMC-1C11.
IMC-1C11 was tested in early phase I trial enrolling 14 pretreated metastatic colorectal cancer (CRC) patients. Even if no grade 3 or higher treatment-related toxicities were reported with a weekly cycle dose range of 0.2-4.0 mg/kg, about 50% of patients developed antibodies against IMC-1C11, limiting the opportunity for further clinical tests (21).
In the scenario, ramucirumab has emerged as a new therapeutic option in solid tumors. In a pioneer phase I trial, 37 heavily pretreated patients received weekly escalating doses of ramucirumab, from 2 to 16 mg/kg, to evaluate the maximum tolerated dose (MTD) in humans. Since patients developed dose-limiting toxicities at 16 mg/kg, MTD was set up at 13 mg/kg. A total of 60% of patients developed grade 3 or higher adverse events (AEs), mainly consisting in hypertension (13.5%), abdominal pain (10.8%), anorexia, vomiting, headache, proteinuria, dyspnea, and deep venous thrombosis. Anticancer activity was seen in 11% of treated patients, with an overall disease control rate (DCR) of 73% (13).
Recently, safety data focusing on the use of ramucirumab in other phase I trials have been presented (22,23). In particular, toxicity data registered in 6 Japanese patients with metastatic breast cancer (mBC) exposed to ramucirumab (10 mg/kg every 3 weeks) plus docetaxel (75 mg/m2 every 3 weeks) revealed that the combination is safe in Eastern patients. Similarly, an ongoing phase I trial aims to evaluate safety of ramucirumab, administered at escalating doses from 6 to 10 mg/kg q14-21 for 6 weeks, in Chinese patients with advanced solid tumors that are resistant to standard therapy (24).
Other early clinical trials are ongoing. A phase Ib/2 trial is currently enrolling patients with advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma, HCC or RCC to receive ramucirumab in combination with LY2875358 (25). Another phase I trial is investigating the safety and tolerability of the combination of ramucirumab and FOLFIRI (irinotecan with fluorouracil and folinic acid) in Japanese metastatic CRC patients.
The positive results of early clinical trials stimulated further researches, as summarized in Table 1. To test its activity and evaluate the safety profile, Zhu et al. accrued 42 previously untreated HCC patients to receive ramucirumab at the dose of 8 mg/kg every other week until disease progression or unacceptable toxicities. Median progression-free survival (PFS) was 4.0 months (95% CI, 2.6-5.7), objective response rate (RR) was 9.5% (95% CI, 2.7-22.6); and median overall survival (OS) was 12.0 months (95% CI, 6.1-19.7). Grade 3 or higher AEs included hypertension (14%), gastrointestinal bleeding (7%), idiosyncratic reactions (7%), and fatigue (5%), suggesting that ramucirumab could be an active and well tolerated first-line option in HCC patients (26).
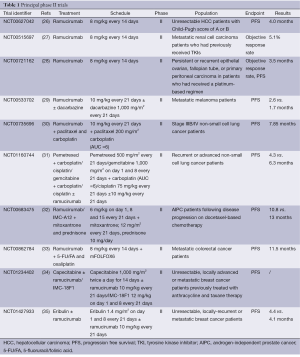
Full table
Another phase II study tested the activity of ramucirumab combined to modified FOLFOX6 (5-fluorouracil, leucovorin, and oxaliplatin) as first-line therapy in 48 advanced CRC patients. Overall RR was 58.3% (95% CI, 43.1-72.4), with a DCR approaching 95%; the median OS was 20.4 months (95% CI, 18.5-25.1). Severe AEs included neutropenia (33.3%), hypertension (16.7%), and neuropathy (12.5%) (33). Camidge et al. reported on the results of a phase II trial evaluating the activity of ramucirumab combined to paclitaxel and carboplatin as first-line treatment in 40 patients with stage IIIb/IV non-small cell lung cancer (NSCLC). Median PFS was 7.5 months (95% CI, 5.5-9.9), RR 55%. The most frequently reported AE were fatigue (53%), neuropathy (33%), nausea (28%), myalgias (23%) and minor bleeding (23%) (30). In another randomized phase II trial (31), 140 patients with recurrent or advanced NSCLC received a platinum-based chemotherapy with or without ramucirumab. Median PFS observed at the first pre-planned interim analysis was 6.3 months for patients who received ramucirumab and 4.3 months for patients enrolled in the control arm. Hypertension was more frequently reported in patients receiving ramucirumab (19% vs. 6%) compared to the others. A similarly designed trial is testing ramucirumab in combination with docetaxel in stage IV NSCLC patients who had reported disease progression after or during a platinum-based therapy (36).
Ramucirumab is being tested in women with metastatic BC as well, and two different phase II trials are ongoing to evaluate the additional benefit of ramucirumab combined to capecitabine (34) or eribulin (35) compared to the antiblastic agent alone. Recently presented data from the latter study demonstrated that there is no statistically significant difference in OS [13.5 vs. 11.5 months, hazard ratio (HR) 0.8; 95% CI, 0.5-1.3; P=0.4], PFS (4.4 vs. 4.1 months, HR 0.8; 95% CI, 0.6-1.2; P=0.4) or overall RR (20% vs. 24%).
A phase II single-arm trial enrolled 39 RCC patients to receive ramucirumab at 8 mg/kg q14 days until disease progression or intolerable toxicities. Overall RR was 5.1%, with a DCR of 64.1%. Median PFS was 7.1 months (95% CI, 4.1-9.7), and the median OS was 24.8 months (95% CI, 18.9-32.6). The most frequent severe AE included hypertension (7.7%) and proteinuria (5.1%) (27).
Ramucirumab was also studied in patients with persistent or recurrent ovarian carcinoma who had failed a platinum-based first-line treatment (28). In castration-resistant metastatic prostate cancer patients, the activity of ramucirumab was evaluated in combination with mitoxantrone and prednisone after failure of a docetaxel-based therapy. A median PFS of 6.7 months (95% CI, 4.5-8.3) was reported, with median OS of 13 months (95% CI, 9.5-16.0) (32). Preliminary data on the activity of the antiangiogenic drug in patients with metastatic cutaneous melanoma have been recently presented. One hundred and six patients were randomly assigned to receive dacarbazine with or without ramucirumab. PFS was 1-month longer (2.6 vs. 1.7 months) in patients who received the combination, and the toxicity profile was acceptable (29).
Phase III trial results: balancing meaningful advances with disappointing missteps
So far, ramucirumab is the only antibody against VEGFR2 to have reached the last phase of clinical development (4). The most notable results have been observed in patients with pretreated advanced gastric and GEJ cancers (37). REGARD is an international phase III placebo-controlled study that enrolled 355 patients with advanced gastric (75%) or GEJ (25%) adenocarcinomas. Patients were randomized with a 2:1 ratio to ramucirumab 8 mg/kg given every 2 weeks plus best supportive care (BSC) or placebo plus BSC as second-line treatment (37). Patients with either measurable or evaluable disease were enrolled. Randomization was stratified by weight loss, anatomic location of the primary tumor and geographical region. The final results were published after 278 patients had died. The addition of ramucirumab to BSC significantly prolonged median OS from 3.8 to 5.2 months, translating into a 22% reduction in the risk of death (HR 0.77; 95% CI, 0.60-0.99; P=0.0473). The study also met its secondary endpoints. Patients enrolled in the experimental arm had a longer PFS (HR 0.48; 95% CI, 0.37-0.62; P<0.0001) and a significant increase in DCR (48.7% with ramucirumab vs. 23.1% with placebo; P<0.0001), although the overall RR was similarly low in both treatment arms (3.4% vs. 2.6%) (37). Subgroup analysis showed consistent treatment effect among almost all subgroups. Interestingly, male patients exposed to ramucirumab seemed to have a greater survival benefit compared to that reported in female patients (HR for OS 0.67; 95% CI, 0.49-0.91 vs. HR 1.43; 95% CI, 0.85-2.40). At the 2014 Gastrointestinal Cancers Symposium, the preliminary results of RAINBOW trial, a large study focused on pretreated patients with advanced gastric or GEJ adenocarcinoma, were reported (38). The study randomized (1:1 ratio) 665 patients who had progressed while on or within 4 months of standard first-line treatment with a platinum-based chemotherapy to receive paclitaxel 80 mg/m2 alone or in combination with ramucirumab 8 mg/kg given every 2 weeks. OS was the primary study endpoint. Stratification factors included geographic region, disease measurability, and time to progression on first-line therapy (<6 vs. >6 months). In the whole trial population, 398 patients were from Europe, Australia or North America, 223 were from the East Asia, and only 44 from South America. The trial met its primary and secondary endpoints with a 19% reduction in the risk of death (P=0.0169) and a 27% reduction in the risk of disease progression (P<0.0001) with the addition of ramucirumab to paclitaxel. Median OS was 9.6 months for the combination vs. 7.4 months for paclitaxel alone and median PFS was 4.4 months vs. 2.9 months, respectively. In addition, the DCR was 80% with paclitaxel plus ramucirumab vs. 64% with paclitaxel alone (P<0.0001). A similar proportion of patient received any post-discontinuation treatment: 47.9% in the ramucirumab plus paclitaxel arm vs. 45.4% in the paclitaxel alone arm.
In both these phase III trials ramucirumab was very well tolerated, with no unexpected toxicities and a safety profile in line with those of other antiangiogenic agents. In the REGARD trial 57% of the patients exposed to ramucirumab had grade 3-4 AEs compared with 58% in the placebo group. The most frequent treatment-related severe AE was hypertension (8% with ramucirumab vs. 3% with placebo); no grade 4 hypertension was recorded. Ramucirumab was not associated with increased rates of proteinuria, bleeding, venous thrombosis, or gastrointestinal perforation. In both groups, 2% of deaths were considered to be treatment-related. The preplanned quality-of-life (QoL) assessment analysis showed that 34% of patients given ramucirumab reported stable or improved conditions at the 6 weeks assessment compared with 13% in the placebo group. In the RAINBOW trial, more severe AEs were reported in the ramucirumab arm compared to the standard arm (82% vs. 63%). More specifically, grade 3-4 neutropenia was 40.7% in the combination arm vs. 18.8% in the other, leucopenia was 17.4% vs. 6.7%, and hypertension was 14.1% vs. 2.4%. However, these AEs did not lead to a higher rate of treatment discontinuation. The rates of treatment-related death were similar across treatment arms (4.0% vs. 4.6%).
Based on these results, on April 21st, 2014 the Food and Drug Administration approved single agent ramucirumab for the treatment of patients with advanced or metastatic, gastric or GEJ adenocarcinoma with disease progression on or after prior treatment with fluoropyrimidine- or platinum-containing chemotherapy (39).
Recently, therapies targeting angiogenesis have been also investigating in NSCLC. In the REVEL trial 1,242 patients diagnosed with NSCLC who had disease progression during or soon after an upfront platinum-based chemotherapy were randomized at a 1:1 ratio to receive docetaxel 75 mg/m2 plus either ramucirumab (10 mg/kg) or placebo every 3 weeks (40,41). The primary endpoint of the study was OS and the secondary endpoints included PFS, overall RR, DCR, patient-reported outcomes, and the assessments of safety and tolerance of ramucirumab. Stratification factors were Eastern Cooperative Group (ECOG) performance status (PS) (0 vs. 1), gender, prior maintenance treatment (yes vs. no), and geographic origin (East-Asia vs. rest of the world). On February 19th 2014, a press release from Eli-Lilly Oncology announced that the study met its primary as well as secondary endpoints (42). The trial results have been recently presented at the 2014 American Society of Clinical Oncology (ASCO) Annual Meeting (43). The intention-to-treat analysis included 1,253 patient allocated to ramucirumab plus paclitaxel arm (n=628) or to the control arm (n=625); 1,245 treated subjects were included in the safety analysis. As expected, patients were well balanced between treatment arms. In particular, epidermal growth factor receptor (EGFR) status showed wild-type for 33% vs 31.5% of treated patients, 24% of patients in both treatment arms had been previously exposed to taxanes and around 14% to bevacizumab. Although the complete RR was negligible in both treatment arms (<1%), the overall RR was statistically superior in those exposed to ramucirumab (22.9% vs. 13.6%, P<0.001), as was the DCR (64% vs. 52.6%, P<0.001). The combination of ramucirumab and docetaxel produced a significant advantage in median PFS (4.5 vs. 3.0 months; HR 0.76; 95% CI, 0.67-0.86; P<0.0001) and in median OS (10.5 vs. 9.1 months; HR 0.85; 95% CI, 0.75-0.97; P=0.02), even if the proportion of patients who received any post-discontinuation therapy was similar between treatment arms (45.4% vs. 48.3%). The OS and PFS were consistent in most treatment subgroups, including squamous and nonsquamous histology. Importantly, the addition of ramucirumab to docetaxel did not result in an increase of serious AEs or AEs leading to death. Accordingly, the safety profile of the combination was not different from what expected, and the increase in specific antiangiogenic-induced toxicities was limited to the doubling of grade 1-2 bleedings.
Over the last decade, the use of antiangiogenic agents in metastatic BC has been intensively tested (44) and the most encouraging data come from the combination of bevacizumab with taxane, either paclitaxel (45) or docetaxel (46).
Accordingly, the ROSE/TRIO-12 trial randomized at a 2:1 ratio 1,144 human epidermal growth factor receptor (HER) 2-negative mBC patients to upfront docetaxel 75 mg/m2 plus ramucirumab 10 mg/kg or placebo every 3 weeks. The primary endpoint of the study was PFS. After a median follow-up of 16.2 months, data from an interim analysis presented at the 2013 San Antonio Breast Cancer Symposium (SABCS) showed no significant advantage in PFS or OS (47). The median PFS as assessed by the investigators was 9.5 months in the combination group and 8.2 months in the docetaxel alone group (HR 0.88; 95% CI, 0.95-1.01; P=0.077); the median OS was 27.3 and 27.2, respectively (HR 1.01; 95% CI, 0.83-1.23; P=0.915). No significant advantage in survival was detected in any subgroup analyzed, including those assessed by age, race, PS, prior taxane treatment, metastases location, hormone receptor status, and geographical region. Moreover, more grade 3-4 AEs were reported with docetaxel plus ramucirumab (61.7%) compared with docetaxel alone (52.4%). In particular, fatigue, hypertension, bleeding, febrile neutropenia, and stomatitis were significantly higher in patients assigned to ramucirumab. So far, no antiangiogenic strategy has improved OS in metastatic BC. The negative results of ROSE mirror those of other trials that failed in demonstrating the benefit of antiangiogenic drugs in this disease (48).
In the near future, the results from the REACH (49) and the RAISE (50) trial will also be available; these two large second-line, phase III studies are evaluating the use of ramucirumab in HCC and advanced CRC patients, respectively. The design of both trials has been presented at the 2012 ASCO Annual Meeting (51).
REACH randomizes to either ramucirumab or placebo 544 HCC patients whose disease progressed during or following first-line therapy with sorafenib or who were intolerant to the latter agent (50,52). The trial enrollment has been recently completed, and results of the study are expected in 2015. However, a press notice released on Jun 11th 2014, announced that OS results favored the ramucirumab arm, but the advantage was not statistically significant.
In the RAISE trial, over 1,000 patients with advanced CRC who have failed a first-line combination including bevacizumab, oxaliplatin, and a fluoropyrimidine are randomized at a 1:1 ratio to receive irinotecan, leucovorin and 5-fluorouracil (FOLFIRI) plus ramucirumab vs. FOLFIRI alone (50). The trial enrollment will be completed by early 2016.
A novel small molecule primarily targeting VEGFR2
Apatinib (YN968D1) is a novel, potent VEGFR inhibitor with an intriguing biologic rationale (15) that is able to circumvent cancer cell resistance to other antineoplastic agent (53). A recently published phase II randomized trial tested apatinib in heavily pretreated gastric cancer patients. Primary objectives were to assess the activity and safety of the daily administration of apatinib and to compare the tolerability of a once-daily vs. a twice-daily regimen. Patients were allocated to placebo (arm A), apatinib 850 mg once daily (arm B), or apatinib 425 mg twice daily (arm C). Outcome results were statistically improved in those patients exposed to apatinib (P<0.001 both for OS and PFS). Specifically, median OS was 2.5 months for arm A (95% CI, 1.87-3.70), 4.8 months for arm B (95% CI, 4.03-5.97), and 4.3 months for arm C (95% CI, 3.83-4.77). Similarly, the median PFS was 1.4 months (95% CI, 1.20-1.83) for arm A, 3.7 months (95% CI, 2.17-6.80) for arm B, and 3.2 months (95% CI, 2.37-4.53) for arm C. Interestingly, 9 patients exposed to apatinib had a partial response. The drug was overall well tolerable, with a limited number of patients complaining of severe AEs (hand-foot syndrome and hypertension); severe hematologic toxicities were occasional (54). Results of a phase 3 randomized trial of apatinib vs. placebo have been recently presented. In the study, 273 heavily pretreated patients were randomized 2:1 to apatinib (at a continuous oral dose of 850 mg per day, n=181) or matching placebo (n=92). The only stratification factor was the number of metastatic sites (less than 3 vs. 3 or above). Key inclusion criteria were age between 18 and 70 years, histologically confirmed advanced or metastatic adenocarcinoma of the stomach, ECOG PS of 0 or 1, the presence of measurable disease according to RECIST, and adequate organ function. Also, patients were required to be able of oral ingestion. Patients with evidence of SNC metastases, prior exposition to any VEGFR inhibitor, uncontrolled hypertension, coronary artery disease or any other significant cardiovascular concurrent morbidity were excluded. OS was the primary endpoint of the study; secondary endpoints included PFS, overall RR, DCR, quality of life, and safety. Median age of included patients was approximately 60 years, and over 75% of the patients had an ECOG PS of 1 (there were 10% more patients with PS 0 in the apatinib arm); 92% had stage IV disease The cohort was clearly heavily pretreated: 65% of the patients had received 2 or 3 previous lines of therapy, 35% were exposed to more than three previous treatments. In the experimental arm, OS was prolonged by 1.8 months compared to placebo (median OS 6.5 vs. 4.7 months, HR 0.71, P=0.01). Accordingly, median PFS was also significantly extended (2.6 vs. 1.8 months, HR 0.44, P<0.001). In the experimental arm, hematological toxicity was increased, but serious AE were equally reported. A significant incidence of grade 3 or 4 HFS reaction (9%) suggests that, despite its specificity, apatinib may have off-target effects. The average survival benefit of the drug to pretreated Chinese patients (1.8 months) appears to be in the same range of that of ramucirumab in the Western population (1.6 months). This successful drug is currently being tested in patients with breast or lung cancers.
Conclusions
In the last decade, the importance of angiogenic inhibitors in cancer therapy has become increasingly clear. VEGF has been recognized as a key angiogenic mediator, and its increased level of expression has been associated with disease aggressiveness. Many preclinical and early clinical studies have provided insights into mechanisms that underlie the complexity of neoplastic angiogenesis. Also they have posed the foundation to develop novel drugs that target VEGF or VEGFR2. Among the scope of these drugs, ramucirumab, the only human antibody that specifically blocks the VEGFR2, has produced notable results in different diseases including gastric cancers and lung carcinomas. In these poor prognosis diseases, even a small absolute survival benefit of 1.5-2 months is clinically valuable. Disappointing clinical results reported for the ROSE study confirm that breast cancer may limitedly benefit from angiogenic inhibitors. While ongoing studies will clarify the role of ramucirumab in CRC, translational research will provide more details about how to properly select optimal candidates and corroborate the evidence for an ethnical difference in benefit. Despite huge efforts have been made to identify a predictive biomarker, no validated predictor is currently available for selecting optimal candidates to antiangiogenic therapy or monitoring treatment response. Future research will possibly increase our knowledge on how to select patients who are more likely to be responsive to antiangiogenic treatment. As well, the role of VEGF in reverting immunosuppression should also be better elucidated. Moreover, novel oral VEGFR2 inhibitors will possibly add some value to this strategy.
Acknowledgements
Giuseppe Aprile was involved as principal investigator in the REGARD study. He participated in advisory boards and was compensated as speaker for Roche, Merck-Serono, Eli-Lilly and Amgen. Gianpiero Fasola participated in advisory boards for Amgen and served as speaker for Amgen, Eli-Lilly, Merck-Serono, Roche, Pfizer, and Glaxo.
Disclosure: The authors declare no conflict of interest.
References
- Sullivan LA, Brekken RA. The VEGF family in cancer and antibody-based strategies for their inhibition. MAbs 2010;2:165-75. [PubMed]
- Youssoufian H, Hicklin DJ, Rowinsky EK. Review: monoclonal antibodies to the vascular endothelial growth factor receptor-2 in cancer therapy. Clin Cancer Res 2007;13:5544s-5548s. [PubMed]
- Kerbel RS. Tumor angiogenesis. N Engl J Med 2008;358:2039-49. [PubMed]
- Aprile G, Bonotto M, Ongaro E, et al. Critical appraisal of ramucirumab (IMC-1121B) for cancer treatment: from benchside to clinical use. Drugs 2013;73:2003-15. [PubMed]
- Pan Q, Chanthery Y, Liang WC, et al. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell 2007;11:53-67. [PubMed]
- Ebos JM, Bocci G, Man S, et al. A naturally occurring soluble form of vascular endothelial growth factor receptor 2 detected in mouse and human plasma. Mol Cancer Res 2004;2:315-26. [PubMed]
- Hamerlik P, Lathia JD, Rasmussen R, et al. Autocrine VEGF-VEGFR2-Neuropilin-1 signaling promotes glioma stem-like cell viability and tumor growth. J Exp Med 2012;209:507-20. [PubMed]
- Witte L, Hicklin DJ, Zhu Z, et al. Monoclonal antibodies targeting the VEGF receptor-2 (Flk1/KDR) as an anti-angiogenic therapeutic strategy. Cancer Metastasis Rev 1998;17:155-61. [PubMed]
- Huang J, Tan Y, Tang Q, et al. A high-affinity human/mouse cross-reactive monoclonal antibody, specific for VEGFR-2 linear and conformational epitopes. Cytotechnology 2010;62:61-71. [PubMed]
- Spratlin JL, Mulder KE, Mackey JR. Ramucirumab (IMC-1121B): a novel attack on angiogenesis. Future Oncol 2010;6:1085-94. [PubMed]
- Lu D, Jimenez X, Zhang H, et al. Selection of high affinity human neutralizing antibodies to VEGFR2 from a large antibody phage display library for antiangiogenesis therapy. Int J Cancer 2002;97:393-9. [PubMed]
- Miao HQ, Hu K, Jimenez X, et al. Potent neutralization of VEGF biological activities with a fully human antibody Fab fragment directed against VEGF receptor 2. Biochem Biophys Res Commun 2006;345:438-45. [PubMed]
- Spratlin JL, Cohen RB, Eadens M, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol 2010;28:780-7. [PubMed]
- Claesson-Welsh L, Welsh M. VEGFA and tumour angiogenesis. J Intern Med 2013;273:114-27. [PubMed]
- Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci 2011;102:1374-80. [PubMed]
- Prewett M, Huber J, Li Y, et al. Antivascular endothelial growth factor receptor (fetal liver kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth of several mouse and human tumors. Cancer Res 1999;59:5209-18. [PubMed]
- Shaheen RM, Tseng WW, Vellagas R, et al. Effects of an antibody to vascular endothelial growth factor receptor-2 on survival, tumor vascularity, and apoptosis in a murine model of colon carcinomatosis. Int J Oncol 2001;18:221-6. [PubMed]
- Zhang L, Yu D, Hicklin DJ, et al. Combined anti-fetal liver kinase 1 monoclonal antibody and continuous low-dose doxorubicin inhibits angiogenesis and growth of human soft tissue sarcoma xenografts by induction of endothelial cell apoptosis. Cancer Res 2002;62:2034-42. [PubMed]
- Bruns CJ, Shrader M, Harbison MT, et al. Effect of the vascular endothelial growth factor receptor-2 antibody DC101 plus gemcitabine on growth, metastasis and angiogenesis of human pancreatic cancer growing orthotopically in nude mice. Int J Cancer 2002;102:101-8. [PubMed]
- Inoue K, Slaton JW, Davis DW, et al. Treatment of human metastatic transitional cell carcinoma of the bladder in a murine model with the anti-vascular endothelial growth factor receptor monoclonal antibody DC101 and paclitaxel. Clin Cancer Res 2000;6:2635-43. [PubMed]
- Posey JA, Ng TC, Yang B, et al. A phase I study of anti-kinase insert domain-containing receptor antibody, IMC-1C11, in patients with liver metastases from colorectal carcinoma. Clin Cancer Res 2003;9:1323-32. [PubMed]
- Abstracts of the 11th Annual Meeting of the Japanese Society of Medical Oncology. August 29-31, 2013. Sendai, Japan. Ann Oncol 2013;24 Suppl 9:ix5-99. [PubMed]
- NCT01256567. A Study of Ramucirumab (IMC-1121B) in Patients With Breast Cancer. Available online: http//:www.clinicaltrials.gov
- NCT01682135. A Study in Participants With Advanced Solid Tumors. Available online: http//:www.clinicaltrials.gov
- NCT02082210. A Study of LY2875358 in Combination With Ramucirumab (LY3009806) in Participants With Advanced Cancer. Available online: http//:www.clinicaltrials.gov
- Zhu AX, Finn RS, Mulcahy M, et al. A phase II and biomarker study of ramucirumab, a human monoclonal antibody targeting the VEGF receptor-2, as first-line monotherapy in patients with advanced hepatocellular cancer. Clin Cancer Res 2013;19:6614-23. [PubMed]
- Garcia JA, Hudes GR, Choueiri TK, et al. A phase 2, single-arm study of ramucirumab in patients with metastatic renal cell carcinoma with disease progression on or intolerance to tyrosine kinase inhibitor therapy. Cancer 2014;120:1647-55. [PubMed]
- Penson RT, Moore KN, Fleming GF, et al. A phase II, open-label, multicenter study of IMC-1121B (ramucirumab; RAM) monotherapy in the treatment of persistent or recurrent epithelial ovarian (EOC), fallopian tube (FTC), or primary peritoneal (PPC) carcinoma (CP12-0711/NCT00721162). ASCO Annual Meeting, 2012.
- Carvajal R, Thompson J, Gordon M, et al. A phase 2 randomized study of ramucirumab (IMC 1121B; RAM) with or without dacarbazine (DTIC) in patients (pts) with metastatic melanoma (MM) (CP12-0604/NCT00533702). ESMO Congress, 2012.
- Camidge DR, Berge EM, Doebele RC, et al. A phase II, open-label study of ramucirumab in combination with paclitaxel and carboplatin as first-line therapy in patients with stage IIIB/IV non-small-cell lung cancer. J Thorac Oncol 2014. [Epub ahead of print]. [PubMed]
- Doebele R, Spiegel D, Tehfe M. A phase 2 randomized open-label study of ramucirumab (IMC 1121b; RAM) in combination with platinum-based chemotherapy in patients (pts) with recurrent or advanced non-small cell lung cancer (NSCLC): results from non-squamous (NSQ) pts (NCT01160744). Vienna, Austria. VESMO 2012. Abstract 1400.
- Hussain M, Rathkopf DE, Liu G, et al. A phase II randomized study of cixutumumab (IMC-A12: CIX) or ramucirumab (IMC-1121B: RAM) plus mitoxantrone (M) and prednisone (P) in patients (pts) with metastatic castrate-resistant prostate cancer (mCRPC) following disease progression (PD) on docetaxel (DCT) therapy. ASCO Meeting Abstracts, 2012:97.
- Garcia-Carbonero R, Rivera F, Maurel J, et al. An open-label phase II study evaluating the safety and efficacy of ramucirumab combined with mFOLFOX-6 as first-line therapy for metastatic colorectal cancer. Oncologist 2014;19:350-1. [PubMed]
- NCT01234402. Study of IMC-18F1 or Ramucirumab DP in Combination With Capecitabine or Capecitabine on Previously Treated Breast Cancer Patients. Available online: http//:www.clinicaltrials.gov
- Yardley DA, Richards PD, Reeves JA, et al. Final results of a phase 2 study of ramucirumab (RAM) plus eribulin (E) versus E in advanced metastatic breast cancer (MBC) (NCT01427933). ASCO Annual Meeting 2014.
- NCT01703091. A Study of Docetaxel and Ramucirumab Versus Docetaxel and Placebo in the Treatment of Stage IV Non-Small Cell Lung Cancer. Available online: http//:www.clinicaltrials.gov
- Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31-9. [PubMed]
- Wilke H, Van Cutsem E, Cheul Oh S, et al. RAINBOW: A global, phase III, randomized, double-blind study of ramucirumab plus paclitaxel versus placebo plus paclitaxel in the treatment of metastatic gastroesophageal junction (GEJ) and gastric adenocarcinoma following disease progression on first-line platinum- and fluoropyrimidine-containing combination therapy rainbow IMCL CP12-0922 (I4T-IE-JVBE). Gastrointestinal Cancers Symposium, 2014.
- FDA news release. Available online: http://www.fda.gov/NewsEvebts/Newsroom/PressAnnouncement/ucm394107.htm
- NCT01168973. A Study of Chemotherapy and Ramucirumab vs. Chemotherapy Alone in Second Line Non-small Cell Lung Cancer Participants Who Received Prior First Line Platinum Based Chemotherapy. Available online: http//:www.clinicaltrials.gov
- Garon EB, Cao D, Alexandris E, et al. A randomized, double-blind, phase III study of Docetaxel and Ramucirumab versus Docetaxel and placebo in the treatment of stage IV non-small-cell lung cancer after disease progression after 1 previous platinum-based therapy (REVEL): treatment rationale and study design. Clin Lung Cancer 2012;13:505-9. [PubMed]
- Ramucirumab Improved Survival in Second-Line Study of Patients with Non-Small Cell Lung Cancer. Available online: https://investor.lilly.com/releasedetail.com
- Pérol M, Ciuleanu TE, Arrieta O, et al. REVEL: A randomized, double-blind, phase III study of docetaxel (DOC) and ramucirumab (RAM; IMC-1121B) versus DOC and placebo (PL) in the second-line treatment of stage IV non-small cell lung cancer (NSCLC) following disease progression after one prior platinum-based therapy. ASCO Annual Meeting, 2014.
- Fakhrejahani E, Toi M. Antiangiogenesis therapy for breast cancer: an update and perspectives from clinical trials. Jpn J Clin Oncol 2014;44:197-207. [PubMed]
- Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 2007;357:2666-76. [PubMed]
- Miles DW, Chan A, Dirix LY, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 2010;28:3239-47. [PubMed]
- Mackey JR, Ramos-Vazquez M, Lipatov O, et al. Primary results of ROSE/TRIO-12, a randomized placebo controlled phase III trial evaluating the addition of ramucirumab to first-line docetaxel chemotherapy in metastatic breast cancer. Available online: http://www.abstracts2view.com/sabcs13/view.php?nu=SABCS13L_238
- D’Agostino RB Sr. Changing end points in breast-cancer drug approval--the Avastin story. N Engl J Med 2011;365:e2. [PubMed]
- NCT01140347. A Study of Ramucirumab (IMC-1121B) Drug Product (DP) and Best Supportive Care (BSC) Versus Placebo and BSC as 2nd-Line Treatment in Patients With Hepatocellular Carcinoma After 1st-Line Therapy With Sorafenib (REACH). Available online: http://www.clinicaltrials.gov
- NCT01183780. A study in second line metastatic colorectal cancer. Available online: http://www.clinicaltrials.gov
- Zhu AX, Chau I, Blanc JF, et al. A multicenter, randomized, double-blind, phase III study of ramucirumab (IMC-1121B; RAM) and best supportive care (BSC) versus placebo (PBO) and BSC as second-line treatment in patients (pts) with hepatocellular carcinoma (HCC) following first-line therapy with sorafenib (SOR). ASCO Annual Meeting, 2012.
- Grothey A, Tabernero J, Rougier P, et al. A randomized, double-blind, phase (Ph) III study of the irinotecan-based chemotherapy FOLFIRI plus ramucirumab (RAM) or placebo (PL) in patients (pts) with metastatic colorectal carcinoma (mCRC) progressive during or following first-line therapy with bevacizumab (BEV), oxaliplatin (OXALI), and a fluoropyrimidine (FP) (RAISE) (NCT01183780). ASCO Annual Meeting, 2014.
- Mi YJ, Liang YJ, Huang HB, et al. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res 2010;70:7981-91. [PubMed]
- Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol 2013;31:3219-25. [PubMed]


