
The current state of navigation in robotic spine surgery
Introduction
Pedicle screw instrumentation has remained the gold standard technique for spinal fixation since its popularization by Roy Camille in the 1970s (1). Despite the benefit of pedicle screw instrumentation, misplaced pedicle screws can result in serious neurovascular injury. Consequently, modalities of image-guidance in spine surgery have evolved over the years (2-10). Although the precision of image-guided screw placement is high (10-13), the constant pursuit to improve clinical outcomes has prompted the introduction of robotics into the field of spine surgery (14-18).
When utilizing robots for surgical procedures, surgeons can interface with robots in three ways (19). In the supervisory-control system, a surgeon plans the procedure and observes the execution of the plan by an autonomously acting robot. An example is the Cyberknife (Accuray, Sunnyvale, California, USA) radiosurgery robot employed by neurosurgeons for the treatment of tumors of the central nervous system (20). The DaVinci (Intuitive, Sunnyvale, California, USA) robot is an example of a tele-surgical robot system. These systems are characterized by the translation of direct real-time surgeon input to robot output (21). Finally, a shared-control system allows the robot to function in concert with the surgeon, who remains in primary control of the procedure. Currently, all FDA approved, commercially available spinal robotic systems are shared-control systems. These systems are theoretically capable of reducing human error through increased precision in execution, indefatigability, motion scaling, and tremor filtration via mechanical actuation (19).
The first shared-control robot designed for use in spine surgery, SpineAssist (Mazor Surgical Technologies, Caesarea, Israel), emerged in the early 2000s. The SpineAssist was developed contemporaneously with computer-assisted spinal navigation systems – both of which were promoted as a solution to the unsatisfactory screw malposition rate and increased radiation exposure associated with minimally invasive spinal instrumentation techniques (14,15,17,22,23). There are many reports of successful implementation of this robotic platform with evidence of increased screw accuracy and reduction in radiation exposure relative to traditional fluoroscopically-guided freehand technique (14,15,17,18). Irrespective of radiographic results, these studies showed that clinical outcomes were not significantly impacted with the use of a robot compared to the fluoroscopically-guided freehand technique. This clinical equipoise combined with the high capital acquisition cost of the system stagnated its widespread adoption (24).
Historically, robots required a certain level of trust from surgeons as the robotic systems did not provide any real-time visual feedback for instrument localization and depth gauging. However, non-robotic 3D computer assisted navigation (CAN) in spine surgery (10-13,25,26) has led to the integration of computer-assisted navigation with modern spine robot platforms. This new combination of technologies appears to be driving the resurgence of spinal robotics. In this article we review the contemporary 3D navigation enabled robotic spine platforms and their current and future applications.
Current integrated navigation and robotics platforms
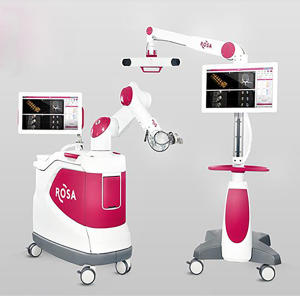
The ROSA Spine Robot (Zimmer Biomet Robotics formerly Medtech SA, Montpellier, France) was launched in 2011 in Europe and has been FDA approved for spine surgery in the USA since 2016 (Figure 1). It is a fully automated robotic arm with 6 degrees of freedom (DOF) deployed from a floor-mounted base station that also houses an integrated CAN interface. A separate optical camera is used for registration and real-time tracking. A removable fiducial array is attached to the robot arm for the registration process. A standard posterior superior iliac spine (PSIS) or spinous process mounted DRB is used for anatomic reference. Pre-operative or intra-operative images are acquired and registered to the patient and robot. The base station is anchored and the robotic arm is deployed. The end effector allows drilling and subsequent insertion of a guide wire. Pedicle tapping and subsequent placement of the screw can all be performed with optional CAN assistance using a tracker array that is affixed to the instrument (27-29). The ROSA spine robot is able to move with patient respiration and surgical manipulation as the optical camera constantly tracks the location of the patient DRB relative to a reference array mounted on the base station next to the arm. The ROSA platform received an FDA approved upgrade in March of 2019, and is now known as Rosa ONE. The upgrade includes a fully integrated navigation interface and Zimmer Biomet instrumentation package and is adaptable across cranial, spinal, and orthopedic knee arthroplasty procedures.
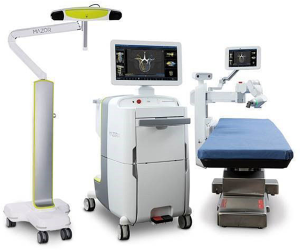
The ExcelsiusGPS® (Globus Medical, Inc., Audubon, PA, USA) launched in late 2017 and at the time was advertised as the first US-built robotic surgical spine and fully integrated navigation platform with real-time instrument tracking—allowing pedicle screw placement without K-wires (Figure 2). The system is anchored on a floor-mounted base station that supports the CAN interface as well as the robotic arm itself. The robotic end effector employs small wirelessly powered LED markers rather than larger standard reflective ball markers, and the instruments that pass through and are held by the end effector also have their own specific tracking arrays (Figure 3). A standard DRB is also mounted to the patient’s PSIS or a spinous process. A separate surveillance DRB is bone mounted as well. An optical camera is used for registration and tracking. Intraoperative CT is the imaging modality of choice for registration for ease of work flow, although the robot is capable of fluoroscopic registration utilizing a pre-operative CT scan. The system can also be used for 3D CAN without the robotic arm, if desired (30).

The Mazor X spine robot (Medtronic Navigation Louisville, CO, USA; Medtronic Spine, Memphis, TN, USA; formerly Mazor Robotics, Caesarea, Israel) initially launched commercially in October 2016 (Figure 4). The X represented an iterative improvement over the Renaissance (Mazor Robotics, Caesarea, Israel) as the robotic arm was upgraded to be fully automated and no longer requires a patient mounted track for deployment. Instead, the X mounts to both the surgical bed frame with rail adaptor attachments, and to the patient with a bone connection bridge from the robotic arm to a pin placed in the PSIS or spinous process. The X has 3 cameras on the arm itself, which first detect and volumetrically defines the operative field in order to prevent collision with the patient. A fiducial marker is then temporarily attached to the arm and registered either to the pre-operative CT scan with AP and oblique fluoroscopic images, or by intra-operative O-arm cone beam CT scan. The robot is then able to guide pedicle cannulation and K-wire insertion. The pedicle is tapped and screw is placed manually with fluoroscopy for depth guidance. Following the acquisition by Medtronic in December of 2018, the co-adaptation of Stealth Navigation and the X Platform was announced and upgrades were launched in early 2019. The O-arm acquisition dataset is reformatted separately by both the X Robotic guidance processing unit and the integrated Stealth Navigation software. This allows parallel integration of fully navigated Medtronic instruments for real-time instrument position feedback. The integrated technical hardware adds a new optical tracking camera as well as a new navigation-specific DRB that is mounted to the base of the robotic arm (Figure 5). The X Stealth Edition otherwise performs identically to the non-navigated version.

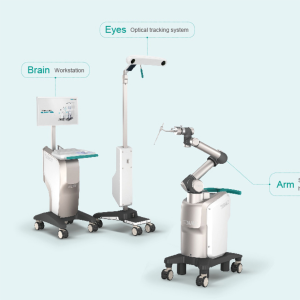
The TiRobot (TINAVI Medical Technologies, Beijing, China) is the first multi-disciplinary orthopedic robot created entirely in China and received China FDA approval in 2016 (Figure 6). It is the most popular surgical robot in China. It has a robotic arm with 6 DOF and registration is completed by cross-referencing a patient mounted (PSIS or spinous process clamp) DRB as well as a DRB on the robotic end effector after 3D iso-centric fluoroscopic image acquisition. An optical camera station tracks the relative positions of the patient and robotic arm. A third and separate CAN station houses the interface for screw planning and visual feedback. The end effector holds a guide tube for pedicle cannulation and implantation of a guidewire. An optical navigation pointer can be used to check trajectories and anatomical landmarks in real time. Pedicle screws can then be placed over this guidewire and implanted with standard techniques (31). This robot can be used for other orthopedic applications as well (32,33).
Analysis of platform nuances and features
These current navigated robotic platforms all implement optical registration and tracking with 3D imaging for pedicle screw trajectory planning and real-time visual feedback. Although the original ROSA spine robot and the TiRobot are capable of real-time 3D navigation with optical pointer tracking, their systems are not capable of full k-wireless 3D image-guided navigation of instrumentation. Both robotic arms have end effector guide tubes that facilitate non-navigated drilling and subsequent placement of a guidewire. Pedicle tapping and screw implantation can then be performed with navigated instruments using attachable tracker arrays. The upgraded ROSA One should be capable of integrated k-wireless 3D guided robotic screw placement, but there is no published literature or media about it at the time of publication. The Mazor X Stealth Edition and Globus Excelsius both have fully independent navigation capabilities, which are unified with their own manufacturer specific instrumentation and allow for K-wireless screw placement.
Unlike the other platforms, which utilize optical tracking for both robot/patient co-localization and 3D navigation registration, the Mazor X Stealth Edition interfaces with the patient directly. As previously described, the robot is mounted to both the patient and the bed. Additionally, the fiducial marker for the robot arm is removed after registration. Together, this allows the robot to move with the patient and bed during respiration and surgical manipulation while still maintaining the target trajectory. This process is not dependent on optical tracking arrays that are susceptible to line-of-sight obscurations or inadvertent DRB movement.
Maintaining accuracy of registration is of utmost importance in 3D navigated spine surgery (30,34). The current navigated robotic platforms all have features to maintain navigation accuracy. The Rosa and TIRobot systems optically track and facilitate real-time end effector compensation for patient movement during respiration and surgical manipulation (27-29,31,35). The Excelsius GPS also employs additional unique features to ensure navigation integrity (30,36). To address unintentional DRB shift, which can go unnoticed and result in screw mal-positioning, an additional optical array with just one reflective marker ball is placed into the contralateral PSIS. Continuously updated in real-time, this surveillance marker serves to detect an offset greater than 1.0 mm from the DRB, which triggers an alert automatically. Additionally, the Excelsius GPS robotic arm is engineered to be rigid, capable of maintaining less than 1.0 mm of deflection under a lateral force of 200 N (30). For unintended shift of the DRB, The Mazor X Stealth Edition features a rapid navigation re-registration feature without the need for additional O-arm image acquisition by referencing the original robotic guidance registration. In addition, the navigation DRB is placed on the base of the robot arm and is physically far from the operative field, reducing the chances of accidental displacement.
Skiving, resulting in lateral pedicle breach, is a well described phenomenon in robotic spine surgery. This occurs when the instrument-bone interface loses orthogonality due to a medial entry point with a lateral trajectory (6,16,22,24). To address this issue, the Globus Excelsius end effector was developed with a unique sensor that detects excessive lateral forces generated by instrument skive (30). Visualization of instrument deflection by real time navigation can also help mitigate skive.
It is important to note that the ROSA One and TINAVI TiRobot are both unified platforms designed for use in multiple neurosurgery and orthopedic subspecialties. This ensures efficient resource utilization considering the high capital acquisition cost of these machines.
Potential future adjunctive navigation modalities
As digital optics continue to improve, high resolution images of the surgical field may be used to register robotic platforms to pre-operative imaging rather than intra-operative fluoroscopy. 7D Surgical (Toronto, ON, Canada) implements digital stereoscopic topographical referencing of exposed bony elements for point-paired surface matching with a pre-operative CT. This allows for rapid registration, elimination of intraoperative radiation exposure, and ease of re-registration. One of the most significant disadvantages of this technology is the technical inability, at the current time, to utilize this method for MIS cases where minimal bony anatomy is exposed.
Augmented reality (AR) has generated significant interest within spine surgery (37). Augmedics Xvision System (Chicago, IL, USA), is a recently FDA approved AR platform that allows a 3D en face “tip of the spear” view of the bony anatomy to be projected to a user-worn translucent heads-up display. This platform allows the surgeon to maintain line-of-sight to the surgical field and all instruments while receiving 3D navigation feedback in real time. This study, which specifically evaluated radiation exposure with the use of this technology for pedicle screw placement showed that the use of AR surgical navigation resulted in minimal radiation exposure to operating room and staff, whereas the radiation exposure to the patient was equivalent to that reported in prior studies using intra-operative CT-based navigation platforms. Other outcomes including operative time, intra- or post-operative complications, accuracy of screw placement, etc. were not reported in this study.
Pedicle screw accuracy in current platforms
The literature regarding pedicle screw accuracy with these navigated platforms is relatively sparse at the current time. No literature exists yet on the pedicle screw accuracy with either the Stealth Edition of Mazor X or the Rosa One Spine platform, but one can infer that the rates will be similar to existing studies that report 100% Ravi Grades A or B (6,7,38).
The original Rosa Spine robot has achieved Gertzbein-Robbin (GR) grades A or B combined accuracy rates of 96.3%, 97.3%, and 98.3% in three different studies (27-29,39).
The Excelsius GPS has been the subject of two large retrospective reviews. Jain et al. reported no screw related complications or returns to the operating room with 643 screws placed. Of the 66 screws that were reviewed with post-operative CT scanning, 100% of those were categorized as GR A or B (40). In their series of 600 screws all reviewed with post-operative CT, Wallace et al. reported a GR A or B grade for 98.2% of the screws. Grade C and D designations were given to 1.5% and 0.3% of screws. These were all laterally breached, and were repositioned successfully by utilizing a more lateral entry point and medial trajectory. The offset from the plan to the final position of the tips and tails of the screws were 1.7±1.3 and 1.8±1.2 mm, respectively. Deviation from planned angle was 2.0±1.6 degrees (36). Elswick et al. also reported 97.6% GR grades A and B for their study involving 125 screws (41).
The TiRobot has been compared to fluoro-guided free hand (FFH) screw placement in a large randomized prospective trial by Han et al. (31). A total of 1,116 screws were placed between the two groups, 532 in the robot guided (RG) cohort and 584 in the FFH group. The TiRobot demonstrated superior results across the board. Gertzbein-Robbin accuracy was 95.3% grade A and 98.7% combined grades A and B for the RG group versus 86.1% grade A and 93.5% grades A and B for the FFH group. Radiation exposure and blood loss were significantly lower in the robot guided group. There were no proximal facet violations in the RG group compared to 2.1% violation in the FFH group. Surgical times between the two groups were not statistically different.
Although no accuracy studies exist for robot navigated pedicle screws placed in the lateral position for single position circumferential thoracolumbar surgery, these data are undoubtedly coming (42). Navigated robotic placement of cortical trajectory lumbar screws has also been reported (43).
Radiation exposure in navigated spine robots
Although non-navigated robotic systems are not commonly utilized, previous studies have shown a significant decrease in radiation exposure compared to fluoroscopic-assisted pedicle screw implantation techniques (15,17,18,44). In a prospective randomized trial of FFH versus 3D navigation versus RG pedicle screw placement, Roser et al. reported half the radiation dose in the RG group vs. FFH. Interestingly, there was even less ionizing radiation emitted in the 3D navigation group, with 35% decreased exposure compared to the RG (44). This could be explained by the obviation of fluoroscopic guidance for depth control with real-time navigation. Still, conflicting evidence exists with Khan et al. reporting higher radiation exposure in the 3D navigation group compared to the non-navigated RG (7). Future studies comparing navigated robotic cohorts to non-navigated robotic cohorts, as well as those comparing navigated robotic cohorts to non-robotic CAN will help further define this potential advantage.
Learning curve
Studies have shown little difference between novice and experienced surgeons with respect to procedural efficiency in placing robotically guided pedicle screws; despite this, a trend towards increasing efficiency after the performance of a certain number of cases or placement of a certain number of screws placed has been reported (45,46). The only study in a full navigation enabled robotic platform that comments on learning curve showed no difference between the attending and the first participating fellow. They both needed to place 30 screws before a noticeable improvement in efficiency was quantified. Notably, the subsequent fellow in that study, now learning from the attending surgeon with newly acquired robotic experience, demonstrated immediate and sustained superior performance compared to the attending, suggesting that the learning curve is easily transferable through observation (47). The visual feedback from real-time navigation could be responsible for this observation. However, further investigation is required to fully understand the learning process of adoption of robotic surgery in practice and how the surgeons’ prior experience with other image guidance and CAN modalities impact this.
Other applications of navigated robotic guidance
Navigated cervical spine applications including successful placement of odontoid screw (48) and C1-2 trans-articular screws (49) have been reported of the TiRobot platform only, but not any of the other robots currently in use. In both the above reports, the DRB was placed on the Mayfield head fixation clamp.
Navigated robot-assisted laminectomy/osteotomy with full decompression will inevitably be a part of the future, with biomechanical studies having been conducted for this application (50,51). These preliminary studies demonstrate that the use of a robot for this purpose has the potential to provide stable and steady bony decompression, with the ability to program the robot to discontinue further drilling when the pre-planned thickness of remaining bone has been achieved in order to prevent complete penetration of the lamina. We envision that this could be implemented by using supervisory-control for bony drilling with automatic disengagement a few millimeters proximal to a predefined 3D area of interest such as the boundaries of the spinal canal. The surgeon could then resume control and complete the remainder of the decompression manually with navigated robotic assistance to avoid critical neural elements. Similarly, robot-guided osteotomies for en-bloc resection of primary spinal column tumors have already been reported for non-navigated spine robots. While these initial studies are promising, the evidence is currently limited due to the small number of cases reported. The integration of navigation with robotics will likely promote an increase in their utilization in this arena given the ability to determine depth with real-time navigation (52,53). This should theoretically improve procedural efficiency and safety.
The strenuous physical demands of surgery also pose a significant occupational risk to surgeons (54). A robotic system with 3D navigation and real-time feedback with potential to perform automated sub-periosteal spine exposure may relieve manual hand strain. Automated pedicle screw trajectory planning (55) and automated insertion could be pre-programmed for execution by the robotic system. The platforms might also integrate tele-robotic control, allowing the surgeon to perform the operation by remote control in an ergonomically optimized command station. Although the use of the DaVinci robot for posterior spine approaches in a porcine model for non-instrumented spinal surgery was reported over a decade ago (56), this platform did not gain traction for spinal surgery likely related to limitations with hardware and software. However, given the recent advances in and increased adoption of other robotic systems for spine surgery, a platform similar to the DaVinci robot, which allows direct translation of real-time surgeon input to robot output may be revisited.
Conclusions
Although spine surgery robots were introduced nearly 20 years ago, their adoption was limited due to a number of factors. Meanwhile, the popularity of 3D navigation in spine surgery has grown considerably. The combination of robotics with real-time 3D navigation has led to a resurgence in the interest of these new robotic platforms and their potential future applications beyond pedicle screw insertion. Initial studies have shown that the use of technology is feasible, safe and effective. It has the potential to decrease occupational radiation exposure, improve accuracy of instrumentation, allow for more efficient surgery, and alleviate some of the physical and mental demands of surgery for the surgeon. Additionally, while a majority of current studies report on the use of this technology for pedicle screw instrumentation, numerous other applications are being explored. This will further add to the utility, versatility and cost-effectiveness of these platforms, and allow for more widespread use. Further studies are necessary to determine the efficacy, efficiency, safety and value of these new combined robotic navigation systems.
Acknowledgments
We would like to acknowledge Kristopher Barberio, DC and Summer Shedd for providing technical educational resources pertaining to current robotic platforms.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Dr. Sheeraz Qureshi) for the series “Current State of Intraoperative Imaging” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-2020-ioi-07). The series “Current State of Intraoperative Imaging” was commissioned by the editorial office without any funding or sponsorship. BNS reports personal fees from Medtronic, outside the submitted work and is a member of the Medtronic Robotics Advisory Board. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Roy-Camille R, Mazel C, Saillant G. Internal Fixation of the Lumbar Spine with Pedicle Screw Plating. Clin Orthop Relat Res 1986.7-17. [Crossref] [PubMed]
- Holly LT, Foley KT. Intraoperative Spinal Navigation. Spine (Phila Pa 1976) 2003;28:S54-61. [Crossref] [PubMed]
- Schwarzenbach O, Berlemann U, Jost B, et al. Accuracy of computer-assisted pedicle screw placement. Spine (Phila Pa 1976) 1997;22:452-8. [Crossref] [PubMed]
- Nolte LP, Visarius H, Arm E, et al. Computer-Aided Fixation of Spinal Implants. J Image Guid Surg 1995;1:88-93. [Crossref] [PubMed]
- Grunert P, Darabi K, Espinosa J, et al. Computer-aided navigation in neurosurgery. Neurosurg Rev 2003;26:73-99. [Crossref] [PubMed]
- Khan A, Meyers JE, Siasios I, et al. Next-Generation Robotic Spine Surgery: First Report on Feasibility, Safety, and Learning Curve. Oper Neurosurg (Hagerstown) 2019;17:61-9. [Crossref] [PubMed]
- Khan A, Meyers JE, Yavorek S, et al. Comparing Next-Generation Robotic Technology with 3-Dimensional Computed Tomography Navigation Technology for the Insertion of Posterior Pedicle Screws. World Neurosurg 2019;123:e474-81. [Crossref] [PubMed]
- Murphy MA, McKenzie RL, Kormos DW, et al. Frameless stereotaxis for the insertion of lumbar pedicle screws. J Clin Neurosci 1994;1:257-60. [Crossref] [PubMed]
- Kalfas IH, Kormos DW, Murphy MA, et al. Application of frameless stereotaxy to pedicle screw fixation of the spine. J Neurosurg 1995;83:641-7. [Crossref] [PubMed]
- Gelalis ID, Paschos NK, Pakos EE, et al. Accuracy of pedicle screw placement: a systematic review of prospective in vivo studies comparing free hand, fluoroscopy guidance and navigation techniques. Eur Spine J 2012;21:247-55. [Crossref] [PubMed]
- Kosmopoulos V, Schizas C. Pedicle Screw Placement Accuracy. Spine (Phila Pa 1976) 2007;32:E111-20. [Crossref] [PubMed]
- Verma R, Krishan S, Haendlmayer K, et al. Functional outcome of computer-assisted spinal pedicle screw placement: a systematic review and meta-analysis of 23 studies including 5,992 pedicle screws. Eur Spine J 2010;19:370-5. [Crossref] [PubMed]
- Tian NF, Xu HZ. Image-guided pedicle screw insertion accuracy: a meta-analysis. Int Orthop 2009;33:895-903. [Crossref] [PubMed]
- Devito DP, Kaplan L, Dietl R, et al. Clinical Acceptance and Accuracy Assessment of Spinal Implants Guided With SpineAssist Surgical Robot. Spine (Phila Pa 1976) 2010;35:2109-15. [Crossref] [PubMed]
- Lieberman IH, Hardenbrook MA, Wang JC, et al. Assessment of Pedicle Screw Placement Accuracy, Procedure Time, and Radiation Exposure Using a Miniature Robotic Guidance System. J Spinal Disord Tech 2012;25:241-8. [Crossref] [PubMed]
- D’Souza M, Gendreau J, Feng A, et al. Robotic-Assisted Spine Surgery: History, Efficacy, Cost, And Future Trends Robot Surg 2019;6:9-23. [published correction appears in Robot Surg 2019 Dec 23;6:25]. [Crossref] [PubMed]
- Hu X, Ohnmeiss DD, Lieberman IH. Robotic-assisted pedicle screw placement: lessons learned from the first 102 patients. Eur Spine J 2013;22:661-6. [Crossref] [PubMed]
- Kantelhardt SR, Martinez R, Baerwinkel S, et al. Perioperative course and accuracy of screw positioning in conventional, open robotic-guided and percutaneous robotic-guided, pedicle screw placement. Eur Spine J 2011;20:860-8. [Crossref] [PubMed]
- Nathoo N, Çavuşoğlu MC, Vogelbaum MA, et al. In Touch with Robotics: Neurosurgery for the Future. Neurosurgery 2005;56:421-33. [Crossref] [PubMed]
- Adler JR, Murphy MJ, Chang SD, et al. Image-guided robotic radiosurgery. Neurosurgery 1999;44:1299-306. [PubMed]
- Bowersox JC, Cornum RL. Remote operative urology using a surgical telemanipulator system: preliminary observations. Urology 1998;52:17-22. [Crossref] [PubMed]
- Ringel F, Stüer C, Reinke A, et al. Accuracy of Robot-Assisted Placement of Lumbar and Sacral Pedicle Screws. Spine (Phila Pa 1976) 2012;37:E496-501. [Crossref] [PubMed]
- Schizas C, Michel J, Kosmopoulos V, et al. Computer tomography assessment of pedicle screw insertion in percutaneous posterior transpedicular stabilization. Eur Spine J 2007;16:613-7. [Crossref] [PubMed]
- Kochanski RB, Lombardi JM, Laratta JL, et al. Image-Guided Navigation and Robotics in Spine Surgery. Neurosurgery 2019;84:1179-89. [Crossref] [PubMed]
- Du JP, Fan Y, Wu QN, et al. Accuracy of Pedicle Screw Insertion Among 3 Image-Guided Navigation Systems: Systematic Review and Meta-Analysis. World Neurosurg 2018;109:24-30. [Crossref] [PubMed]
- Tian NF, Huang QS, Zhou P, et al. Pedicle screw insertion accuracy with different assisted methods: a systematic review and meta-analysis of comparative studies. Eur Spine J 2011;20:846-59. [Crossref] [PubMed]
- Lonjon N, Chan-Seng E, Costalat V, et al. Robot-assisted spine surgery: feasibility study through a prospective case-matched analysis. Eur Spine J 2016;25:947-55. [Crossref] [PubMed]
- Lefranc M, Peltier J. Evaluation of the ROSATM Spine robot for minimally invasive surgical procedures. Expert Rev Med Devices 2016;13:899-906. [Crossref] [PubMed]
- Chenin L, Capel C, Fichten A, et al. Evaluation of Screw Placement Accuracy in Circumferential Lumbar Arthrodesis Using Robotic Assistance and Intraoperative Flat-Panel Computed Tomography. World Neurosurg 2017;105:86-94. [Crossref] [PubMed]
- Crawford N, Johnson N, Theodore N. Ensuring navigation integrity using robotics in spine surgery. J Robot Surg 2020;14:177-83. [Crossref] [PubMed]
- Han X, Tian W, Liu Y, et al. Safety and accuracy of robot-assisted versus fluoroscopy-assisted pedicle screw insertion in thoracolumbar spinal surgery: a prospective randomized controlled trial. J Neurosurg Spine 2019;30:1-8. [Crossref] [PubMed]
- Wang JQ, Wang Y, Feng Y, et al. Percutaneous Sacroiliac Screw Placement: A Prospective Randomized Comparison of Robot-assisted Navigation Procedures with a Conventional Technique. Chin Med J (Engl) 2017;130:2527-34. [Crossref] [PubMed]
- Tian W, Han X, Liu B, et al. A robot-assisted surgical system using a force-image control method for pedicle screw insertion. PLoS One 2014;9:e86346. [Crossref] [PubMed]
- Rahmathulla G, Nottmeier EW, Pirris SM, et al. Intraoperative image-guided spinal navigation: technical pitfalls and their avoidance. Neurosurg Focus 2014;36:E3. [Crossref] [PubMed]
- Zhang Q, Han XG, Xu YF, et al. Robotic navigation during spine surgery. Expert Rev Med Devices 2020;17:27-32. [Crossref] [PubMed]
- Wallace DJ, Vardiman AB, Booher GA, et al. Navigated Robotic Assistance Improves Pedicle Screw Accuracy in Minimally Invasive Surgery of the Lumbosacral Spine: 600 Pedicle Screws in a Single Institution. Int J Med Robot 2020;16:e2054. [Crossref] [PubMed]
- Edström E, Burström G, Omar A, et al. Augmented Reality Surgical Navigation in Spine Surgery to Minimize Staff Radiation Exposure. Spine (Phila Pa 1976) 2020;45:E45-53. [Crossref] [PubMed]
- Ravi B, Zahrai A, Rampersaud R. Clinical accuracy of computer-assisted two-dimensional fluoroscopy for the percutaneous placement of lumbosacral pedicle screws. Spine (Phila Pa 1976) 2011;36:84-91. [Crossref] [PubMed]
- Lonjon N, Russo V, Barbarisi M, et al. Spinal Cervical Meningiomas: The Challenge Posed by Ventral Location. World Neurosurg 2016;89:464-73. [Crossref] [PubMed]
- Jain D, Manning J, Lord E, et al. Initial Single-Institution Experience With a Novel Robotic-Navigation System for Thoracolumbar Pedicle Screw and Pelvic Screw Placement With 643 Screws. Int J Spine Surg 2019;13:459-63. [Crossref] [PubMed]
- Elswick CM, Strong MJ, Joseph JR, et al. Robotic-Assisted Spinal Surgery: Current Generation Instrumentation and New Applications. Neurosurg Clin N Am 2020;31:103-10. [Crossref] [PubMed]
- Quiceno E, Hartman C, Godzik J, et al. Single position spinal surgery for the treatment of grade II spondylolisthesis: A technical note. J Clin Neurosci 2019;65:145-7. [Crossref] [PubMed]
- Agyei JO, Khan A, Jowdy PK, et al. Robot-Assisted Cortical Bone Trajectory Insertion of Pedicle Screws: 2-Dimensional Operative Video. Oper Neurosurg (Hagerstown) 2020;18:E171. [Crossref] [PubMed]
- Roser F, Tatagiba M, Maier G. Spinal Robotics. Neurosurgery 2013;72 Suppl 1:12-8. [Crossref] [PubMed]
- Urakov TM, Chang KH, Burks SS, et al. Initial academic experience and learning curve with robotic spine instrumentation. Neurosurg Focus 2017;42:E4. [Crossref] [PubMed]
- Kam JKT, Gan C, Dimou S, et al. Learning Curve for Robot-Assisted Percutaneous Pedicle Screw Placement in Thoracolumbar Surgery. Asian Spine J 2019;13:920-7. [Crossref] [PubMed]
- Siddiqui MI, Wallace DJ, Salazar LM, et al. Robot-Assisted Pedicle Screw Placement: Learning Curve Experience. World Neurosurg 2019;130:e417-22. [Crossref] [PubMed]
- Tian W, Wang H, Liu YJ. Robot-assisted Anterior Odontoid Screw Fixation: A Case Report. Orthop Surg 2016;8:400-4. [Crossref] [PubMed]
- Tian W. Robot-Assisted Posterior C1-2 Transarticular Screw Fixation for Atlantoaxial Instability: A Case Report. Spine (Phila Pa 1976) 2016;41 Suppl 19:B2-5. [Crossref] [PubMed]
- Wang T, Luan S, Hu L, et al. Force-based control of a compact spinal milling robot. Int J Med Robot 2010;6:178-85. [Crossref] [PubMed]
- Qi X, Sun Y, Ma X, et al. Multilevel Fuzzy Control Based on Force Information in Robot-Assisted Decompressive Laminectomy. Adv Exp Med Biol 2018;1093:263-79. [Crossref] [PubMed]
- Bederman SS, Lopez G, Ji T, et al. Robotic guidance for en bloc sacrectomy: a case report. Spine (Phila Pa 1976) 2014;39:E1398-401. [Crossref] [PubMed]
- Trybula SJ, Oyon DE, Wolinsky JP. Robotic Tissue Manipulation and Resection in Spine Surgery. Neurosurg Clin N Am 2020;31:121-9. [Crossref] [PubMed]
- Memon AG, Naeem Z, Zaman A, et al. Occupational Health Related Concerns among Surgeons. Int J Health Sci (Qassim) 2016;10:279-91. [Crossref] [PubMed]
- Vijayan R, Silva TD, Han R, et al. Automatic pedicle screw planning using atlas-based registration of anatomy and reference trajectories. Phys Med Biol 2019;64:165020. [Crossref] [PubMed]
- Ponnusamy K, Chewning S, Mohr C. Robotic Approaches to the Posterior Spine. Spine (Phila Pa 1976) 2009;34:2104-9. [Crossref] [PubMed]



