Analysis of the clinical characteristics of 176 patients with pathologically confirmed cryptogenic organizing pneumonia
Introduction
Cryptogenic organizing pneumonia (COP), which was formerly termed idiopathic bronchiolitis obliterans with organizing pneumonia (iBOOP), is typically diagnosed based on based on the presence of pathological organizing pneumonia (OP) and the absence of the etiological clinical and medical characteristics of OP (1). COP is an idiopathic interstitial pneumonia (IIP) and is diagnosed by clinical-radiologic-pathologic (CRP) diagnosis (1). In the 2002 IIP Consensus of the American Thoracic Society (ATS) and European Respiratory Society (ERS), it was listed as having the third-highest incidence of any IIP (2). The 2013 IIP Consensus of the ATS and ERS revealed COP to be the fifth most common type of IIP (3). Earlier studies found the incidence rate of OP to be 6–7 per 100,000 people (4), with COP accounting for more than half of OP cases (5). The recent incidence rate of COP was not available (6).
In the last two decades, the number of COP cases has been reported to have gradually increased, which may be related to the more frequent use of lung biopsy. In 2011, Yoo et al. reported 76 COP cases (7), and Yilmaz et al. described 100 COP cases in 2017 (8). Those studies were the most recent literature with large patient groups. The condition is called “cryptogenic” because in most cases, the cause is unknown. Studied have shown that there are many possible causes including radiation therapy, exposure to certain chemicals, post respiratory infections, as a side-effect of organ transplantation or as a side effect from taking certain medications (9-12). Therefore, COP is only diagnosed when all other possible causes of pneumonia have been eliminated.
This study retrospectively analyzed the clinical data of 1,346 patients with OP who were diagnosed by lung biopsy in our hospital between January 1, 2000, and December 31, 2013. After undergoing strict CRP diagnostic consideration, 176 patients were confirmed to have COP and followed up to December 31, 2017. We aim to summarize the clinical, imaging, pathological, and prognostic characteristics of 176 patients with confirmed COP. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4490).
Methods
Study design
This was a retrospective observational study. The study was conducted in accordance with the Declaration of Helsinki. The study protocols were approved by the Institutional Review Boards of Shanghai Pulmonary Hospital (Approval No: k15-192). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Diagnostic criteria
COP was diagnosed according to the 2013 ATS guidelines (3). The diagnosis of COP requires multidisciplinary CRP consideration, known causes of OP to be eliminated, and other possible diseases, such as infectious/non-infectious granulomatous diseases, connective tissue diseases, allergic pneumonia, tumors, and occupational pneumoconiosis (examined by polarized light microscopy), to be excluded. If secondary causes of OP were identified during follow-up, the diagnosis was promptly revised. Patients who showed symptoms such as Raynaud phenomenon, heliotrope rash, mechanic hands, erosive arthritis, telangiectasias calcinosis, erosive Gottron’s papules, or periungual erythema, were referred to the Department of Rheumatology to rule out connective tissue disease.
Study participants
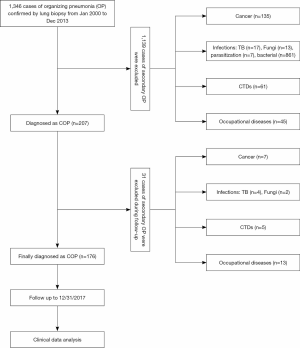
A total of 1,346 patients with a confirmed diagnosis of OP who were treated in Shanghai Pulmonary Hospital between January, 2000, and December, 2013, were screened. The clinical presentations, imaging examination results, pathological features, and laboratory test results of each patient were independently reviewed by 3 pulmonologists, 3 radiologists, and 3 pathologists. The patient screening process is displayed in Figure 1. A total of 207 cases were diagnosed as COP. Each case was followed up by telephone or clinic visits. Among them, 31 patients were found to have secondary OP during the follow-up and thus were excluded. Therefore, only 176 patients were diagnosed with COP and were followed up until December 31, 2017.
Imaging examination
The patients underwent chest X-ray and chest CT examination. Each patient had at least two imaging examinations: one before treatment and at least one after treatment.
Pulmonary function test
Pulmonary ventilation function was estimated using the percentage of the first second forced expiratory volume (FEV1) over the predicted value (FEV1%). Lung capacity was estimated using the percentage of residual gas volume over the total lung volume. Diffusion function was estimated using the percentage of diffusion of carbon monoxide (DLCO) over the predicted value (DLCO%).
Arterial blood gas analyses
The arterial partial pressure of oxygen (PaO2), arterial carbon dioxide partial pressure (PaCO2), arterial oxygen saturation (SaO2), and blood pH (pH) of the patients were measured.
Blood tests
The patients regularly underwent routine blood tests, blood biochemical examinations, rheumatoid factor tests, and other autoimmune antibody tests and the result were negative. [Including anti-centromere antibodies, perinuclear and cytoplasmic anti-neutrophil cytoplasmic antibodies, indirect immunofluorescence anti-nuclear antibody detection, immunoblotting to detect ENA polypeptide antibodies (anti-Sm antibody, anti-U1RNP antibody, anti-Scl-70 antibody, anti-ribosomal antibody), anti-cardiolipin antibody, and serum immunoglobulin electrophoresis].
Other tests
Sputum and bronchoalveolar lavage fluid (BALF) samples were sent for cytologic evaluation and culture to exclude tuberculosis, tumor, and other potential infectious diseases. And all of the results were negative.
Pathology
All lung specimens underwent hematoxylin and eosin (H&E) staining, and the pathological changes associated with OP were observed under light microscopy. The specimens also underwent special acid staining, hexamine silver staining, reticular fiber staining, and Periodic Acid-Schiff (PAS) stain for observation under a polarized light microscope.
Treatment and criteria for therapeutic efficacy evaluation
Treatments included glucocorticoid treatment (Figure 2) and other supplementary therapies. All patients were followed up after surgery.
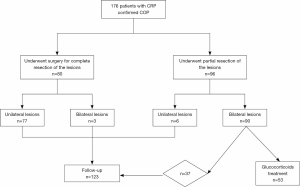
In 80 cases, the patient showed no obvious symptoms at the time of disease onset but lung lesions were detected by high-resolution chest CT during routine physical examination. These patients underwent video-assisted thoracoscopic surgery (VATS) for complete resection of their lesions (77 cases of unilateral resection and 3 cases of bilateral resection). OP was confirmed by surgical pathology.
There were 96 patients [6 cases of unilateral lesions (ULs) and 90 cases of bilateral lesions (BLs)] who underwent partial resection of their lesions. These patients underwent surgery, transbronchial lung biopsy (TBLB), or percutaneous needle lung biopsy (PNLB). Of these 96 cases, 43 (6 ULs and 37 BLs) cases presented with no prominent symptoms. The post-treatment residue lesions of these patients involved a limited area, and they were followed up after surgery. The other 53 patients had BLs and presented with apparent symptoms or had extensive lesions shown on chest CT. These patients underwent steroid therapy after partial resection: 36 out of 53 patients began steroid therapy immediately after diagnosis, and 17 cases whose condition was shown by CT examination or pulmonary function tests to have progressed commenced steroid therapy during follow-up.
Statistical analyses
All data were analyzed using the IBM SPSS Statistics 20.0 (SPSS, Inc., Chicago, IL). Continuous variables were presented as mean ± standard deviation (SD) and were compared using Student’s t-test. The chi-square test was used for the categorical variables. A P value of <0.05 was considered to be statistically significant.
Results
General clinical data
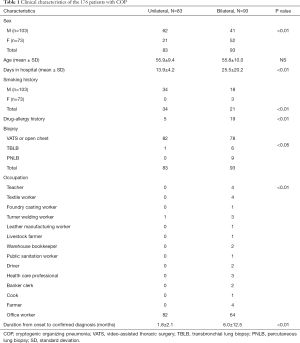
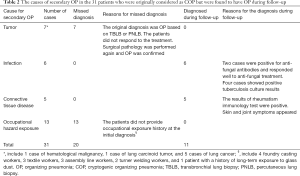
The general clinical information of the patients is displayed in Tables 1 and 2. Of the 176 patients with COP (103 men, aged 19–78 years), 160 underwent thoracoscopy or VATS, and 9 had PNLB and 7 had TBLB. All pathological specimens were re-examined by pathologists to exclude secondary OP. Of the patients, 17 patients had an exposure history to industrial dust, 26 had a history of allergies, and 55 had a smoking history. The patients had an extremely diverse range of occupations. The duration from the onset of symptoms to confirmed diagnosis ranged from 1–72 months (average: 4.0±9.4 months; median: 1.5 months, Table 1). The length of hospital stay ranged from 7–96 days (average: 20.2±16.2 days). The follow-up time ranged from 51–215 months. There were 31 patients who were originally diagnosed with COP who were found to have secondary OP during follow-up; their diagnoses were subsequently amended to secondary OP (Table 2).
Full table
Full table
Imaging presentations
All of the patients in this study received chest X-ray and chest CT. Chest CT examination of the patients with COP showed diverse patterns (Figure 3). The most common patterns were patchy ground-glass opacity (63.6%), consolidation (with or without air bronchogram, 56.8%), nodules (39.8%), and fibrous stripes (39.2%), and 6.8% (12/176) had a reversed halo sign (Table 3). Also, 65.3% of the patients showed mixed patterns. Patchy ground-glass lesions, fibrous stripes, and mixed types of lesions were seen more commonly in cases with BLs than in cases with ULs. Cases with ULs comprised 47% of the study population, which might be attributable to the majority of cases being identified by lung biopsy.
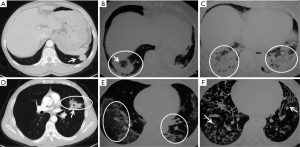
Full table
Lesions were found in the upper, middle, and lower lungs of the patients; however, upper lung lesions were 38.7% (117/302). The distribution of lesions was not substantially different between the left and the right lung. Half of the patients had involvement of the pleura, which was more common in cases with BLs than in cases with ULs. Most of the lesions (59.5%, 50/84) were migratory, especially in patients with BLs (84%, 42/50).
Histopathology
Light microscopy revealed that the most typical pathological change was patchy granulomatous polypoid hyperplasia in the distal airways and alveolar cavity along the small airways (Figure 4A). The contents of the alveoli were composed of loose collagen and fusiform cells, and the alveoli had clear boundaries. The tissue surrounding the alveoli showed chronic inflammatory cell infiltration (Figure 4B). Lymphocyte and plasma cell infiltration was observed in the interalveolar space of the lesion area. Type II alveolar epithelial hyperplasia was also seen. Some cases exhibited fibrous tissue hyperplasia to varying degrees. However, severe fibrosis and honeycomb lung were rarely seen.
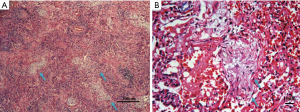
Acid-fast staining, hexamine silver staining, reticular fiber staining, and PAS staining were performed on all of the tissue specimens. Then, the specimens were examined with polarized light microscopy and showed no significant dust particle deposition. Tuberculosis, fungal infection, and tumors were ruled out. One patient with severe COP presented clinical and radiographic changes similar to those of usual interstitial pneumonia (UIP); however, the patient’s biopsy showed characteristics typical of OP. The histopathology of this patient also revealed fibrosis and honeycomb lung.
Pulmonary function and arterial blood gas status
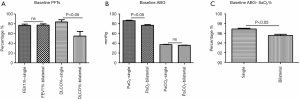
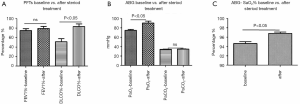
The baseline lung function test results showed mild airway dysfunction. The mean FEV1% was 77.4%±1.7%, and there was no statistical difference between the patients with ULs and BLs. The patients with BLs had significantly lower DLCO% (P<0.05, Figure 5A), lower PaO2 (77.3±1.2 mmHg, P<0.05, Figure 5B), and lower SaO2% (P<0.05, Figure 5C) than the patients with ULs. The other parameters were similar in the two groups. For the 53 patients who received steroid therapies, the DLCO% was improved after the treatment (83.4%±6.0% vs. 51.8%±6.4%, P<0.05, Figure 6A), as were their PaO2 (90.7±4.1 vs. 75.2±1.8 mmHg, P<0.05, Figure 6B) and SaO2% (96.8%±0.3% vs. 94.7%±0.4%, P<0.05, Figure 6C).
Rheumatology blood test, BALF, and other test results
The results of an autoimmune antibody test were negative for all patients. BALF samples were cultured and used for smear tests. No tumor cells, tubercle bacilli, or fungi were found, and the smear tests were all negative. Lymphocyte subtype, serum immunoglobulin, urine, fecal examination, and routine blood biochemistry tests produced normal results. No acid-fast bacilli or cancer cells were found in the sputum samples.
Therapeutic outcomes
The therapeutic outcomes of the patients are displayed in Table 4. All 80 patients who received complete resection were radically cured. Of the 96 patients who received partial resection, the follow-up examination results showed that 43 had been cured. The remaining 53 patients were treated with steroids and their conditions improved to various extents within one week after the treatment. Of these 53 patients, 35 had COP recurrence when the dose of steroid was reduced or stopped, and unfortunately, 3 patients died. The overall 5-year survival rate of our patient cohort was 98.3%.

Full table
Discussion
Pathological biopsy is not commonly performed in patients with COP, and it is challenging to clinically distinguish COP from the secondary OP. Consequently, COP is often misdiagnosed as pulmonary infection, tuberculosis, or cancer (7). The increasing application of pathological biopsy in current practice may leads to the increasing number of COP cases. In 2017, Yilmaz et al. conducted a comparative study on a group of 65 patients with pathologically confirmed secondary OP and 100 patients with COP (8). They retrospectively compared the clinical and imaging characteristics of the two patient groups and concluded that COP and secondary OP display similar imaging features on CT scan. However, their study was limited by a lack of follow-up data, treatment information, and outcome results. In 2011, Yoo et al. also reported a comparative study of 76 cases of COP and 24 cases of connective tissue disease-related OP (CTD-OP). Their findings suggested that the clinical features and prognosis of CTD-OP are similar to COP, however, lower complete recovery rate with a tendency towards higher recurrence rate in CTD-OP compared with COP (7).
The current study included 1,346 cases of the pathologically diagnosed OP, of which 176 cases of COP were diagnosed by the CRP method. Although it is a retrospective study, to our knowledge, the current study involved the largest patient group from a single-center with the longest follow-up time.
The majority of the patients in our study were male, which is consistent with the previous report (1), and were aged in the range of 50–60 years old. Some the patients had histories of drug allergies (26/176), exposure to industrial dust (17/176), and smoking (52/176), which indicated that COP initiation might be associated with dust, allergy, and smoking. Various conditions or factors can cause the pathological changes associated with OP or BOOP (13); these include infection (fungus, tuberculosis, parasites, and viruses), drugs, occupational hazard exposure, chest radiotherapy, organ transplantation, connective tissue diseases, and tumors. Furthermore, COP initiation has been increasingly found to be associated with environmental factors (9-12).
In our study, 207 COP cases were initially identified; however, during follow-up, 31 of them were diagnosed with secondary OP. It included 7 cases of pulmonary tumor, 4 cases of pulmonary tuberculosis, and 2 cases of pulmonary fungal infection. Meanwhile, 13 cases had occupational diseases and 5 cases had connective tissue disease. Among the 176 cases of COP, 17 patients had a history of exposure to industrial dust. These findings suggest a possible correlation between occupational hazard exposure and COP. Our results indicate that COP could be a self-limiting condition, and to confirm its diagnosis, patients may require long-term follow-up to exclude secondary OP.
COP onset in our patients was mostly subacute. The initial diagnosis for most of these patients was a pulmonary infection for which antibiotics were ineffective. No unique patterns were revealed in the patients’ clinical presentations, which is consistent with the report by Cordier (1). In our patient cohort, 53% had bilateral lesions and the imaging characteristics of these lesions were multiple, polymorphic (patchy, consolidation, lumps, and/or fibrous strips), migratory, and recurrent, which was consistent with the lesions described in previous studies (1,4,5). Nodules with a reversed halo sign were seen in 12 of 176 cases. Moreover, 47% of our patients had ULs, which was relatively higher than the percentage previously reported (14). The possible reason for the discrepancy may be related to the increasing use of surgical biopsy in patients with such lesions. The ULs were mostly focal consolidation and some were hollow. The majority of the patients with such lesions underwent surgery for suspected lung cancer or tuberculosis and were later confirmed as OP through a surgical biopsy. Usually, the lesions were not migratory and were rarely recurrent. The low recurrence rate could be associated with most of the patients with ULs receiving complete resection.
Glucocorticoids are often used to treat COP. Steroid therapy can be low dosage and short course (1) or high dosage and long course (13). There is no consensus on the issue of glucocorticoid therapy for COP are unavailable. In the current study, 53 patients received intravenous methylprednisolone followed by oral prednisone. The clinical symptoms of the other patients alleviated within 1–3 days of the treatment except for one patient with late-stage COP who responded poorly. For the majority of the patients, chest X-ray performed one week after steroid treatment revealed substantial remission of the lesion and after two weeks of treatment, chest X-ray and CT showed that most of the lesions had disappeared. The patients were followed up closely, and the dosage of oral prednisone was personalized and reduced as patients’ condition improved. Oral steroid treatment was continued until the lesions were completely resolved. Most of the patients accepted 1-year steroid therapy for COP. In this study, 35 patients experienced reoccurrence when the oral prednisone dosage was reduced to 5–15 mg/d but eventually showed an improvement when the dosage was increased to 30 mg/d. Therefore, the premature termination of steroid therapy could increase the risk of recurrence. If patients have already developed severe fibrosis and honeycomb lung, steroid therapy should be extended to a minimum of 6 months after the disease becomes stable.
COP is reported to have a good prognosis (1,15). The 5-year survival rate of our patients was 98.3%, which is similar to the survival rates previously reported (range, 73–98%) (16,17). Some patients can achieve spontaneous remission (18,19). In the current study, 43 (44.8%) of the 96 patients who received partial resection had spontaneous remission after the surgery, and the 80 patients who underwent complete resection did not experience any recurrence during follow-up. Furthermore, 35 of the 53 patients (66%) who received steroid therapy had recurrence when the steroid dosage was reduced or the steroid therapy was stopped.
Conclusions
Among 1,346 cases of confirmed OP, the majority (86.9%) were secondary OP, and COP only accounted for 13.1% (176/1,346) of cases. ULs were common in our COP patients. CRP diagnosis and continuous follow-up may be the key for the accurate diagnosis of COP.
Acknowledgments
The authors thank Dr. Y. Huang (The Center of Thyroid Diseases, the Shanghai Tenth People’s Hospital, Tongji University, School of Medicine, Shanghai, China) for her helpful discussions. We also thank our patients for their cooperation, and all of the authors who contributed their time and effort.
Funding: This study was funded by the National Science Foundation of China (No: 81500052, 81730002, 81670055, 81670056, 91442103 and 81570057), Ministry of Science and Technology of the People’s Republic of China (2016YFC1100200, 2016YFC1100204), National Science Foundation of Shanghai (18ZR1431400) and Shanghai Hospital Development Center (16CR3054A).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-4490
Data Sharing Statement: http://dx.doi.org/10.21037/atm-20-4490
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4490). The authors have no conflicts of interest to declare.
(English Language Editor: J. Reynolds)
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocols were approved by the Institutional Review Boards of Shanghai Pulmonary Hospital (Approval No: k15-192). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cordier JF. Cryptogenic organizing pneumonia. Clin Chest Med 2004;25:727-38. vi-vii. [Crossref] [PubMed]
- American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 2002;165:277-304. [Crossref] [PubMed]
- Travis WD, Costabel U, Hansell DM, et al. An official american thoracic society/european respiratory society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. [Crossref] [PubMed]
- Zhou X, Chen Y, Zhao L. Organizing pneumonia: a rare pulmonary manifestation of well-controlled ulcerative colitis. J Thorac Dis 2018;10:E634-8. [Crossref] [PubMed]
- Oymak FS, Demirbas HM, Mavili E, et al. Bronchiolitis obliterans organizing pneumonia. Clinical and roentgenological features in 26 cases. Respiration 2005;72:254-62. [Crossref] [PubMed]
- Gudmundsson G, Sveinsson O, Isaksson HJ, et al. Epidemiology of organising pneumonia in Iceland. Thorax 2006;61:805-8. [Crossref] [PubMed]
- Yoo JW, Song JW, Jang SJ, et al. Comparison between cryptogenic organizing pneumonia and connective tissue disease-related organizing pneumonia. Rheumatology (Oxford) 2011;50:932-8. [Crossref] [PubMed]
- Yılmaz S, Akıncı Özyürek B, Erdoğan Y, et al. Retrospective evaluation of patients with organizing pneumonia: is cryptogenic organizing pneumonia different from secondary organizing pneumonia? Tuberk Toraks 2017;65:1-8. [Crossref] [PubMed]
- Garibaldi BT, West NE, Illei PB, et al. Bronchiolitis obliterans organizing pneumonia following a jalapeño grease fire. Chest 2015;147:e31-3. [Crossref] [PubMed]
- Camus P, Nemery B. A novel cause for bronchiolitis obliterans organizing pneumonia: exposure to paint aerosols in textile workshops. Eur Respir J 1998;11:259-62. [Crossref] [PubMed]
- Romero S, Hernandez L, Gil J, et al. Organizing pneumonia in textile printing workers. A clinical description. Eur Respir J 1998;11:265-71. [Crossref] [PubMed]
- Lee LT, Ho CH, Putti TC. Bronchiolitis obliterans organizing pneumonia following nitric acid fume exposure. Occup Med (Lond) 2014;64:136-8. [Crossref] [PubMed]
- Epler GR. Bronchiolitis obliterans organizing pneumonia. Arch Intern Med 2001;161:158-64. [Crossref] [PubMed]
- Li H, Fan F, Li Q, et al. Clinical analysis of 25 subjects with biopsy-proven Cryptogenic Organizing pneumonia Zhonghua Jie He He Hu Xi Za Zhi 2007;30:259-64. (in Chinese). [PubMed]
- Chung MP, Nam BD, Lee KS, et al. Serial chest CT in cryptogenic organizing pneumonia: Evolutional changes and prognostic determinants. Respirology 2018;23:325-30. [Crossref] [PubMed]
- Lazor R, Vandevenne A, Pelletier A, et al. Cryptogenic Organizing Pneumonia-Characteristics of Relapses in a Series of 48 Patients. Am J Respir Crit Care Med 2000;162:571-7. [Crossref] [PubMed]
- Lohr RH, Boland BJ, Douglas WW, et al. Organizing pneumonia: features and prognosis of cryptogenic, secondary, and focal variants. Arch Intern Med 1997;157:1323-9. [Crossref] [PubMed]
- Oymak FS, Demirbas HM, Mavili E, et al. Bronchiolitis obliterans organizing pneumonia. Clinical and roentgenological features in 26 cases. Respiration 2005;72:254-62. [Crossref] [PubMed]
- Chang J, Han J, Kim DW, et al. Bronchiolitis Obliterans Organizing Pneumonia: Clinicopathologic Review of a Series of 45 Korean Patients Including Rapidly Progressive Form. J Korean Med Sci 2002;17:179-86. [Crossref] [PubMed]
(English Language Editor: J. Reynolds)