
Relationship between prediagnostic body mass index trajectory and colorectal adenomas: an analysis of the PLCO cancer screening trial
Introduction
Colorectal cancer is the third most common cancer worldwide. About 1.8 million new cases were recorded globally in 2018 (1), and it is also a leading cause of cancer death in the United States. However, age-adjusted incidence of colorectal cancer has shown a downward trend since 1985 (2), which may be attributed to screening programs. Regular screening can identify individuals with early stage disease and improve outcome. Colorectal adenomas are considered premalignant lesions to colorectal cancer, and their discernible endoscopic features make them ideal candidates for population screening. Early detection and removal of colorectal adenomas can stop the progression of adenomas to cancer, thereby reducing the incidence of colorectal cancer (3).
Incidence of obesity and overweight is on the rise globally (4), potentially impacting worldwide cancer incidence and cancer-related mortality (5). Continuous updates of scientific researches have confirmed the association between high body mass index (BMI) and risk of twelve cancers, and half of which are gastrointestinal tumors such as esophageal cancer, stomach cancer, pancreatic cancer, liver cancer, and colorectal cancer (6-11). The estimated increase in risk of these cancers due to high BMI ranges from 3% to 10% per unit increase in BMI (12). Epidemiological evidence suggests that obesity or overweight is a potentially modifiable risk factor associated with colorectal premalignant lesions (13-17). Yet, the weight of an individual may change over his/her lifetime. Studies on the relationship between adulthood BMI and colorectal adenoma incidence are limited, and the relationship between changes in BMI and the risk of colorectal premalignant lesions has not been investigated.
Studies about timing of obesity and weight changes across lifetime may help explain the biological mechanisms of colorectal adenoma. Therefore, in this study, we assessed the relationship between adulthood BMI trajectory and the risk of colorectal adenomas, using large prospective cohort data from the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-19-4634).
Methods
Study population
The PLCO cancer screening trial was a randomized controlled clinical trial that aimed to evaluate current screening methods for prostate, lung, colorectal, and ovarian cancer. In total, 155,000 subjects between the ages of 55 and 74 years in ten research centers across the United States were enrolled, and they were randomly divided into the intervention and control arms. Subjects in the intervention arms would receive prostate cancer screening [i.e., prostate-specific antigen (PSA) exams], lung cancer screening (i.e., lung computed tomography), colorectal cancer screening (i.e., colonoscopy or sigmoidoscopy), and ovarian cancer screening (i.e., ovarian palpation). Follow-up activity continued as long as the reports of cancers were not abstracted and confirmed. The median follow-up time was 12.4 years.
We enrolled a subset of the intervention group from this trial. Subjects enrolled were required to have undergone at least one colonoscopy or sigmoidoscopy examination with a confirmed diagnosis of colorectal adenomas. They were also required to have completed all baseline and supplemental questionnaires, including completed BMI data at the age of 20, 30, 40, and 50 and at the age of adenomas diagnosis. Baseline information such as marital status (married, widowed, divorced, separated, and single), education level (high school or less, some college, college graduate, and postgraduate), race (non-Hispanic white/black, Hispanic, Asian, Pacific islander, and American Indian), and cigarette status (never, ever, and former) were collected. We excluded individuals based on the following extreme criteria: (I) weight at enrollment less than 60 lbs, (II) height under 48 inches, (III) female height over 78 inches, (IV) male height over 84 inches, and (V) calculated BMI less than 15 kg/m2 or more than 70 kg/m2.
BMI assessment
At the time of enrollment, participants recorded their current height (m) and body weight (kg) and recalled this information when they were at the age of 20, 30, 40, and 50. BMI during each age period was calculated using the formula body weight (kg)/height (m2). Patients were classified according to their BMI in each age period according to the World Health Organization criteria: underweight (less than 18 kg/m2), normal weight (18 to 24.9 kg/m2, reference), overweight (25 to 29.9 kg/m2), and obese (greater than 30 kg/m2). Since subjects enrolled were over 55 years of age, we assessed the relationship between prediagnostic BMI change and the risk of colorectal adenomas at the age of 20, 30, 40, and 50. Changes in weight (ΔW) from age 20 to 50 years were classified as loss (ΔW ≤–2 kg), or stable (–2 kg< ΔW <5 kg, reference), or notable gain (ΔW ≥5 kg).
Assessment of colorectal adenomas
According to PLCO protocol, endoscopic screenings were respectively performed at baseline and 3 or 5 years after enrollment. Participants were not diagnosed with colorectal adenomas at the time of enrollment and any incidental diagnosis of adenomas was histologically verified after endoscopic biopsy. Pathological types of adenomas include tubular adenomas, villous adenomas, serrated adenomas, and hyperplastic polyps.
Statistical analysis
Composition ratio was applied to describe sociodemographic and clinical features of the participants enrolled. Descriptive statistics on ages of the participants and BMI of each age group were given as mean (SD). Multivariate logistic regression was used to analyze the relationship between BMI at age 20, 30, 40, 50 years, and colorectal adenomas risk, respectively, adjusted for race, gender, age, education level, married and cigarette smoking.
To identify BMI changes through age 20 to 50 years, BMI trajectories based on the latent class group model were generated using SAS Proc Traj (SAS Institute, Cary, NC, USA). Initially, we applied the Bayesian information criterion (BIC) to correct the fitted three to five trajectories permitted, with a minimum of 1% of subjects per trajectory. Linear and quadratic polynomials were used during the fitting. The BMI trajectory with the smallest BIC was selected as the final trajectory. Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs), to evaluate the relationship of prediagnostic BMI trajectory with the risk of colorectal adenomas. All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA). Statistical significance was set at P<0.05, and all the P values were two sided.
Results
Subject characteristics
Baseline characteristics are shown in Table 1. The median follow-up time of PLCO cancer screening trial was 12.4 years. Of the 39,824 participants, 20,358 (51.5%) were males. The average age at randomization was 61.8±5.1 years, and more than two-thirds of the enrolled participants were under 65 years of age at the baseline. Most of them were non-Hispanic white (91.8%) and married (79.7%). In all, 21,210 (53.3%) participants reported a family history of cancer, and 4,122 (10.4%) had a family history of colorectal cancer. In addition, 5,439 (13.7%) participants reported that adenomas were identified during sigmoidoscopic or colonoscopic examinations.

Full table
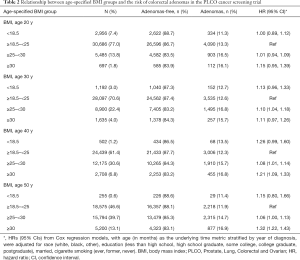
Age-specified BMI groups and the risk of colorectal adenomas
As shown in Table 2, the proportion of overweight and obese individuals increased with age. The proportion of those with a BMI ranges from 25 to 30 kg/m2 at the age of 20, 30, 40, and 50 was 13.8%, 22.4%, 30.6%, and 39.7%, respectively. The proportion of those with a BMI over 30 kg/m2 at the age of 20, 30, 40, and 50 was 1.8%, 4.0%, 6.8%, and 13.1%, respectively. Compared with normal weight, overweight and obesity significantly increased the risk of colorectal adenomas in each age period, starting at age 30 years. Importantly, obesity at age 50 years showed the strongest relationship with colorectal adenomas [HR: 1.32, 95% CI: (1.22, 1.43)].
Full table
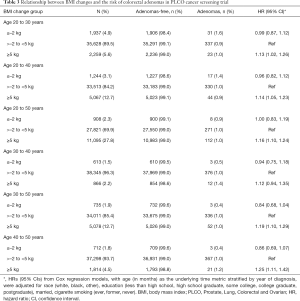
BMI change and the risk of colorectal adenomas
The BMI changes in different age periods are shown in Table 3. BMI changes from the age of 30 to 40 did not increase the risk of colorectal adenomas. However, BMI notably gains from the age of 40 to 50 [HR: 1.25, 95% CI: (1.11, 1.42)] showed the strongest relationship with an increased incidence of colorectal adenomas, followed by that from the age of 30 to 50 [HR: 1.19, 95% CI: (1.10, 1.29)], the age of 20 to 50 [HR: 1.16, 95% CI: (1.10, 1.24)], the age of 20 to 40 [HR: 1.14, 95% CI: (1.05, 1.23)], and the age of 20 to 30 [HR: 1.13, 95% CI: (1.02, 1.26)]. In contrast, BMI decreases from the age of 30 to 50 and the age of 40 to 50 numerically but not significantly decreased the risk of colorectal adenomas [HR: 0.84, 95% CI: (0.68, 1.04); HR: 0.86, 95% CI: (0.69, 1.07)].
Full table
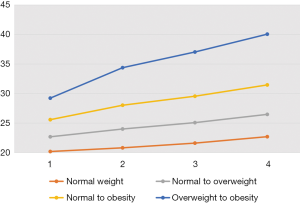
BMI trajectories and the risk of colorectal adenomas
Adulthood BMI trajectories are illustrated in Figure 1. The normal weight maintained, normal to overweight, normal to obesity, and overweight to obesity groups made up 41.2%, 44.1%, 12.9%, and 1.9% of the cohort, respectively (Table 4). Compared with individuals who maintained normal weight throughout their adulthood, those who progressed from normal weight to obesity [HR: 1.15, 95% CI: (1.06, 1.25)] and from overweight to obesity [HR: 1.33, 95% CI: (1.11, 1.61)] had a higher risk of colorectal adenomas.

Full table
Discussion
This study established a significant positive relationship between high BMI and the risk of colorectal adenomas. We found that being overweight and/or obese at different stages of adulthood, especially after the age of 30, was strongly associated with an increased risk of colorectal adenomas. In addition, compared with individuals who maintained normal weight throughout their adulthood, those who progressed from normal weight to overweight did not increase the risk of colorectal adenomas, while those who progressed from normal weight or overweight to obesity did significantly increase the risk.
Our results confirmed findings from prior observational research showing that obesity and weight gained in adulthood are independent risk factors of colorectal adenomas (18-20). We detected an increased risk of colorectal adenomas as BMI increased. Although underlying mechanisms linking high BMI to increased risk of colorectal adenomas are unclear, evidence suggests that hormones in the insulin-like growth factor/growth hormone (IGF/GH) axis may have a role downstream of high BMI. A positive correlation between BMI and IGF could cause elevated IGF levels as BMI increases (21). Obesity influence the amount of IGF available to the cell, and higher IGF levels may contribute to increased cancer growth (22). When IGF binds to its cognate receptor, it triggers a signaling cascade that results to proliferative and anti-apoptotic events (23). The IGF axis is reported to involve in several cancers development and progression, including colorectal, breast, and endometrioid cancers (23-25). Similar mechanisms could explain how high BMI in turn could increase the risk of colorectal adenomas (26-28).
In this study, we found that the association between obesity and colorectal adenomas become more prominent with advancing age, especially from age 30 years. As to age 20 years, several studies reported a strong association between weight gained in adulthood, especially obesity in early adulthood, and a high incidence of colorectal adenomas (19,20,29). However, we did not find an increased risk of colorectal adenomas despite a significant increase in BMI during early adulthood (at age 20 years). This could be attributed to random error due to disproportionately fewer underweight, overweight, and obese individuals in the age of 20 compared to normal weight individuals in our study. Our analysis revealed that notable weight gained at the age of 40 to 50 significantly increased the risk of colorectal adenomas. Simultaneously, the risk of colorectal adenomas increased, as notable BMI gained in shorter time before the age of 50. This can be explained by an age-related change in body fat accumulation pattern, from subcutaneous to visceral, which is more likely to increase IGF levels.
The relationship between BMI trajectory and cancers has long been a heat topic in the field of epidemiology. Zheng et al. (25) study the relationship between BMI trajectory and colorectal cancer using data from PLCO cancer screening trial. According to the BIC, they divided the participants into four groups: normal weight maintained, normal to overweight, normal to obesity, and overweight to obesity groups. Consistent to their study, we divided the participants into the same four groups in accordance with the trend of BMI change. We found that individuals who progressed from normal weight or overweight to obesity did significantly increase the risk of colorectal adenomas, when compared to those who maintained normal weight in their lifespan. In particular, weight change from normal to obesity showed a higher risk. Similar findings have been reported in colorectal and prostate cancers (6,25). Possible explanation may be that the growth rate from normal weight to obesity is more obvious than that of overweight to obesity, so the effect of IGF axis may be more serious.
To the best of our knowledge, this is the first study to investigate the relationship between changed BMI trajectory and colorectal premalignant lesion risk. Apart from the relationship between age-specific BMI and colorectal adenomas, we highlighted the association of prediagnostic BMI trajectory and colorectal adenomas, which provided helpful clues for better understanding of the relationship between BMI and colorectal adenomas. Another advantage of our study was that the PLCO cancer screening trial, as a cohort study, had a large sample size and involved extended follow-up. Also, the BMI trajectory model tracks data before the occurrence of colorectal adenomas, demonstrating high reliability of risk estimates.
However, our study has some limitations. First, the study relied on self-reported BMI; thus, any recall bias could artifactually affect our risk estimates. Second, this study enrolled mainly non-Hispanic white individuals resulting in little racial diversity among the participants. Further investigation is required to determine whether similar patterns are observed among other racial populations. Finally, because the sample size of the change from overweight to obesity group was too small, the relationship between this change and the risk of colorectal adenomas needs to be verified in a larger population.
Conclusions
Our study showed that overweight and obesity increased the risk of colorectal adenomas, and this risk increased with advancing age. Moreover, prediagnostic BMI trajectory modeling demonstrated that excessive weight gain during adulthood could lead to an elevated risk of colorectal adenomas. In contrast to individuals who maintained normal weight throughout their adulthood, those who progressed from normal weight to overweight did not increase the risk of colorectal adenomas, while those who progressed from normal weight to obesity and from overweight to obesity did significantly increase the risk, especially those progressed from normal weight to obesity.
Acknowledgments
We thank everyone who provided support for this study. We would like to thank Editage (www.editage.com) for English language editing. We apologize for any omission of citations and references due to space limitation.
Funding: This research was supported by the National Key R&D Program of China (2018YFC1313300), Natural Science Foundation of Guangdong Province (2017A030313485, 2014A030312015), Science and Technology Program of Guangdong (2015B020232008), Science and Technology Program of Guangzhou (201508020250, 201604020003, 2019B020227002), and the Fundamental Research Funds for the Central Universities (17ykpy82).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-19-4634
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-19-4634). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). There is no need to go through an ethical review process, because this is a research that extracts information from the PLCO database, which is open to those who applied. PLCO has the following five ClinicalTrials.gov registration numbers: NCT00002540 (Prostate), NCT01696968 (Lung), NCT01696981 (Colorectal), NCT01696994 (Ovarian), and NCT00339495 (EEMS). Individual consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet 2014;383:1490-502. [Crossref] [PubMed]
- Leslie A, Carey FA, Pratt NR, et al. The colorectal adenoma-carcinoma sequence. Br J Surg 2002;89:845-60. [Crossref] [PubMed]
- Stevens GA, Singh GM, Lu Y, et al. National, regional, and global trends in adult overweight and obesity prevalences. Popul Health Metr 2012;10:22. [Crossref] [PubMed]
- Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2224-60. [Crossref] [PubMed]
- Kelly SP, Graubard BI, Andreotti G, et al. Prediagnostic body mass index trajectories in relation to prostate cancer incidence and mortality in the PLCO cancer screening trial. J Natl Cancer Inst 2016;109:djw225. [Crossref] [PubMed]
- World Cancer Research Fund International, American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Oesophageal Cancer. 2018.
- World Cancer Research Fund International, American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Stomach Cancer. 2018.
- World Cancer Research Fund International, American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Pancreatic Cancer. 2018.
- World Cancer Research Fund International, American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Liver Cancer. 2018.
- World Cancer Research Fund International, American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Colorectal Cancer. 2018.
- Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569-78. [Crossref] [PubMed]
- Wallace K, Baron JA, Karagas MR, et al. The association of physical activity and body mass index with the risk of large bowel polyps. Cancer Epidemiol Biomarkers Prev 2005;14:2082-6. [Crossref] [PubMed]
- Laake I, Thune I, Selmer R, et al. A prospective study of body mass index, weight change, and risk of cancer in the proximal and distal colon. Cancer Epidemiol Biomarkers Prev 2010;19:1511-22. [Crossref] [PubMed]
- Matsuo K, Mizoue T, Tanaka K, et al. Association between body mass index and the colorectal cancer risk in Japan: pooled analysis of population-based cohort studies in Japan. Ann Oncol 2012;23:479-90. [Crossref] [PubMed]
- Laiyemo AO. The risk of colonic adenomas and colonic cancer in obesity. Best Pract Res Clin Gastroenterol 2014;28:655-63. [Crossref] [PubMed]
- Kyrgiou M, Kalliala I, Markozannes G, et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ 2017;356:j477. [Crossref] [PubMed]
- Neugut AI, Lee WC, Garbowski GC, et al. Obesity and colorectal adenomatous polyps. J Natl Cancer Inst 1991;83:359-61. [Crossref] [PubMed]
- Bird CL, Frankl HD, Lee ER, et al. Obesity, weight gain, large weight changes, and adenomatous polyps of the left colon and rectum. Am J Epidemiol 1998;147:670-80. [Crossref] [PubMed]
- Sedjo RL, Byers T, Levin TR, et al. Change in body size and the risk of colorectal adenomas. Cancer Epidemiol Biomarkers Prev 2007;16:526-31. [Crossref] [PubMed]
- Sandhu MS, Dunger DB, Giovannucci EL. Insulin, insulin-like growth factor-I (IGF-I), IGF binding proteins, their biologic interactions, and colorectal cancer. J Natl Cancer Inst 2002;94:972-80. [Crossref] [PubMed]
- Zelenko Z, Gallagher EJ. Diabetes and cancer. Endocrinol Metab Clin North Am 2014;43:167-85. [Crossref] [PubMed]
- Christopoulos PF, Msaouel P, Koutsilieris M. The role of the insulin-like growth factor-1 system in breast cancer. Mol Cancer 2015;14:43. [Crossref] [PubMed]
- Gunter MJ, Hoover DR, Yu H, et al. A prospective evaluation of insulin and insulin-like growth factor-I as risk factors for endometrial cancer. Cancer Epidemiol Biomarkers Prev 2008;17:921-9. [Crossref] [PubMed]
- Zheng R, Du M, Zhang B, et al. Body mass index (BMI) trajectories and risk of colorectal cancer in the PLCO cohort. Br J Cancer 2018;119:130-2. [Crossref] [PubMed]
- Kaaks R, Lukanova A. Energy balance and cancer: the role of insulin and insulin-like growth factor-I. Proc Nutr Soc 2001;60:91-106. [Crossref] [PubMed]
- Renehan AG, Zwahlen M, Minder C, et al. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet 2004;363:1346-53. [Crossref] [PubMed]
- Matano Y, Okada T, Suzuki A, et al. Risk of colorectal neoplasm in patients with acromegaly and its relationship with serum growth hormone levels. Am J Gastroenterol 2005;100:1154-60. [Crossref] [PubMed]
- Wernli KJ, Newcomb PA, Wang Y, et al. Body size, IGF and growth hormone polymorphisms, and colorectal adenomas and hyperplastic polyps. Growth Horm IGF Res 2010;20:305-9. [Crossref] [PubMed]


