
Personalized-medicine on carotid endarterectomy and stenting
Background
In the last thirty years evidence based medicine (EBM) progressively emerged as a milestone for promotion of good clinical practice (1). Sackett et al. in their memorable editorial published in BMJ in 1996 “Evidence based medicine: what it is and what it isn't” affirm “Evidence based medicine is the conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients.” (2). In this paper the three EBM milestones are reported: (I) best evidence; (II) individual clinical expertise; (III) patients’ choice (2). With this view, EBM is at the basis of both precision medicine and guidelines for clinical practice.
The main purpose of a medical guideline is to use research (the best evidence) to create an easily accessible instrument that helps clinicians to decide the treatment for achieving the best outcome in routine practice (3). There commendations that are spelled out in guidelines are stronger the more they are based on evidence or proof of efficacy deriving from randomized control trials (RCTs) on patient cohorts. However, RCTs often do not take into account comorbidities, totally excluding patients presenting with multiple pathologies (4,5). In fact, too often the guidelines are geared to a basic and idealized patient (4). In real world, clinicians are involved in the medicine of complexity in which patients often present multiple comorbidities (not represented in most RCTs) and have different risk factors, lifestyles and socio-cultural backgrounds that influence their preferences and choices. For this, even if RCTs are essential and represent the apex of the famous EBM pyramid, the reduction of EBM and guidelines merely to the use of RCTs frequently leads to a non-application to the complex patient (5). In fact, guidelines based only on best available evidence may not encompass the real-life scenario; moreover too often EBM is misinterpreted because it is frequently considered limited only to the first milestone. This conducted EBM to a crisis point (6). In order to overthrow this limit personalized medicine (PM) was born, it has been proposed as new paradigm that, with its holistic systems approach, is able to consider individual patient with her/his specific characteristic in a predictive, preventive, personalized, and participatory model (7).
In literature there are multiple guidelines on carotid endarterectomy (CEA) and carotid artery stenting (CAS) for primary and secondary stroke prevention. The recommendations were based only on results of RCTs which were often conducted over 10 years ago, medical treatment improvements were not taken into account and often potential CAS hazards were understated (3).
RCTs versus observational studies: current controversials
One of the main questions to ask is whether the results obtained by RCTs are therefore confirmed in the “real world”. Paraskevas and Naylor evaluated the external validation of carotid revascularization RCTs (8). They highlighted discrepancy in outcome measures (OM) observed in CEA and CAS RCTs with respect to data provided by administrative dataset and registries. When they discussed the reasons of these incongruities, they identified several features that determined it, such as the skills of interventionists, the patient selection and the improvements in CAS technique. They concluded that it is desirable the introduction of prospective, randomized, controlled clinical registries (8). In a recent paper, Paraskevas et al. confirmed the previous observations, remarking their point of view about the reasons because the trials do not reflect reality (9). In particular, they empathized the need to a correct interpretation of RCTs writing guidelines. Moreover, they underlined that most available RCTs on carotid revascularization should be considered obsolete because of improvement in medical and surgical treatments (9). After reporting the reasons why the RCTs could be misleading, they concluded that industry should not be involved in design and performance of RCTs, OM and patient selection should be carefully planned. This help to improve the external validation of RCTs, consequently RCTs and “real life” observational studies results may be closer and more superimposable (9). In a recent paper entitled “Real-world studies no substitute for RCTs in establishing efficacy” published on Lancet the authors described the relationship between observational data and RCTs (10). “Real world” data may aid to recognize the link between the intervention and OM and they could be used to assess the strength of relations. On the other hand, confounders can bias observational findings such that the true results might be diminished or increased and, although statistical analysis can mitigate some confounding factors, several factors are not take into account because unknown (10). The randomization process of large RCTs overcomes this source of bias balancing known or unknown risk factor, because a well-performed randomization creates two or more homogeneous groups. If randomization is not executed, the data from the observational studies may be instrumental to generate hypothesis. However when an element shows an extreme relative risks (less than 0.25 or greater than 4), probably its relationships is not due to chance, therefore it do not represent a confounding factor and a RCT created to asses this issue may be needless (10). The authors sustained that RCTs are evolving to become less arduous, less expensive, and more generalizable by being embedded within real-world settings (10). Another eminent work recently published on NEJM entitled “The magic of randomization versus the myth of real word evidence” emphasized that the replacement of RCTs with non-randomized observational studies is a false solution to provide the patients the right treatment that ensures the best balance between risk and benefit (11). As above described, large observational studies can detect rare severe adverse effect that cannot be ascribed to chance since the relative risk is extreme, as they also can find beneficial effects is very large and easily assessed (11) (e.g., Acetyl-cholinesterase inhibitor treatment for myasthenia gravis that non need RCTs, observational studies is so clear that a randomised controlled trial depriving participants in a placebo arm of treatment would be difficult to justify) (12). Except for these extreme situations observational studies, although large, may lead to misleading associations of a treatment, indeed right sized RCTs are required to identify any moderate effect (both benefits and harms) (11). There are the need to remove the obstacles to RCTs in order to protect the patients, to achieve this result it is necessary reduce costs and complexity of conducting RCTs, in this view the Clinical Trials Transformations Initiative has shown that it is possible to develop guidance that can help improve specific aspect of the design and conduct of RCTs (11) but a step forward must be moved.
Objectives
In this paper we start from the main evidence available in the literature on this issue and reported in the most widespread guidelines, analyse the most controversial and grey aspects, such as CEA versus CAS versus optimal medical therapy alone in symptomatic and asymptomatic patient, which are the most fertile ground for medicine of complexity, and then analyse subgroups and risk/benefit scores from meta-analysis and RCTs in order to provide some good practice point (GPP), as suggestions based on expert consensus rather than recommendations in classical guidelines, in attempt to apply PM in this area that more than others may benefit from the application of PM (13).
Methods
Authors from direct searches on Medline, Cochrane Library and Google Scholar carried out the data collection. Being a narrative review, the search for the literature was not systematic, no inclusion and exclusion criteria were used, nor a systematic evaluation of the quality of the works. Not only the results of the main trials and registries, but also the most significant subgroup analyses and risk/benefit scores were considered.
Results
Symptomatic carotid stenosis (SCS)
Although this may seem like a simple concept, there is no uniformly accepted definition of SCS. Guideline generally referred SCS to patients with previous stroke or non-disabling stroke or TIA, or a combination of these terms, but there are discrepancies in term of degree of stenosis and timing of any previous stroke or TIA (3).
About twenty years ago, the North American Symptomatic Carotid Endarterectomy Trial (NASCET) (14) and the European Carotid Surgery Trial (ECST) (15) were published. These two studies, although dated, represent the pillars of the treatment of SCS. They showed for the first time the benefit from surgical treatment with respect to medical treatment, above all in patient with moderate-severe stenosis and with low perioperative risk (14,15).
Our group (ISO-SPREAD guideline), based on these two studies and on the two fundamental meta-analysis conducted by Rothwell and colleagues (16,17), defined symptomatic stenosis “… if the last ipsilateral cerebral or retinal ischemic episode occurred in the previous 6 months. Based on recent reviews of the same studies, the ISO-SPREAD group believes it is appropriate to reduce this interval to no more than 3 months.” (18). In fact, looking at Rothwell systematic review it is clear that the advantage of revascularization (CEA) from SCS becomes similar to the one obtained in ACS after three months from index event.
NASCET and ECST enrolled patient with minor stroke or TIA and carotid stenosis, included men and women with various degrees of stenosis. These two trials established the net benefit of surgical procedure in stenosis greater than 70% and a questionable benefit in patient with stenosis 50–70% (14,15). On the other hand, the surgery benefits were partially counterweigh by its peri-procedural risks of major cardio-cerebrovascular complications such as ischaemic stroke, myocardial infarction and death. In the surgical groups this ranged from 4.5% to 7.0%, while in the medical therapy groups it is from 2.4% to 6.1% (19). Looking at these data, the patient undergoing CEA should have a perioperative risk less than 6% (4). If a period of three years of follow-up is taken into account, patients with minor or moderate SCS not benefitted from CEA, while the recurrence of ipsilateral stroke and death in SCS ≥80% (ECST method) was lower in CEA group (6.8%) compared with medical group (20.6%) (19,20). It is important to note that these studies do not include the best current medical treatment, considering the current guidelines on statins, antiplatelet agents, blood pressure control, smoking cessation, and glycaemic control. Therefore, the risk with best medical treatment could now be reduced.
The above-mentioned two meta-analysis conducted by Rothwell et al. demonstrated that CEA is highly beneficial in patients with SCS ≥70%, participants with SCS of 50–69% have some benefit, while CEA is not effective in those with stenosis less than 50%, the advantage in near-occlusion is borderline in the short-term and undefined in the long-term follow-up (16). Moreover, the risk reduction of stroke recurrence is highest if CEA is achieved within 2 weeks of the minor stroke/TIA onset (17).
This huge amount of evidence provides high-level of proof to recommend the use in clinical practice of CEA in patients with moderate-severe SCS. However, patients with a low perioperative risk should be selected.
In order to evaluate the risk/benefit balance between medical and surgical treatments, predictive variables were identified; they were incorporated into the following risk-factor scores, reported in Table 1.
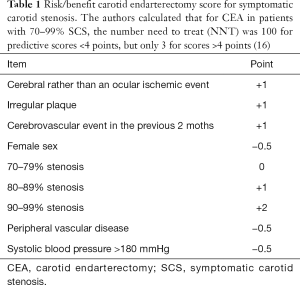
Full table
In a cumulative analysis of ECST and NASCET data, the benefit of surgery was clearly higher for male patients older than 75, and for those randomized within 2 weeks from the ischaemic event; NNT at 5 years were 9 for men and 36 for women, 5 for patients older than 75 and 18 for those younger than 65; 5 for those randomized within 2 weeks from the ischaemic event and 125 for those randomized more than 12 weeks after the event (16,17).
On the other hand, Ferguson et al. in their analysis of NASCET recognized participants baselines variables that were prognostic of increased surgical risk: (I) hemispheric ischemia vs. retinal event; (II) contralateral carotid occlusion; (III) ipsilateral ischemic lesion on neuroimaging and (IV) irregular plaque (21). Melin et al. demonstrated that “frailty” is a predictor of increased stroke, mortality, myocardial infarct, and length of stay after CEA and an implemented risk analysis index (RAI), considering variables such as age, cancer, sex, congestive heart failure, dyspnea, renal insufficiency, functional status, may be able to recognize a limited subclass of patients who have an higher post-surgery complications risk and may not benefit from CEA both in symptomatic and asymptomatic patients (22). Also, criteria for indication may include the presence of microemboli despite best medical therapy, whereas the presence of a recent ipsilateral territorial lesion on MR may add to the risk score for operation (22).
Asymptomatic carotid stenosis (ACS)
Medical therapy (risk factor control, lifestyle changes and pharmacological treatment) for stroke prevention has greatly improved in the last years. It is likely that a minority of ACS persons will now benefit from a carotid procedure. Abbott et al. reviewed the literature about medical intervention alone versus the association with revascularization treatment in persons with ACS (23). Therefore, the main conclusions in majority of guidelines are that CEA showed a significant benefit, with respect to medical intervention alone, in men with normal surgical risk, aged less than 75 year and with a degree of stenosis more than 60%.
However, it is necessary to underline that the most recent evidences show that the stroke rate in ACS patients treated with the best medical treatment is lower than for those who had revascularization treatment in RCTs and RCTs of CEA versus CAS in patients with ACS had an undersized sample size. Furthermore, the current evidences available indicate a basically higher risk of stroke and death with CAS. Randomized trials planned to compare current optimal medical intervention with CEA or CAS are not available. Commonly cited markers of high stroke risk in relation to ACS lack specificity have not been assessed in conjunction with benefit from a carotid procedure in addition to current optimal medical intervention Stroke risk stratification using current optimal medical therapy is the main priority in order to identify patients with ACS likely to benefit from adding CEA or CAS (23).
Until now the Asymptomatic Carotid Stenosis and Risk of Stroke (ACSRS) study defines the risk of ipsilateral ischaemic events in relation to the degree of ACS and other vascular risk factors (24). In addition to the stenosis degree the characteristics of the plaques were reported, in relation to texture analysis were classified on a scale from 1 (soft) to 5 (hard) according to a modified Geroulakos classification (25). The study group found that 94% of major events occurred in patients with plaques of types 1 to 3 (26).
The predictive value of microemboli detection on trans-cranial-Doppler (TCD) for the identification of patients with ACS at high risk for future stroke is well established (27), it is supported by autonomous studies and systematic review with meta-analysis (28,29). In particular, Spence et al. showed that patients with at least 2 microemboli on TCD were more likely to have cerebrovascular event in the first year (30,31). Moreover, they affirmed that patient with ACS and without micro-embolic signals probably did not benefit from interventional approach (CEA or CAS), in these patients the risk of stroke in the first year is about 1% (31). The same study group demonstrated that microemboli at TCD and above all cerebrovascular event have significantly reduced in ACS patients with the use of intensive medical therapy. The reduction in microemboli overlapped with better treatment of hypercholesterolemia and slower progression of carotid plaque. In this study they confirm that the patients, who took intensive medical treatment without microemboli, had the risk of cerebral ischaemic events less than the risk during CEA or CAS. They affirmed that the patients with ACS should receive best medical therapy alone and only be candidate for CEA or CAS if microemboli at TCD persist (32). In 2016 Italian Stroke Organization and Stroke Prevention and Education Awareness Diffusion (SPREAD) introduced in its guideline ipsilateral TCD microemboli as criterion for intervention in ACS (4); in 2017 the European Society for Vascular Surgery carotid guidelines endorse revascularization treatment stratifying patients with the detection of TCD microemboli (33).
In addition to microembolic signals, several other plaque features should be considered in order to guarantee the best characterization for the patient. One of these is the plaque echolucency, which since 30 years ago were considered related to lipid-rich necrotic core or intra-plaque haemorrhage and it is a find more frequent in SCS than in ACS (33). The majority of studies reported a strong association between plaque echolucency and increased risk of stroke in patients with ACS (for review see Paraskevas et al. 2018) (27). Moreover the ACES study showed that combine microembolic signal with plaque echogenicity allows a greater prediction than each measure alone and better classifies the highest-risk group (34).
Another plaque characteristic is the progression of severity of stenosis. The above-mentioned ACSRS study demonstrated a risk of ipsilateral ischaemic stroke near double in participants with progression of stenosis compared with patients with stable plaque and this is confirmed at every degree of stenosis (24). In the same study, that represents one of the biggest prospective studies on ACS, asymptomatic embolic cerebral infarcts on neuroimaging are showed as an independent predictor of ipsilateral cerebrovascular event (24). This results was confirmed by another study group (35), the risk of stroke appears to be triple in subjects with silent brain infarct on neuroimaging (36).
In another analysis of ACSRS study the juxtaluminal hypo-echoic area has a linearly correlation with the risk of ipsilateral ischaemic stroke (37). This finding represents another element of plaque instability and can be used for better stratify patients with ACS at higher risk of stroke.
We cannot finish talking about plaque instability without discussing ulceration. Already in NASCET it was shown that the presence of ulceration on angiography was associated with an increased risk of stroke (38). Colour-Doppler ultrasound represents a non-invasive method to identify carotid ulceration. This method can be strengthened using contrast-enhanced ultrasound, that ensures a better identification of ulcerated and vulnerable plaque associated with a high embolic potential (39). Furthermore 3D ultrasound plaque measurements could be considered for stroke risk stratification in ACS (40) but this method as well as more expensive procedures, like identification of intra-plaque haemorrhage using MRI, are not widespread and available in clinical practice.
Another low-cost method useful for stratifying individuals is the study of the cerebrovascular reserve. This instrument has lower evidence than the over-mentioned in relation a several bias of primary studies, but a systematic review with meta-analysis concluded that impairment of cerebrovascular reserve may identify patients with ACS at high risk for cerebrovascular ischaemic event (41).
The treatment of ACS is very individualized, it is necessary to stratify the patients in order to provide the best medical treatment and to identify the few patients with a very high risk of stroke, who are the subject candidate to revascularization treatment.
On the other hand, in order to evaluate the right risk/benefit of surgical procedure Gupta et al. proposed a validated perioperative risk score. In particular the items are: (I) <60 years 0 point; (II) 60–79 years −1 point; (III) ≥80 year +2 points; (IV) dyspnea +2 points; (V) chronic obstructive pulmonary disease +3 points; (VI) previous peripheral revascularization or amputation +3 points; (VII) angina pectoris in the last month.
Considering the combined outcome: stroke, myocardial infarction and death at 30 days, the patients can be classified at low risk (corresponding to a peri-procedural risk <3%) with total point score <4, intermediate risk (between 3% and 6%) with a score between 4 and 7, and at high risk (>6%), with a score greater than 7 (42).
In any case, surgery for patient with ACS should not be routinely proposed outside of “low surgical risk” patients that have a “high risk” of stroke if not operated on. In fact, a systematic review reported that best medical treatment alone can be now best for stroke prevention in patients with severe ACS (23).
Endarterectomy vs. stenting
When patient is eligible to revascularization treatment, a decision between CAS and CEA should be achieved. Several studies have been conducted on this issue and summarized in systematic review and meta-analysis. One of the more extensive concluded that CEA is more effective than CAS in reducing perioperative stroke/death, restenosis, and stroke/death at 10-years follow-ups (43), this is consistent with a previous Cochrane review, though it is more cautious in conclusion on the risk of restenosis and long term efficacy of CAS (that are “unclear”) (44).
On the other hand, a recent individual patient data meta-analysis of 4 trials: Stent-Protected Percutaneous Angioplasty of the Carotid Artery versus Endarterectomy trial (SPACE) (45), Endarterectomy versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis trial (EVA-3S) (46), Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) (47), and the International Carotid Stenting Study (ICSS) (48), shows that the risk of ipsilateral ischaemic stroke up to 10 years is similar in CEA and CAS, the cumulative peri- and post-procedural risk remains better in CEA (49). On these bases, authors conclude that improvement in CAS procedural safety might provide similar outcomes of the two interventions (49).
In a recent systematic review of 20 RCTs, the 30 days death/stroke rate was significantly higher after CAS both in ACS and in SCS (45). If we considered 9 years of follow-up the risk of ipsilateral stroke was the same after CEA or CAS, it is about 4%. In order to reduce the peri-procedural risk of stroke the right selection of patient is fundamental. In particular, CEA should be preferred to CAS if the subject is older than 70 years and the surgery is performed within the first 2 weeks from index event. Moreover, there are some conditions, such as plaque length more than 13 mm and remote plaques, where more stents are necessary, in which CAS is more risky than CEA. In addition, new white matter lesions were significantly more common after CAS and were associated with higher rates of late cerebrovascular events (50).
Moreover another systematic review centred on patient selection for CAS or CEA, including patients considered at ‘high risk’ of complications, reported that CAS could be superior in cases with restenosis, tracheostomy, hostile neck anatomy, paralysis of the contralateral laryngeal nerve, high bifurcation or stenosis extending toward the cranium, and after radiotherapy of the neck (51). Two Italian consensus statements on CAS highlighted the crucial role of operator experience and center performance volume (52,53).
Several procedural risk scores have been proposed for CAS. The Siena CAS score identified three categories of low (<1%), medium (1–3%), and high (>3%) peri-procedural risk depending on the following variables: cardiac disease; SCS; diabetes; calcification or ulceration of plaque; lesion length >15 mm; the need for pre-dilation; presence of a type III arch, bovine arch, or arch calcification; procedure time >30 minutes; and operator experience <50 CAS procedures (54). Similar items are considered in Stenting and Angioplasty With Protection in Patients at HIgh Risk for Endarterectomy (SAPPHIRE) trial, this study has found as independent predictors of adverse outcomes: advanced age, previous stroke or TIA, myocardial infarction, dialysis, need for cardiac surgery in addition to carotid revascularization, a longer carotid plaque, type II or III aortic arch, and tortuosity of the carotid (55).
Gender medicine
Men and women have several differences, in the epidemiology of stroke medicine too. In their lifetime, women have a greater risk of stroke (56); moreover, they have more complications after CEA or CAS than men, in particular the risk of stroke recurrence and death is higher (20,57,58).
Furthermore, the carotid stenosis was different between sexes; in particular, the plaque in women is more stable with more smooth-muscle e minor inflammatory infiltration. Also the other features of instability and risk of embolization, such as lipid-rich necrotic cores, are less represented in women (59). The causes of the propensity to develop more peri-procedural risk is still debated, a reason may be found in the susceptibility to collect more comorbidities during their life (60). In randomized clinical trials the benefit of CEA in women with ACS is uncertain (61,62); on the other hand CEA for women with SCS (stenosis ≥70% excluding near occlusion) has substantial benefit in term of stoke recurrence prevention (17). Inter alia in a meta-analysis that explore sex differences in CEA for SCS, including data from NASCET and ECST, women with 70-99% stenosis show benefit from revascularization in the first two week from index event. The benefit declines rapidly with increasing delay in women and no differences are found between medical treatment and surgery after 14 days (63). The main determinant of this sex difference was a more rapid fall with time in the risk of stroke in the medical group in women than in men (63). A meta-analysis of symptomatic women who underwent CEA or CAS shown a peri-procedural rate of stroke higher after CAS than after CEA (44). In the CREST, that enrolled both SCS and ACS, women assigned to CAS presented more peri-procedural ischaemic stroke with respect to CEA and this difference is more evident than in men (64). Emboli protection devices could aid to overcome this limitation of CAS and improve the outcome in women with ACS undergoing CAS with respect to CEA (65).
Moreover, a recent study shows that females with carotid disease less frequently receive optimal medical treatment and among symptomatic CEA patients, the female sex is associated with higher mortality, among asymptomatic CAS patients, females experience higher rates of stroke/death. The authors suggest that careful patients selection is necessary in the treatment of female patients (66).
In contrast with main RCTs, in a large real-world analysis, the Society for Vascular Surgery-Vascular Registry, women and men demonstrated similar rates of perioperative events after CEA and CAS and were independent of symptomatic status (67). But several limitations of this analysis need to be addressed, as underlined by authors: the inherent weaknesses in the use of a registry, the potential for treatment bias, absence of certain anatomic information (such as plaque morphology), and reporting bias, the lack of a comparison group for patients treated with best medical therapy (67).
PM—GPP
The following points represent indications stated by our group (derived from SPREAD Italian guideline group), based on low degree of evidence but useful to stratify patients and personalize treatment.
PM—GPP 1
For SCS referred to surgery, the patient’s risk score for stroke upon medical therapy alone—and thus the benefit gained from surgery—should be considered: in patients with a high score (≥4, according to the NASCET and ECST revisions) (see Table 1).
PM—GPP 2
When performing CEA or CAS for SCS, the following prognostic factors of higher peri-procedural risk should be considered: female gender, non-ocular cerebral ischaemic event; ipsilateral ischaemic lesion at neuroimaging; contralateral carotid occlusion, especially for CEA; and instable carotid plaque, especially for CAS.
PM—GPP 3
CEA or CAS should be taken into account for the treatment of ACS judged “at risk” for medical therapy alone if at least one of the followings are present: (I) previous stroke or territorial lesion at neuroimaging; (II) vulnerable, ulcerated or rapidly growing plaque; (III) pre-occlusive stenosis; (IV) 70–80% stenosis in the presence of contralateral carotid occlusion; or (V) ipsilateral microemboli at TCD, despite best medical treatment.
On the other hand, best medical treatment alone should be considered the best choice in female patient or if survival is estimated to be shorter than that presumably obtained by CAS or CEA for example in ultra-octogenarians and in patients with comorbidity (insulin-dependent diabetes, serious cardiomyopathy, respiratory diseases or chronic renal insufficiency receiving dialysis).
PM—GPP 4
In order to assess the risks/benefits from surgical intervention for ACS, the predictive peri-procedural score for major complications (stroke, myocardial infarction, death) should be considered.
According to the most recent models, the expected benefit from invasive procedure is marked in patients with a low score (<4%); marginal in patients with an intermediate score [4–7]; and negligible in patients with a high score (>7), in which case the best medical treatment alone should be administered.
Score points are assigned as follows: 0, for age <60 years; −1, for age 60–79 years; +2, for age ≥80 years; +2, for the presence of dyspnea; +3, for the presence of chronic obstructive pulmonary disease; +3, for previous revascularization of the legs or amputation of an extremity; +4, for angina pectoris in the previous month; and +5, if the patient is totally dependent on others for his/her everyday activities.
PM—GPP 5
CAS—executed with appropriate procedural quality—should be performed on patients presenting with a major cardiac and/or pulmonary comorbidity or with at least one of the following: (I) paralysis of the contralateral laryngeal nerve; (II) stenosis extending cranially or clavically; (III) restenosis; and (IV) prior tracheotomy or neck surgery/radiotherapy. By convention, major cardiac comorbidity includes: congestive heart failure and/or left ventricular dysfunction; heart surgery in the previous 6 weeks; myocardial infarction in the previous month; and unstable angina. In ultra-septuagenarians not presenting with a major comorbidity, it should be considered to prefer CEA over CAS for the surgical treatment of carotid stenosis, especially if SCS and if the surgery is done within a short interval from symptoms.
PM—GPP 6
For CAS, the following predictive factors of higher peri-procedural risk for major complications (stroke, myocardial infarction, death) should be considered: (I) ischaemic or dilated cardiomyopathy; (II) female gender; (III) diabetes mellitus; (IV) SCS; (V) plaque calcification or ulceration; (VI) stenosis with a length >13 mm; (VII) bovine or type III aortic arch; (VIII) calcification of the aortic arch; (IX) pre-occlusive stenosis; and evidence of (X) major lesions of the white matter upon neuroimaging.
PM—GPP 7
When the choice between performing CAS or CEA for revascularization is not clear, it is opportune to: use an integrated interdisciplinary approach, with specialists in the cerebro- and cardio-vascular diseases, imaging, traditional and endovascular surgery and anaesthesiology. The experience of the centre and of the operator must be taken in consideration; it is necessary adopt the locally agreed, coordinated, and shared standard operating procedure; consider the option of best medical therapy alone; and to consider enrolling the patient in a controlled, comparative, prospective study.
Conclusions
The current guidelines on CEA versus CAS versus optimal medical therapy alone in patient with SCS and ACS, classically based on EBM pyramid, with classical recommendations for idealized “median patient” as resulting from large trials of comparison, may be conjugated with for good clinical practice. Moreover, there are grey zones in literature about these areas that current guidelines are not able to encompass and clarify. PM and tailored indications for single patient, often characterized by comorbidity, can offer a valid solution by adding to classical recommendations some GPPs and suggestions derived by analysing data of subgroups of patients from meta-analysis, RCTs, registries and applying risk/benefit scores.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Dr. Kosmas I. Paraskevas) for the series “Carotid Artery Stenosis and Stroke: Prevention and Treatment Part I” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-1126). The series “Carotid Artery Stenosis and Stroke: Prevention and Treatment Part I” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Evidence-Based Medicine Working Group. Evidence-Based Medicine. JAMA 1992;268:2420. [Crossref] [PubMed]
- Sackett DL, Rosenberg WMC, Gray JAM, et al. Evidence based medicine: What it is and what it isn’t. It’s about integrating individual clinical expertise and the best external evidence. Br Med J 1996;312:71-2. [Crossref]
- Abbott AL, Paraskevas KI, Kakkos SK, et al. Systematic Review of Guidelines for the Management of Asymptomatic and Symptomatic Carotid Stenosis. Stroke 2015;46:3288-301. [Crossref] [PubMed]
- Lanza G, Lanza J, Ricci S, et al. Personalized medicine: New enhancement to guidelines on carotid endarterectomy and stenting. Ital J Vasc Endovasc Surg 2019;26:151-5. [Crossref]
- Tinetti ME, Bogardus ST, Agostini J V. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004;351:2870-4. [Crossref] [PubMed]
- Greenhalgh T, Howick J, Maskrey N, et al. Evidence based medicine: A movement in crisis? BMJ 2014;348:g3725. [Crossref]
- Hood L, Balling R, Auffray C. Revolutionizing medicine in the 21st century through systems approaches. Biotechnol J 2012;7:992-1001. [Crossref] [PubMed]
- Paraskevas KI, Naylor AR. External Validation of Randomized Trial Outcomes Following Carotid Interventions in the Modern Era. Angiology 2017;68:669-74. [Crossref] [PubMed]
- Paraskevas KI, de Borst GJ, Veith FJ. Why randomized controlled trials do not always reflect reality. J Vasc Surg 2019;70:607-614.e3. [Crossref] [PubMed]
- Gerstein HC, McMurray J, Holman RR. Real-world studies no substitute for RCTs in establishing efficacy. Lancet 2019;393:210-1. [Crossref] [PubMed]
- Collins R, Bowman L, Landray M, et al. The Magic of Randomization versus the Myth of Real-World Evidence. N Engl J Med 2020;382:674-8. [Crossref] [PubMed]
- Mehndiratta MM, Pandey S, Kuntzer T. Acetylcholinesterase inhibitor treatment for myasthenia gravis. Cochrane Database Syst Rev 2014.CD006986. [PubMed]
- Setacci C, De Rango P. A light in the shadows of carotid artery stenting. Eur J Vasc Endovasc Surg 2010;39:527-8. [Crossref] [PubMed]
- North American Symptomatic Carotid Endarterectomy Trial Collaborators, Barnett HJ, Taylor DW, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445-53. [Crossref] [PubMed]
- Warlow C. MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70-99%) or with mild (0-29%) carotid stenosis. Lancet 1991;337:1235-43. [Crossref] [PubMed]
- Rothwell PM, Eliasziw M, Gutnikov SA, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet 2003;361:107-16. [Crossref] [PubMed]
- Rothwell PM, Eliasziw M, Gutnikov SA, et al. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet 2004;363:915-24. [Crossref] [PubMed]
- SPREAD. Ictus cerebrale. Linee Guida Italiane di Prevenzione e Trattamento dell'Ictus Ischemico 2016;1-151. Available online: http://www.iso-stroke.it
- Morris DR, Ayabe K, Inoue T, et al. Evidence-based carotid interventions for stroke prevention: State-of-the-art review. J Atheroscler Thromb 2017;24:373-87. [Crossref] [PubMed]
- Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998;351:1379-87. [Crossref] [PubMed]
- Ferguson GG, Eliasziw M, et al. The North American Symptomatic Carotid Endarterectomy Trial: surgical results in 1415 patients. Stroke 1999;30:1751-8. [Crossref] [PubMed]
- Melin AA, Schmid KK, Lynch TG, et al. Preoperative frailty Risk Analysis Index to stratify patients undergoing carotid endarterectomy. J Vasc Surg 2015;61:683-9. [Crossref] [PubMed]
- Abbott AL, Brunser AM, Giannoukas A, et al. Misconceptions regarding the adequacy of best medical intervention alone for asymptomatic carotid stenosis. J Vasc Surg 2020;71:257-69. [Crossref] [PubMed]
- Nicolaides AN, Kakkos SK, Griffin M, et al. Severity of Asymptomatic Carotid Stenosis and Risk of Ipsilateral Hemispheric Ischaemic Events: Results from the ACSRS Study. Eur J Vasc Endovasc Surg 2005;30:275-84. [Crossref] [PubMed]
- Geroulakos G, Ramaswami G, Nicolaides A, et al. Characterization of symptomatic and asymptomatic carotid plaques using high-resolution real-time ultrasonography. Br J Surg 1993;80:1274-7. [Crossref] [PubMed]
- Nicolaides AN, Kakkos SK, Griffin M, et al. Effect of Image Normalization on Carotid Plaque Classification and the Risk of Ipsilateral Hemispheric Ischemic Events: Results from the Asymptomatic Carotid Stenosis and Risk of Stroke Study. Vascular 2005;13:211-21. [Crossref] [PubMed]
- Paraskevas KI, Veith FJ, Spence JD. How to identify which patients with asymptomatic carotid stenosis could benefit from endarterectomy or stenting. Stroke Vasc Neurol 2018;3:92-100. [Crossref] [PubMed]
- Paraskevas KI, Spence JD, Veith FJ, et al. Identifying which patients with asymptomatic carotid stenosis could benefit from intervention. Stroke 2014;45:3720-4. [Crossref] [PubMed]
- King A, Markus HS. Doppler embolic signals in cerebrovascular disease and prediction of stroke risk: a systematic review and meta-analysis. Stroke 2009;40:3711-7. [Crossref] [PubMed]
- Paraskevas KI, Gloviczki P. Prognostic factors of long-term survival to guide selection of asymptomatic patients for carotid endarterectomy. Int Angiol 2020;39:29-36. [Crossref] [PubMed]
- Spence JD, Tamayo A, Lownie SP, et al. Absence of microemboli on transcranial Doppler identifies low-risk patients with asymptomatic carotid stenosis. Stroke 2005;36:2373-8. [Crossref] [PubMed]
- Spence JD, Coates V, Li H, et al. Effects of intensive medical therapy on microemboli and cardiovascular risk in asymptomatic carotid stenosis. Arch Neurol 2010;67:180-6. [Crossref] [PubMed]
- Grønholdt MLL, Nordestgaard BG, Wiebe BM, et al. Echo-lucency of computerized ultrasound images of carotid atherosclerotic plaques are associated with increased levels of triglyceride-rich lipoproteins as well as increased plaque lipid content. Circulation 1998;97:34-40. [Crossref] [PubMed]
- Topakian R, King A, Kwon SU, et al. Ultrasonic plaque echolucency and emboli signals predict stroke in asymptomatic carotid stenosis. Neurology 2011;77:751-8. [Crossref] [PubMed]
- Miwa K, Hoshi T, Hougaku H, et al. Silent cerebral infarction is associated with incident stroke and TIA independent of carotid intima-media thickness. Intern Med 2010;49:817-22. [Crossref] [PubMed]
- Kakkos SK, Sabetai M, Tegos T, et al. Silent embolic infarcts on computed tomography brain scans and risk of ipsilateral hemispheric events in patients with asymptomatic internal carotid artery stenosis. J Vasc Surg 2009;49:902-9. [Crossref] [PubMed]
- Kakkos SK, Griffin MB, Nicolaides AN, et al. The size of juxtaluminal hypoechoic area in ultrasound images of asymptomatic carotid plaques predicts the occurrence of stroke. J Vasc Surg 2013;57:609-18.e1; discussion 617-8.
- Eliasziw M, Streifler JY, Fox AJ, et al. Significance of plaque ulceration in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial. Stroke 1994;25:304-8. [Crossref] [PubMed]
- Faggioli GL, Pini R, Mauro R, et al. Identification of carotid “vulnerable plaque” by contrast-enhanced ultrasonography: correlation with plaque histology, symptoms and cerebral computed tomography. Eur J Vasc Endovasc Surg 2011;41:238-48. [Crossref] [PubMed]
- van Engelen A, Wannarong T, Parraga G, et al. Three-dimensional carotid ultrasound plaque texture predicts vascular events. Stroke 2014;45:2695-701. [Crossref] [PubMed]
- Gupta A, Chazen JL, Hartman M, et al. Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion: a systematic review and meta-analysis. Stroke 2012;43:2884-91. [Crossref] [PubMed]
- Gupta PK, Ramanan B, Mactaggart JN, et al. Risk index for predicting perioperative stroke, myocardial infarction, or death risk in asymptomatic patients undergoing carotid endarterectomy. J Vasc Surg 2013;57:318-26. [Crossref] [PubMed]
- Zhang L, Zhao Z, Ouyang Y, et al. Systematic Review and Meta-Analysis of Carotid Artery Stenting Versus Endarterectomy for Carotid Stenosis: A Chronological and Worldwide Study. Medicine 2015;94:e1060. [Crossref] [PubMed]
- Bonati LH, Lyrer P, Ederle J, et al. Percutaneous transluminal balloon angioplasty and stenting for carotid artery stenosis. Cochrane database Syst Rev 2012.CD000515. [PubMed]
- SPACE Collaborative Group, Ringleb PA, Allenberg J, et al. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet 2006;368:1239-47. [Crossref] [PubMed]
- Mas JL, Trinquart L, Leys D, et al. Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: results up to 4 years from a randomised, multicentre trial. Lancet Neurol 2008;7:885-92. [Crossref] [PubMed]
- Mantese VA, Timaran CH, Chiu D, et al. CREST Investigators. The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST): stenting versus carotid endarterectomy for carotid disease. Stroke 2010;41:S31-4. [Crossref] [PubMed]
- Bonati LH, Dobson J, Featherstone RL, et al. Long-term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: the International Carotid Stenting Study (ICSS) randomised trial. Lancet 2015;385:529-38. [Crossref] [PubMed]
- Brott TG, Calvet D, Howard G, et al. Long-term outcomes of stenting and endarterectomy for symptomatic carotid stenosis: a preplanned pooled analysis of individual patient data. Lancet Neurol 2019;18:348-56. [Crossref] [PubMed]
- Batchelder AJ, Saratzis A, Ross Naylor A. Editor’s Choice - Overview of Primary and Secondary Analyses From 20 Randomised Controlled Trials Comparing Carotid Artery Stenting With Carotid Endarterectomy. Eur J Vasc Endovasc Surg 2019;58:479-93. [Crossref] [PubMed]
- Narins CR, Illig KA. Patient selection for carotid stenting versus endarterectomy: a systematic review. J Vasc Surg 2006;44:661-72. [Crossref] [PubMed]
- Cremonesi A, Setacci C, Bignamini A, et al. Carotid artery stenting: first consensus document of the ICCS-SPREAD Joint Committee. Stroke 2006;37:2400-9. [Crossref] [PubMed]
- Lanza G, Setacci C, Cremonesi A, et al. Carotid artery stenting: second consensus document of the ICCS/ISO-SPREAD joint committee. Cerebrovasc Dis 2014;38:77-93. [Crossref] [PubMed]
- Setacci C, Chisci E, Setacci F, et al. Siena carotid artery stenting score: a risk modelling study for individual patients. Stroke 2010;41:1259-65. [Crossref] [PubMed]
- Wimmer NJ, Yeh RW, Cutlip DE. at al. Risk prediction for adverse events after carotid artery stenting in higher surgical risk patients. Stroke 2012;43:3218-24. [Crossref] [PubMed]
- Seshadri S, Beiser A, Kelly-Hayes M, et al. The lifetime risk of stroke: estimates from the Framingham Study. Stroke 2006;37:345-50. [Crossref] [PubMed]
- Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998;339:1415-25. [Crossref] [PubMed]
- McArdle MJ, Abbott AL, Krajcer Z. Carotid artery stenosis in women. Tex Heart Inst J 2018;45:243-5. [Crossref] [PubMed]
- Hellings WE, Pasterkamp G, Verhoeven BAN, et al. Gender-associated differences in plaque phenotype of patients undergoing carotid endarterectomy. J Vasc Surg 2007;45:289-96; discussion 296-7. [Crossref] [PubMed]
- Poisson SN, Johnston SC, Sidney S, et al. Gender differences in treatment of severe carotid stenosis after transient ischemic attack. Stroke 2010;41:1891-5. [Crossref] [PubMed]
- Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995;273:1421-8. [PubMed]
- Halliday A, Harrison M, Hayter E, et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet 2010;376:1074-84. [Crossref] [PubMed]
- Rothwell PM, Eliasziw M, Gutnikov SA. at al. Sex difference in the effect of time from symptoms to surgery on benefit from carotid endarterectomy for transient ischemic attack and nondisabling stroke. Stroke 2004;35:2855-61. [Crossref] [PubMed]
- Howard VJ, Lutsep HL, Mackey A, et al. Influence of sex on outcomes of stenting versus endarterectomy: a subgroup analysis of the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST). Lancet Neurol 2011;10:530-7. [Crossref] [PubMed]
- Weinberg I, Beckman JA, Matsumura JS, et al. Carotid Stent Fractures Are Not Associated With Adverse Events: Results From the ACT-1 Multicenter Randomized Trial (Carotid Angioplasty and Stenting Versus Endarterectomy in Asymptomatic Subjects Who Are at Standard Risk for Carotid Endarterectomy With S. Circulation 2018;137:49-56. [Crossref] [PubMed]
- Dansey KD, Pothof AB, Zettervall SL, et al. Clinical impact of sex on carotid revascularization. J Vasc Surg 2020;71:1587-1594.e2. [Crossref] [PubMed]
- Jim J, Dillavou ED, Upchurch GR, et al. Gender-specific 30-day outcomes after carotid endarterectomy and carotid artery stenting in the Society for Vascular Surgery Vascular Registry. J Vasc Surg 2014;59:742-8. [Crossref] [PubMed]


