
Hemodynamic monitoring using trans esophageal echocardiography in patients with shock
Introduction
Circulatory shock is a life-threatening condition responsible for inadequate tissue perfusion (1), that can quickly lead to multiorgan failure. The objectives of hemodynamic monitoring in this setting are multiple: identifying the mechanisms of shock (hypovolemic, distributive, cardiogenic, obstructive); choosing the adequate therapeutic intervention, and evaluating the patient’s response (2).
Echocardiography is proposed as a first line tool for this assessment in the intensive care unit (ICU) (1,3) and in the emergency department (4,5). It is used as “point-of-care ultrasonography” (POCUS), which means that the exam is performed in order to answer specific clinical questions. Hemodynamically unstable patients should receive Critical Care echocardiography (CCE) at least for initial evaluation [recommendation Grade 1B, (6)], and CCE could be considered as a semi-continuous hemodynamic monitoring tool thereafter (7).
CCE is divided into basic and advanced (8,9). Basic CCE is useful for the intensivist to guide in the etiological diagnosis of hemodynamic insufficiency. Trans-Thoracic Echocardiography (TTE) is the first recommended technique in non-ventilated patients, but Trans Esophageal Echocardiography (TEE) should be used when TTE does not provide the answer. Some authors evaluated its feasibility in the emergency department for intubated patients in the case of undifferentiated hypotension (5). A scoring system was proposed to evaluate skills of intensivists for TEE hemodynamic monitoring (10).
Role of TEE as a monitoring tool
Training period is shorter for TEE than for TTE, and the technique is less operator-dependent. It can be quickly and safely performed by fellows with faculty supervision (11). The use of computerized echocardiographic simulation can also help improve the learning curve (12). TEE performed by advanced intensivists showed a good accuracy compared to the gold standard of a cardiology-led TTE or TEE (13). For advanced CCE, a minimum of 100 TTE studies and more than 30 TEE studies is required (depending on countries and type of accreditation) (9,14), and its aim is a more detailed and comprehensive approach of circulatory failure, from diagnosis to monitoring of treatments. As compared to TTE, TEE offers a better echogenicity in case of invasive mechanical ventilation, obesity, surgical dressings, chest tubes and is feasible in prone position (15). It is the best way to evaluate deep anatomic structures and their alterations (including superior vena cava, patent foramen ovale, valve morphology and endocarditis) with a much better sensitivity than TTE (16,17). It is also superior to TTE for the diagnosis of aortic dissection (18), localized pericardial hematoma (after cardiac surgery), left atrial thrombus, and extracorporeal membrane oxygenation (ECMO) canula position (19).
Diagnostic accuracy and therapeutic implications of TEE
In a review of 20 studies in 2,508 patients, the diagnostic impact of TEE in ICU patients was estimated at 67.2% (20). In a study by Vignon et al., the diagnostic accuracy of TEE in patients under mechanical ventilation was superior to TTE, but TEE required a longer physician’s presence. When TTE and TEE were scheduled, TEE yielded an additional diagnosis or excluded with more confidence a suspected diagnosis, except in two cases, and TEE had a therapeutic impact more frequently than TTE (21). Other studies reported the superiority of TEE as compared to TTE for the diagnosis of unexplained hypotension (22,23). TEE also detected more accurately acute cor pulmonale (ACP) and patent foramen ovale than TTE (24).
The therapeutic implication of TEE was confirmed in several studies, leading to change in clinical management between 38% (11) and 79% of cases (25). Similar findings were reported when patients were monitored simultaneously by a pulmonary artery catheter, with a therapeutic implication of TEE in 44% of cases (26).
Comparison with thermodilution based hemodynamic monitoring
TEE allows depicting sources of inaccuracy of thermodilution-based hemodynamic monitoring (27) like ACP, severe left-sided valvulopathies, dynamic left ventricular outflow tract obstruction, and severe low flow state. In this study, comparing hemodynamic assessment of patients in septic shock with transpulmonary thermodilution versus CCE, the interpretation of the two techniques was concordant in 87/132 patients without ACP for bedside clinicians (66%), with a moderate agreement (kappa 0.48), and up to 77.5% (kappa 0.66) for experts (27). A similar weak agreement between pulmonary artery catheter and TEE was found in others studies. Benjamin et al. (28) reported dissimilar recommendations after pulmonary artery catheter vs. TEE evaluation in 58% of patients.
Accuracy of cardiac output measurement with TEE
As compared to the gold standard of thermodilution technique, echocardiography (29) shows an adequate estimation of cardiac output and of its variations, using pulsed doppler at the level of left ventricle outflow track (LVOT) with TEE (30,31), and TTE (32). A systematic review (33) found a percentage of error between 16% and 48% for the measurement of cardiac output with TEE at the level of LVOT, as compared to thermodilution. These findings were not confirmed in another systematic review (34) in the subgroup of patients undergoing TEE, including 13 studies with 19 sets of data and 606 patients, where there was no significant differences observed between TEE and thermodilution (random effects model: MD, 0.00; 95% CI, −0.12 to 0.11; P=0.98). One of the limits of the measure of cardiac output by echography is the assumption of a symmetrical flow pattern and parabolic flow profile. It is thus more reliable to track the aortic velocity time index (VTI) and its changes (33). The importance of averaging 3 measures of VTI in sinus rhythm and 5 in case of atrial fibrillation to obtain an acceptable precision (interquartile range highest value <10%) was confirmed by a recent study by Jozwiak et al. (35). In this study, the least significant change (LSC) of the VTI between two examinations, performed by the same operator was 11% (5–18%), and was 14% (8–26%) when performed by 2 different operators. These values are close to the definition of positive response to fluid loading, defined by an increase in VTI ≥10% to 15% (depending on the test). In such instances, changes in VTI should be assessed by the same operator, without moving the probe during the whole duration of the test (35). The thermodilution method also has limits in case of tricuspid regurgitation or intracardiac shunt, leading to recycling of the indicator fluid across tricuspid valve and underestimation of cardiac output.
Limitations of TEE
TEE is more time consuming than TTE because of setup time and need for probe decontamination. Although less invasive that other monitoring techniques requiring vessel catheterization, TEE is more invasive than TTE, with a risk of complications including displacement of tracheal tube, and esophageal, hypopharyngeal (36) or gastric injury (37). The complication rate was 2.6%, in a literature review of 2,508 patients (20), but scarce in other series. No major complication was reported in a series of 152 TEE performed by fellows (11).
The risk of bacteremia induced by TEE is low and does not require antimicrobial prophylaxis (38). A recent study showed no significant impact of TEE on microaspiration markers of gastric contents and oropharyngeal secretions and on VAP in intubated critically-ill patients (39).
Absolute contraindications of TEE are esophageal stricture, tumor, perforation, diverticulum and active upper gastrointestinal (GI) bleeding (40). Relative contraindications are history of radiation to neck and mediastinum, gastro-intestinal surgery, recent upper gastro-intestinal bleeding, Barrett’s esophagus, dysphagia, restriction of neck mobility, symptomatic hiatal hernia, coagulopathy, thrombocytopenia, active esophagitis or peptic ulcer. Esophageal varices grade 1 or 2 without red signs are not considered a contraindication (41,42).
Practical use of TEE in the hemodynamic evaluation of shock
An expert round table listed the relevant following clinical questions to be addressed with advanced CCE in patients with circulatory compromise (9):
- Is tamponade present?
- What is the stroke volume and cardiac output? Is it decreased?
- Is the heart preload sensitive? What is the efficacy and tolerance of fluid challenge?
- Is LV systolic dysfunction present? Are there regional wall motion abnormalities? Is this LV dysfunction acute (and potentially reversible, e.g., septic myocardial dysfunction, or acute myocarditis)?
- Is right ventricle (RV) systolic dysfunction present? Is ACP present? Is it related to a proximal pulmonary embolism?
- Is LV diastolic dysfunction present?
- Is a severe valvular disease or prosthetic dysfunction present?
- Is there a relevant obstruction to LV ejection?
In specific settings:
- Acute myocardial infarction:
- Are LV regional wall motion abnormalities extended? Is an LV pseudoaneurysm, thrombus, or pericardial effusion present? A ventricular septal defect with active shunting? A rupture of papillary muscle with massive mitral regurgitation?
- Endocarditis:
- Are there vegetations? Are there obstructive? Is there an annular abscess, valvular lesions with severe regurgitation, intracardiac or great vessels anatomical shunt?
- Acute aortic syndrome:
- Are there signs of blood extravasation (hemopericardium, hemothorax)? Is the aorta abnormal (dissection, wall hematoma, ulcer)?
- Severe chest trauma:
- Is there myocardial contusion, hemopericardium, acute valvular insufficiency, septal defect, aortic injury (isthmus), hemomediastinum, left hemothorax? (43).
- Postcardiac surgery:
- Is there a compressive mediastinal hematoma or loculated pericardial effusion?
An examination sequence designed to rapidly assess the patient with hemodynamic failure has been proposed by Charron et al. (10,40).
Early phase of shock
The first step is to characterize the mechanisms of circulatory failure among hypovolemia, vasoplegia, cardiac dysfunction, and obstruction (Figure 1). Briefly, echocardiographic findings will be different depending on the mechanism of shock:
- Hypovolemia: normal or hyperkinetic LVEF, low LV filling pressure, collapse of vena cava, LV dynamic obstruction, telesystolic obliteration of LV;
- Vasoplegia: normal or hyperkinetic LVEF, low LV filling pressure;
- Cardiac dysfunction: LV or RV hypokinesia, low cardiac output, low or elevated LV filling pressure;
- Obstruction: ACP (RV dilation and paradoxical interventricular septum) secondary to pulmonary embolism, or compressive pericardial effusion with impaired RV and LV relaxation.
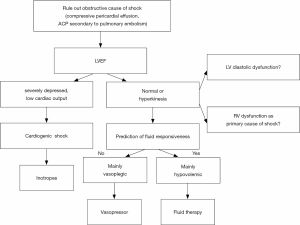
Within this first step, the first question to answer is whether the shock is cardiogenic/obstructive or not. TEE allows ruling out a compressive pericardial effusion, including localized hematoma after cardiac surgery (44). In a series of 61 patients, Heidenreich et al. showed that a diagnosis of valvular or pericardial cause of hypotension, leading to a rapid and specific treatment, was associated with a better prognosis than a diagnosis of ventricular cause or vasoplegia (22). Among cardiogenic causes of circulatory failure, TEE can diagnose left ventricular dysfunction and low cardiac output, right ventricular failure (RV myocardial infarction, ACP), severe valve heart diseases (regurgitation, endocarditis). It can also diagnose aortic dissection, and obstructive shock secondary to pulmonary embolism (when a thrombus is seen in the proximal pulmonary artery (45,46) or cardiac tamponade (when a pericardial effusion is compressive) (47).
TEE qualitative evaluation of LV systolic function (normal, moderately or severely depressed), RV diastolic size (normal, moderately or markedly enlarged), respiratory changes of the superior vena cava (no variation, minor, major respiratory variations), and RV pressure overload (absence or presence of dyskinesia of interventricular septum) showed a good accuracy compared to a quantitative evaluation (48). These parameters can help scrutinize the various mechanisms of shock, as during sepsis.
Hemodynamic profiles
Several mechanisms of circulatory failure can be intricated, as seen in septic shock. Geri et al described five cardiovascular phenotypes, using a cluster analysis of TEE parameters recorded in the 12 first hours of shock (49): left ventricular (LV) systolic dysfunction (LVEF <40% and Aortic VTI <14 cm and LV fractional area contraction <33%), LV hyperkinesia (aortic VTI >20 cm and heart rate <106 bpm and LV fractional area contraction >58%), still hypovolemia (aortic VTI <16 cm and E wave <67 cm/s and superior vena cava respiratory variation >39%), right ventricular failure (RV/LV end diastolic area >0.8 and systolic arterial blood pressure <100 mmHg and diastolic arterial blood pressure <51 mmHg) and well-resuscitated phenotype. This could help physicians individualize their hemodynamic support.
LV systolic function
Qualitative evaluation of LV ejection fraction (LVEF) is easy and quick to assess and to learn (28,48,50,51). LV fractional area contraction at the mid part of the LV is also easy to assess as a surrogate of LVEF. However, LVEF is dependent of loading conditions, and does not reflect intrinsic myocardial contractility, but the coupling between LV contractility and LV afterload (52), the latter being particularly reduced during septic or other vasoplegic shocks. Normal LVEF may be thus observed when afterload is severely impaired, despite seriously decreased intrinsic LV contractility. Arterial tone restoration may unmask depressed LVEF (53). Precise evaluation of afterload is crucial to adequately interpret LV systolic function in this setting (54), and echographic evaluation at the early phase of shock should be thus repeated after correction of hypovolemia and vasoplegia.
Among other contractility parameters, tissue Doppler peak systolic wave at the lateral mitral valve annulus did not significantly correlate with afterload, suggesting its relative independence from loading conditions (54,55). Measure of strain by speckle tracking was more than twice as often depressed than LVEF in septic shock patients, probably revealing covert myocardial dysfunction (54). This could be an interesting tool but the feasibility is limited in clinical practice. A recent meta-analysis using speckle tracking suggested an association of depressed strain with death in septic shock (56), whereas this association was not found with LVEF (57).
LV diastolic function
The assessment of LV diastolic function includes the evaluation of LV relaxation and filling pressures, as well as the assessment of LV obstruction.
Evaluation of LV relaxation (with tissue Doppler peak diastolic e’ wave velocity at the lateral mitral valve annulus <10 cm/s) and other parameters recommended by experts (58,59), is important in advanced cardiac monitoring. It has been suggested that it could have a prognostic role in septic shock (57,60). Other mechanisms of shock may indirectly impair diastolic function and filling of the heart, such as tamponade or ACP, as well as tachycardia related to shock.
Anatomic (severe mitral or aortic stenosis, severe hypertrophic cardiomyopathy) or functional LV obstruction (severe hypovolemia) can also lead to hemodynamic compromise. LV intraventricular gradient is detected with continuous Doppler. Presence of dynamic LV intraventricular obstruction triggered by hypovolemia in hyperdynamic patients at the early stage of septic shock has been found to be associated with a worse prognosis (61).
At the early phase of shock, LV filling pressures are often low or normal, except in cardiogenic shock. The evaluation of LV filling pressure with TEE uses the ratio E/e’ (peak Doppler velocity of early diastolic transmitral flow measured with pulsed Doppler, and early diastolic mitral annular velocity measured with tissue Doppler), and is well correlated to pulmonary artery occlusion pression (62,63). E/é <8 predicts normal or low LV filling pressure, whereas E/é >14 predicts elevated pressure, and a value between 8 and 14 cannot predict reliably the LV filling pressure (58,64).
RV function and pericardium
RV failure may be the primary cause of shock, following ACP or RV infarction.
ACP, defined as the association of RV dilation and paradoxical interventricular septal motion, is secondary to a sudden increase in RV afterload, observed in pulmonary embolism or ARDS. During ARDS, ACP is associated with worse outcome, and a score has been proposed to identify patients at risk of ACP and requiring TEE (65,66).
Isolated RV infarction is rare, but one third of infero-posterior infarctions involve the RV. TEE findings can associate right ventricular dilation, hypokinesis, akinesis or dyskinesis of the right ventricular free wall, paradoxical motion of the interventricular and interatrial septum, right atrial enlargement and dysfunction, tricuspid regurgitation, ventricular septal defect and shunting across patent foramen ovale. The pulmonary artery pressure is normal in the case of acute RV infarction. Ischemic RV leads to decrease in RV compliance, reduced filling and decreased RV stroke volume. This results in decrease in LV filling and low cardiac output despite normal LV contractility. The LV compliance is decreased and biventricular diastolic dysfunction contributes to significant hemodynamic compromise (67).
The early recognition of RV failure in patients with shock is crucial to avoid therapies that could lower RV preload or increase RV outflow impedance, and to use specific therapies such as revascularization in case of infarction (65). Echocardiography can help optimize RV fluid loading, although the location of the failing RV in the Franck-Starling relationship can be difficult to estimate.
TEE has some limitations in the evaluation of RV, because of misalignment with tricuspid annulus and tricuspid regurgitation, and often requires a complementary examination with TTE, to measure tissue Doppler peak systolic wave or systolic excursion at the lateral tricuspid valve annulus, and to estimate pulmonary artery systolic pressure with the tricuspid regurgitation (64). In case of low cardiac output, the tricuspid regurgitant gradient may be low, leading to underestimation of pulmonary vascular impedance.
In case of cardiac tamponade, echocardiography can document pulsus paradoxus, with the inspiratory increase of right-sided flows (tricuspid or pulmonary) and the concomitant decrease on the left side (mitral or aortic) in patients breathing spontaneously. This pulsus paradoxus is inverted and complex to analyze during mechanical ventilation. Other echocardiographic signs include inferior vena cava dilation, paradoxical motion of the interventricular septum, LV relaxation impairment and diminished stroke volume (47). TEE is the key examination to detect post-operative compressive localized hematoma, usually unseen with TTE.
Cardiac output and stroke volume
As mentioned above, calculation of the velocity-time integral (VTI) of the LVOT and the area of the subaortic tract crossed by this flow provides estimation of stroke volume, and hence cardiac output when multiplicated by heart rate. Since the area of the subaortic tract does not change over time, it is sufficient to follow VTI to track changes of stroke volume when assessing the efficacity of a therapeutic intervention. There are no “normal” values of cardiac output (CO) during shock. The question is not whether the CO is in a numerical range considered as “normal”, but whether it is adapted to the patient’s needs and tissue perfusion. To answer this question, assessment of clinical or biological signs of hypoperfusion is necessary. Low stroke volume and cardiac output have two main causes: preload insufficiency (relative or absolute hypovolemia) and intrinsic cardiac dysfunction.
Hypovolemia and fluid responsiveness
Absolute (hypovolemic, hemorrhagic shock) or relative (vasoplegic shock) severe hypovolemia can be diagnosed by TEE with indices such as: telesystolic obliteration of LV with papillary muscles kissing at the mid part of the LV in short axis view; LV intraventricular dynamic obstruction; inspiratory collapse of superior vena cava in patients under invasive mechanical ventilation (68-70). After the initial resuscitation, assessment of the benefit/risk balance to continue fluid therapy is important, as fluid overload was shown to be associated with increased mortality (71). In a recent study reevaluating echocardiographic indices used to predict fluid responsiveness, respiratory variation of maximal Doppler velocity in the LVOT was the most sensitive parameter to predict fluid responsiveness in mechanically ventilated patients in shock, and respiratory variation of vena cava diameter was the most specific dynamic index (72).
Hemodynamic monitoring during shock
TEE can be used as a semi-continuous monitoring tool and can be repeated before and after therapeutic interventions (vasopressors, inotropes, fluid therapy, specific treatment such as pericardial effusion evacuation) to evaluate efficacy and tolerance of therapeutic interventions. Several studies showed the feasibility of miniature TEE probe during 72 hours (73-75), although it had no clear impact on the prognosis (73).
There are no prospective randomized trials that have shown an effect of goal directed therapies on patient outcome and mortality (76). A retrospective database study found that the use of TTE was associated with an improvement in 28-day mortality, but the study was restricted to septic patients, with only 37% receiving vasopressors (77). Trans-pulmonary thermodilution has been proposed as a continuous monitoring in patients remaining unstable after initial resuscitation or in case of association with ARDS (3,78). However, there are no studies showing an impact on outcome of these goal-directed strategies, whatever the techniques used: echography (27,73), pulmonary artery catheter (79,80), or transpulmonary thermodilution versus pulmonary artery catheter (81). The choices of the studied populations, of the standardized protocols, and of the studied outcome variables may explain these results.
In a recent monocentric randomized study by Merz et al. (73), 550 patients in circulatory shock were randomized to receive either continuous TEE during 72 hours, or a standard care. The primary outcome, which was time to resolution of hemodynamic instability, did not differ between the two groups. However, time to resolution of hemodynamic instability was shorter with TEE during the 72 hours with the probe in place. Of note, frequency of assessment had no influence on the outcome. However, in this study, more than 60% of patients in each group had a hemodynamic monitoring by pulmonary artery catheter.
Conclusions
TEE plays an important role in the different phases of management of circulatory failure when TTE is not enough to answer the questions (etiological diagnosis, choice of treatment, evaluation of efficacity and tolerance of therapeutics), although it is not a continuous tool of monitoring. One must remind also that TEE results must be integrated in a global evaluation, the first step being clinical examination. Whether goal directed therapy and close hemodynamic monitoring of shock has an impact on outcome remains debated.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Glenn Hernández and Guo-Wei Tu) for the series “Hemodynamic monitoring in critically ill patients” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-2020-hdm-23). The series “Hemodynamic monitoring in critically ill patients” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vincent JL, De Backer D. Circulatory shock. N Engl J Med 2013;369:1726-34. [Crossref] [PubMed]
- Cecconi M, De Backer D, Antonelli M, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med 2014;40:1795-815. [Crossref] [PubMed]
- Teboul JL, Saugel B, Cecconi M, et al. Less invasive hemodynamic monitoring in critically ill patients. Intensive Care Med 2016;42:1350-9. [Crossref] [PubMed]
- Shokoohi H, Boniface KS, Pourmand A, et al. Bedside Ultrasound Reduces Diagnostic Uncertainty and Guides Resuscitation in Patients With Undifferentiated Hypotension. Crit Care Med 2015;43:2562-9. [Crossref] [PubMed]
- Arntfield R, Pace J, Hewak M, et al. Focused Transesophageal Echocardiography by Emergency Physicians is Feasible and Clinically Influential: Observational Results from a Novel Ultrasound Program. J Emerg Med 2016;50:286-94. [Crossref] [PubMed]
- Levitov A, Frankel HL, Blaivas M, et al. Guidelines for the Appropriate Use of Bedside General and Cardiac Ultrasonography in the Evaluation of Critically Ill Patients-Part II: Cardiac Ultrasonography. Crit Care Med 2016;44:1206-27. [Crossref] [PubMed]
- Vieillard-Baron A, Millington SJ, Sanfilippo F, et al. A decade of progress in critical care echocardiography: a narrative review. Intensive Care Med 2019;45:770-88. [Crossref] [PubMed]
- Mayo PH, Beaulieu Y, Doelken P, et al. American College of Chest Physicians/La Societe de Reanimation de Langue Francaise statement on competence in critical care ultrasonography. Chest 2009;135:1050-60. [Crossref] [PubMed]
- Expert Round Table on Echocardiography in ICU. International consensus statement on training standards for advanced critical care echocardiography. Intensive Care Med 2014;40:654-66. [Crossref] [PubMed]
- Charron C, Prat G, Caille V, et al. Validation of a skills assessment scoring system for transesophageal echocardiographic monitoring of hemodynamics. Intensive Care Med 2007;33:1712-8. [Crossref] [PubMed]
- Garcia YA, Quintero L, Singh K, et al. Feasibility, Safety, and Utility of Advanced Critical Care Transesophageal Echocardiography Performed by Pulmonary/Critical Care Fellows in a Medical ICU. Chest 2017;152:736-41. [Crossref] [PubMed]
- Prat G, Charron C, Repesse X, et al. The use of computerized echocardiographic simulation improves the learning curve for transesophageal hemodynamic assessment in critically ill patients. Ann Intensive Care 2016;6:27. [Crossref] [PubMed]
- Lau V, Priestap F, Landry Y, et al. Diagnostic Accuracy of Critical Care Transesophageal Echocardiography vs Cardiology-Led Echocardiography in ICU Patients. Chest 2019;155:491-501. [Crossref] [PubMed]
- Charron C, Vignon P, Prat G, et al. Number of supervised studies required to reach competence in advanced critical care transesophageal echocardiography. Intensive Care Med 2013;39:1019-24. [Crossref] [PubMed]
- Mekontso Dessap A, Proost O, Boissier F, et al. Transesophageal echocardiography in prone position during severe acute respiratory distress syndrome. Intensive Care Med 2011;37:430-4. [Crossref] [PubMed]
- Shively BK, Gurule FT, Roldan CA, et al. Diagnostic value of transesophageal compared with transthoracic echocardiography in infective endocarditis. J Am Coll Cardiol 1991;18:391-7. [Crossref] [PubMed]
- Pedersen WR, Walker M, Olson JD, et al. Value of transesophageal echocardiography as an adjunct to transthoracic echocardiography in evaluation of native and prosthetic valve endocarditis. Chest 1991;100:351-6. [Crossref] [PubMed]
- Willens HJ, Kessler KM. Transesophageal echocardiography in the diagnosis of diseases of the thoracic aorta: part 1. Aortic dissection, aortic intramural hematoma, and penetrating atherosclerotic ulcer of the aorta. Chest 1999;116:1772-9. [Crossref] [PubMed]
- Vignon P, Merz TM, Vieillard-Baron A. Ten reasons for performing hemodynamic monitoring using transesophageal echocardiography. Intensive Care Med 2017;43:1048-1051. [Crossref] [PubMed]
- Hüttemann E, Schelenz C, Kara F, et al. The use and safety of transoesophageal echocardiography in the general ICU -- a minireview. Acta Anaesthesiol Scand 2004;48:827-36. [Crossref] [PubMed]
- Vignon P, Mentec H, Terre S, et al. Diagnostic accuracy and therapeutic impact of transthoracic and transesophageal echocardiography in mechanically ventilated patients in the ICU. Chest 1994;106:1829-34. [Crossref] [PubMed]
- Heidenreich PA, Stainback RF, Redberg RF, et al. Transesophageal echocardiography predicts mortality in critically ill patients with unexplained hypotension. J Am Coll Cardiol 1995;26:152-8. [Crossref] [PubMed]
- Slama MA, Novara A, Van de Putte P, et al. Diagnostic and therapeutic implications of transesophageal echocardiography in medical ICU patients with unexplained shock, hypoxemia, or suspected endocarditis. Intensive Care Med 1996;22:916-22. [Crossref] [PubMed]
- Lhéritier G, Legras A, Caille A, et al. Prevalence and prognostic value of acute cor pulmonale and patent foramen ovale in ventilated patients with early acute respiratory distress syndrome: a multicenter study. Intensive Care Med 2013;39:1734-42. [Crossref] [PubMed]
- Arntfield R, Lau V, Landry Y, et al. Impact of Critical Care Transesophageal Echocardiography in Medical-Surgical ICU Patients: Characteristics and Results From 274 Consecutive Examinations. J Intensive Care Med 2018. [Crossref] [PubMed]
- Poelaert JI, Trouerbach J, De Buyzere M, et al. Evaluation of transesophageal echocardiography as a diagnostic and therapeutic aid in a critical care setting. Chest 1995;107:774-9. [Crossref] [PubMed]
- Vignon P, Begot E, Mari A, et al. Hemodynamic Assessment of Patients With Septic Shock Using Transpulmonary Thermodilution and Critical Care Echocardiography: A Comparative Study. Chest 2018;153:55-64. [Crossref] [PubMed]
- Benjamin E, Griffin K, Leibowitz AB, et al. Goal-directed transesophageal echocardiography performed by intensivists to assess left ventricular function: comparison with pulmonary artery catheterization. J Cardiothorac Vasc Anesth 1998;12:10-5. [Crossref] [PubMed]
- Narasimhan M, Koenig SJ, Mayo PH. Advanced echocardiography for the critical care physician: part 1. Chest 2014;145:129-34. [Crossref] [PubMed]
- Poelaert J, Schmidt C, Van Aken H, et al. A comparison of transoesophageal echocardiographic Doppler across the aortic valve and the thermodilution technique for estimating cardiac output. Anaesthesia 1999;54:128-36. [Crossref] [PubMed]
- Estagnasié P, Djedaini K, Mier L, et al. Measurement of cardiac output by transesophageal echocardiography in mechanically ventilated patients. Comparison with thermodilution. Intensive Care Med 1997;23:753-9. [Crossref] [PubMed]
- Mercado P, Maizel J, Beyls C, et al. Transthoracic echocardiography: an accurate and precise method for estimating cardiac output in the critically ill patient. Crit Care 2017;21:136. [Crossref] [PubMed]
- Wetterslev M, Moller-Sorensen H, Johansen RR, et al. Systematic review of cardiac output measurements by echocardiography vs. thermodilution: the techniques are not interchangeable. Intensive Care Med 2016;42:1223-33. [Crossref] [PubMed]
- Zhang Y, Wang Y, Shi J, et al. Cardiac output measurements via echocardiography versus thermodilution: A systematic review and meta-analysis. PLoS One 2019;14:e0222105. [Crossref] [PubMed]
- Jozwiak M, Mercado P, Teboul JL, et al. What is the lowest change in cardiac output that transthoracic echocardiography can detect? Crit Care 2019;23:116. [Crossref] [PubMed]
- Aviv JE, Di Tullio MR, Homma S, et al. Hypopharyngeal perforation near-miss during transesophageal echocardiography. Laryngoscope 2004;114:821-6. [Crossref] [PubMed]
- Hilberath JN, Oakes DA, Shernan SK, et al. Safety of transesophageal echocardiography. J Am Soc Echocardiogr 2010;23:1115-27; quiz 1220-11.
- Mentec H, Vignon P, Terre S, et al. Frequency of bacteremia associated with transesophageal echocardiography in intensive care unit patients: a prospective study of 139 patients. Crit Care Med 1995;23:1194-9. [Crossref] [PubMed]
- Bagate F RA, Zerimech F. Effect of transesophageal echocardiography on tracheal microaspiration and ventilator-associated pneumonia in intubated critically-ill patients: a multicenter prospective observational study. Annals of Intensive Care 2020;10:16. [PubMed]
- Mayo PH, Narasimhan M, Koenig S. Critical Care Transesophageal Echocardiography. Chest 2015;148:1323-32. [Crossref] [PubMed]
- Nigatu A, Yap JE, Lee Chuy K, et al. Bleeding Risk of Transesophageal Echocardiography in Patients With Esophageal Varices. J Am Soc Echocardiogr 2019;32:674-6.e2. [Crossref] [PubMed]
- Liu E, Guha A, Dunleavy M, et al. Safety of Transesophageal Echocardiography in Patients with Esophageal Varices. J Am Soc Echocardiogr 2019;32:676-7. [Crossref] [PubMed]
- Chirillo F, Totis O, Cavarzerani A, et al. Usefulness of transthoracic and transoesophageal echocardiography in recognition and management of cardiovascular injuries after blunt chest trauma. Heart 1996;75:301-6. [Crossref] [PubMed]
- Chan KL. Transesophageal echocardiography for assessing cause of hypotension after cardiac surgery. Am J Cardiol 1988;62:1142-3. [Crossref] [PubMed]
- Pruszczyk P, Torbicki A, Pacho R, et al. Noninvasive diagnosis of suspected severe pulmonary embolism: transesophageal echocardiography vs spiral CT. Chest 1997;112:722-8. [Crossref] [PubMed]
- Krivec B, Voga G, Zuran I, et al. Diagnosis and treatment of shock due to massive pulmonary embolism: approach with transesophageal echocardiography and intrapulmonary thrombolysis. Chest 1997;112:1310-6. [Crossref] [PubMed]
- Mekontso Dessap A, Chew MS. Cardiac tamponade. Intensive Care Med 2018;44:936-9. [Crossref] [PubMed]
- Vieillard-Baron A, Charron C, Chergui K, et al. Bedside echocardiographic evaluation of hemodynamics in sepsis: is a qualitative evaluation sufficient? Intensive Care Med 2006;32:1547-52. [Crossref] [PubMed]
- Geri G, Vignon P, Aubry A, et al. Cardiovascular clusters in septic shock combining clinical and echocardiographic parameters: a post hoc analysis. Intensive Care Med 2019;45:657-67. [Crossref] [PubMed]
- McGowan JH, Cleland JG. Reliability of reporting left ventricular systolic function by echocardiography: a systematic review of 3 methods. Am Heart J 2003;146:388-97. [Crossref] [PubMed]
- Johnson BK, Tierney DM, Rosborough TK, et al. Internal medicine point-of-care ultrasound assessment of left ventricular function correlates with formal echocardiography. J Clin Ultrasound 2016;44:92-9. [Crossref] [PubMed]
- Robotham JL, Takata M, Berman M, et al. Ejection fraction revisited. Anesthesiology 1991;74:172-83. [Crossref] [PubMed]
- Jardin F, Brun-Ney D, Auvert B, et al. Sepsis-related cardiogenic shock. Crit Care Med 1990;18:1055-60. [Crossref] [PubMed]
- Boissier F, Razazi K, Seemann A, et al. Left ventricular systolic dysfunction during septic shock: the role of loading conditions. Intensive Care Med 2017;43:633-42. [Crossref] [PubMed]
- Aissaoui N, Guerot E, Combes A, et al. Two-dimensional strain rate and Doppler tissue myocardial velocities: analysis by echocardiography of hemodynamic and functional changes of the failed left ventricle during different degrees of extracorporeal life support. J Am Soc Echocardiogr 2012;25:632-40. [Crossref] [PubMed]
- Sanfilippo F, Corredor C, Fletcher N, et al. Left ventricular systolic function evaluated by strain echocardiography and relationship with mortality in patients with severe sepsis or septic shock: a systematic review and meta-analysis. Crit Care 2018;22:183. [Crossref] [PubMed]
- Sanfilippo F, Corredor C, Fletcher N, et al. Diastolic dysfunction and mortality in septic patients: a systematic review and meta-analysis. Intensive Care Med 2015;41:1004-13. [Crossref] [PubMed]
- Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:1321-60. [Crossref] [PubMed]
- Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 2009;10:165-93. [Crossref] [PubMed]
- Sanfilippo F, Corredor C, Arcadipane A, et al. Tissue Doppler assessment of diastolic function and relationship with mortality in critically ill septic patients: a systematic review and meta-analysis. Br J Anaesth 2017;119:583-94. [Crossref] [PubMed]
- Chauvet JL, El-Dash S, Delastre O, et al. Early dynamic left intraventricular obstruction is associated with hypovolemia and high mortality in septic shock patients. Crit Care 2015;19:262. [Crossref] [PubMed]
- Vignon P. Echocardiographic assessment of pulmonary artery occlusion pressure in ventilated patients: a transoesophageal study. Crit Care 2008;12:R18. [Crossref] [PubMed]
- Nagueh SF, Abraham TP, Aurigemma GP, et al. Interobserver Variability in Applying American Society of Echocardiography/European Association of Cardiovascular Imaging 2016 Guidelines for Estimation of Left Ventricular Filling Pressure. Circ Cardiovasc Imaging 2019;12:e008122. [Crossref] [PubMed]
- Narasimhan M. S JK, Mayo PH. Advanced echocardiography for the critical care physician: part 2. Chest 2014;145:135-42. [Crossref] [PubMed]
- Vieillard-Baron A, Naeije R, Haddad F, et al. Diagnostic workup, etiologies and management of acute right ventricle failure: A state-of-the-art paper. Intensive Care Med 2018;44:774-90. [Crossref] [PubMed]
- Mekontso Dessap A, Boissier F, Charron C, et al. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensive Care Med 2016;42:862-70. [Crossref] [PubMed]
- Namana V, Gupta SS, Abbasi AA, et al. Right ventricular infarction. Cardiovasc Revasc Med 2018;19:43-50. [Crossref] [PubMed]
- Hüttemann E. Transoesophageal echocardiography in critical care. Minerva Anestesiol 2006;72:891-913. [PubMed]
- Leung JM, Levine EH. Left ventricular end-systolic cavity obliteration as an estimate of intraoperative hypovolemia. Anesthesiology 1994;81:1102-9. [Crossref] [PubMed]
- Oren-Grinberg A, Talmor D, Brown SM. Focused critical care echocardiography. Crit Care Med 2013;41:2618-26. [Crossref] [PubMed]
- Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 2006;34:344-53. [Crossref] [PubMed]
- Vignon P, Repesse X, Begot E, et al. Comparison of Echocardiographic Indices Used to Predict Fluid Responsiveness in Ventilated Patients. Am J Respir Crit Care Med 2017;195:1022-32. [Crossref] [PubMed]
- Merz TM, Cioccari L, Frey PM, et al. Continual hemodynamic monitoring with a single-use transesophageal echocardiography probe in critically ill patients with shock: a randomized controlled clinical trial. Intensive Care Med 2019;45:1093-102. [Crossref] [PubMed]
- Vieillard-Baron A, Slama M, Mayo P, et al. A pilot study on safety and clinical utility of a single-use 72-hour indwelling transesophageal echocardiography probe. Intensive Care Med 2013;39:629-35. [Crossref] [PubMed]
- Begot E, Dalmay F, Etchecopar C, et al. Hemodynamic assessment of ventilated ICU patients with cardiorespiratory failure using a miniaturized multiplane transesophageal echocardiography probe. Intensive Care Med 2015;41:1886-94. [Crossref] [PubMed]
- Singh K, Mayo P. Critical care echocardiography and outcomes in the critically ill. Curr Opin Crit Care 2018;24:316-21. [Crossref] [PubMed]
- Feng M, McSparron JI, Kien DT, et al. Transthoracic echocardiography and mortality in sepsis: analysis of the MIMIC-III database. Intensive Care Med 2018;44:884-92. [Crossref] [PubMed]
- Vieillard-Baron A, Matthay M, Teboul JL, et al. Experts' opinion on management of hemodynamics in ARDS patients: focus on the effects of mechanical ventilation. Intensive Care Med 2016;42:739-49. [Crossref] [PubMed]
- Rajaram SS, Desai NK, Kalra A, et al. Pulmonary artery catheters for adult patients in intensive care. Cochrane Database Syst Rev 2013;2013:CD003408.
- Shah MR, Hasselblad V, Stevenson LW, et al. Impact of the pulmonary artery catheter in critically ill patients: meta-analysis of randomized clinical trials. JAMA 2005;294:1664-70. [Crossref] [PubMed]
- Trof RJ, Beishuizen A, Cornet AD, et al. Volume-limited versus pressure-limited hemodynamic management in septic and nonseptic shock. Crit Care Med 2012;40:1177-85. [Crossref] [PubMed]


