
Carotid intraplaque haemorrhage: pathogenesis, histological classification, imaging methods and clinical value
Background
Stroke is the second most frequent cause of death in the world and the primary cause of long-term disabilities in western countries (1). At least 80% of all strokes are ischemic and 20% of them are the consequence of the rupture of a vulnerable carotid atherosclerotic plaque located at the carotid bifurcation (1). As strokes resulting from carotid plaque rupture are known to be linked to embolism (2), plaque vulnerability assessment is likely an essential element in the detection of patients at risk for ischemic stroke and should be a factor when considering carotid endarterectomy (CEA) surgery (3). Currently, the decision to perform CEA surgery is only based on the degree of carotid stenosis and cerebrovascular medical history (4). The link between stenosis degree and stroke risk has been widely demonstrated (5), and many national guidelines still claim that stenosis should remain the main criteria to classify plaques and thus the decision of CEA (6) even if evidence has been provided otherwise for asymptomatic patients (7,8) thus this sole factor may be insufficient to evaluate the risk-benefit ratio of CEA surgery in these patients (3,9). A meta-analysis showed that for most asymptomatic patients, a very few ischemic events happened after CEA, thus the risk-benefit ratio resulting from the CEA might be unfavourable (3,7,10). Moreover, the 10 years risk of stroke is only 4.6%, suggesting that 95% of the CEA performed on asymptomatic patient are unnecessary (11,12). Thus, it appears that management of CEA in asymptomatic patients need to be rethought and in vivo identification of vulnerable plaque has to be improved to be commonly used in a clinical setting for the decision of CEA surgery. In this context, non-invasive imaging methods and more specifically magnetic resonance imaging (MRI) appear to be the most sensitive and specific to identify IPH in vivo (13,14), especially in a clinical perspective to predict stroke risk (15-17). In this regards, ESVS guidelines encourage surgeons to use imaging to identify plaque with vulnerable factors in particular intraplaque haemorrhage (IPH) that needs CEA surgery (18). Several vulnerability factors were reported: thin fibrous cap, large lipid-necrotic core volume, monocytes infiltration (19) and IPH (19,20) resulting from immature neovascularization (21). It has been shown that all these factors are unequally involved in plaque destabilisation (19,22,23). Since 1936, IPH has been described as a risk factor of plaque rupture (24). During the 1980s several studies have demonstrated a relationship between carotid IPH and history of ischemic events (25,26). IPH is more prevalent in symptomatic stroke patients, regardless of the time since the event, than in asymptomatic patients (27-30). Carotid IPH has been documented by histological studies over the last 20 years, now it is likely an important factor to consider when classifying vulnerable plaques (26). Currently no drug treatment targeting specifically IPH is available; however statin use is associated with lower prevalence of plaque with neovascularisation (31) and IPH (32). On the contrary, platelet antiaggregant is associated with higher prevalence of IPH (32). In this context, in vivo detection of IPH appears to be one of the most reliable factors to predict cerebral ischemic events (15-17).
Carotid IPH
Pathophysiology
IPH is defined by the accumulation of blood components within the atheromatous plaque (26). IPHs are closely linked to the microcirculation within the plaque. McCarthy et al. showed that symptomatic plaques showed more, larger and more irregular neo-vessels than asymptomatic plaques (33). Indeed an increased neo-vessels density is associated with IPH and rupture of the plaque (33). In advanced atherosclerotic lesions, hypoxia, along with macrophages triggering inflammation and oxidative stress promoting low density lipoprotein (LDL) oxidation into oxidized LDL (Ox-LDL) processes are merged. All these factors lead to the chronic secretion of vascular endothelial growth factor (VEGF) increasing pathological impaired neoangiogenesis (19). Neo-vessels originating from the vasa vasorum develop through the medium and large arteries from adventitia to the intima (19). These neo-vessels, which lack smooth muscle cells and endothelial gap junctions, are disorganized and incomplete (21,34), and thus are prompt to leak. This results in IPH formation (20) and expansion (35) and the transfer of blood cells that promote plaque rupture (36). IPH carries inflammatory cells (37) that increase the necrotic core volume (38) also indirectly leading to vulnerable plaques rupture and subsequent clinic ischemic events (39).
During IPH, leucocytes, platelets and erythrocytes are released. The leaked erythrocytes break down into iron, cholesterol, glycophorin A and ceroids (24). The erythrocytes and leucocytes (37) extravasated from the lumen of neo-vessels into the atherosclerotic plaque, self-sustain inflammation and pro-oxidant mechanisms (19,40) (Figure 1). Indeed, in the hypoxic environment of the plaque, IPH-released neutrophils secrete angiogenic factors such as VEGF and lipid peroxidation by-products, (41,42) known risk-factors for future ischemic events (43). Neutrophils also abundantly express myeloperoxidase, which produce hypochlorous acid and H2O2, leading to a decrease in NO bioavailability thus enhancing endothelial dysfunction (44,45). Ultimately, the lysis of neutrophils release highly pro-oxidant materials, such as DNA histones (46). Moreover, the activation of NAD(P)H oxidases and myeloperoxidases in macrophages are known to produce superoxide (O2•-) enhancing a pro-oxidant environment. To reduce IPH, the leaked erythrocytes are phagocyted mainly by macrophages. This results in the release of haemoglobin and iron which are highly pro-oxidant (24) and pro-inflammatory through the conversion of H2O2 into the highly toxic hydroxyl (OH•). In addition, the erythrocytes plasma membrane, which is composed of 40 percent cholesterol, is the primary source of necrotic core expansion during phagocytosis (20,24,47). Therefore regulation mechanisms are implemented to reduce IPH, such as the anti-inflammatory, cytoprotective shift in macrophages phenotype and the recruitment of haptoglobin that metabolise haemoglobin and recycle iron (48). Unfortunately, they are quickly depleted and become inefficient. Consequently, these closely integrated pro-inflammatory and pro-oxidant processes persist which not only enhances IPH but also promotes the growth of the necrotic core (19,49), increasing the risk of ischemic event (36).
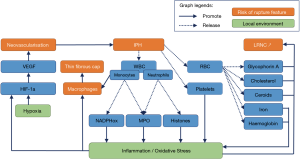
Typically the presence of blood in the atherosclerotic plaque is due to leaky neovessels localized to the plaque shoulder (50). However, in different areas, other mechanisms can lead to the presence of blood within the plaque. Cholesterol crystals due to eryptosis can mechanically break neovessels, which bleed into the atheromatous plaque (51,52). Moreover, cholesterol crystal content are independent predictors of thrombus and cardiovascular events (52). Intraplaque blood can also originate from the integration of a luminal erythrocyte-rich thrombus with the plaque (24,53) or entry of luminal blood (20). Plaque fissures are observed even in plaques with an intact fibrous cap and those that are co-localised with fresh IPH (54). In this case, the thrombus is entrapped into extracellular matrix leading to a narrow lumen, yet it also appears to be a healing process. Histologically, this healing process can be hard to discriminate from IPH (24).
Histology
IPH determination by histology
Histology is the gold standard in studying plaque components like IPH, lipid rich necrotic core (LRNC) and inflammation. Usually, the surgically removed atheromatous plaque also includes the middle part of the media and internal part of the media. In clinical context, surgical pathologists generally evaluate IPH on plaque slices after hematoxylin-eosin (H&E) or Masson trichrome stains, which are non-specific staining for haemorrhage (25,55).
To improve the histological IPHs identifications, several biomarkers are used. The most frequently used is iron, as it links to haemoglobin and is released during erythrocyte phagocytosis; it is highlighted in the adventitia by the Perls technique with Prussian blue stain (Figure 1). Red blood cell (RBC) membrane specific protein cholesterol crystals are detected by immunohistochemistry with glycophorin A, allowing the discrimination of lipid-rich and erythrocyte-rich parts of the necrotic core (Figure 1). Ceroids (56,57) are often co-localised with haem (24) and are markers of senescent RBC but are less specific of IPH than cholesterol crystals (24). They are identified by Raman or fluorescence spectroscopy and are completed by other peroxidation markers. Neutrophils markers such as matrix metallopeptidase-9 (MMP-9), neutrophil gelatinase-associated lipocalin/MMP-9 (NGAL/MMP-9), elastase, CD66b, proteinases 3, myeloperoxidase (MPO) or α-defensins are colocalised with IPH (58). Neutrophils are predictors of recurrent ischemic events.
As these markers are specific to different forms and localisations of RBC, multiple consecutive slice staining could elucidate RBC trafficking and IPH. Moreover, in vulnerable plaques, neo-angiogenesis-derived IPH could be difficult to discriminate from integrate coagulum which is a sign of plaque regression (24). CD34 immunostaining allows for a precise quantification of the micro-vessels density (59). Plaque neo-vascularisation can also be visualised by H&E staining of endothelial cells (60) or von Willebrand factor (factor VIII) staining (61). To characterise leaky neo-vessels, SMC should also be stained for smooth muscle antibody (SMA), as the presence of factor VIII and absence of SMA indicates that the vessel is leaky.
IPH and vulnerable carotid atherosclerotic plaques in histology
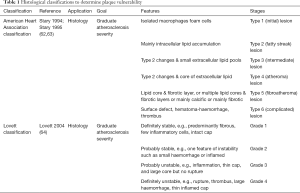
Vulnerable carotid atherosclerotic plaques are composed of various components (Figure 1). The lipid-rich necrotic core harbours lipid content such as cholesterol crystals, but also calcifications and haemorrhagic components. As vulnerable plaques continually evolve, different classifications have been established (Table 1) (62-64) in order to stratify the associated plaque instability and thus the subsequent ischemic risk.
Full table
After surgery, histopathological classifications assess the vulnerability of the carotid atherosclerotic plaque. The American Heart Association classification was the first that aimed to graduate plaque vulnerability (62,63), and more recently, Lovett proposed his own classification, adding the inflammation item (64). IPH is an evolving process due to the different pathophysiological processes that are chronologically involved and the subsequent progression of ischemic risk.
In the preclinical setting, mice (ApoE-/- and LDLR-/- under high cholesterol diet) are the most commonly used animals to study atherosclerosis. However, they are poor experimental models of IPH, neovascularisation and plaque rupture. Therefore, IPH data essentially originate from human clinical studies (24) or from other animal species that develop vulnerable carotid atherosclerotic plaques similar to human plaques. For example, annexin V (which is highly released during eryptosis) injection in aortic atheromatous plaque of de-endothelialized rabbits under cholesterol diet leads to the necrotic core growth, free cholesterol crystals formation and higher macrophage recruitment (65) suggesting the role of eryptosis in unstable atherosclerotic plaques. This suggests that in addition to a direct increase in plaque rupture risk (15), IPH might participate in a pathophysiological vicious circle leading to plaque rupture.
Analysing the plaque components, IPH presence is generally associated with a thin fibrous cap and a large necrotic core (19). Pelisek et al. found in symptomatic and asymptomatic patients that neo-vessels were present in 93.8% of the plaques with over 70% stenosis and 97.1% of these neo-vessels were immature and leaky leading to IPH (66). A histological study (n=526) led by Redgrave et al. on patients with symptomatic carotid plaques who underwent CEA reported that 64.6% of the plaques showed IPH (67). This study also demonstrated that IPH was associated with fibrous cap rupture, independent of other risk factors [OR =3.00 (1.64–5.51)] suggesting that IPH is a contributing factor to plaque vulnerability (67). Vrijenhoek’s team reported that IPH was more frequent in men (67%) than in same aged post-menopausal women (54%), while micro-vessels density was not significantly different (68). Moreover, in the same study IPH leads more often to plaque rupture in men than in women [HR =1.5 (1.1; 2.1)] (68). In most cases, IPH presence was associated with other known risks factors of rupture: necrotic core expansion (19), leaky neo-vessels (20), macrophages accumulation and oxidative stress (19,26).
An Anglo-Dutch group published a histochemistry analysis on symptomatic patients with moderate stenosis. They studied the link between risk factors traditionally associated with plaque rupture and cerebral cardiovascular outcomes (69). Statistical analysis of 1,087 carotid plaques revealed that macrophages infiltration, thrombosis and micro-vessels density [OR (micro-vessels density) 1.49 (1.05; 2.11)] was significantly correlated with plaque vulnerability. On the contrary, no relationship was observed between plaque vulnerability and fibrous cap, lymphocyte infiltration or IPH [OR (IPH) 1.15 (0.84; 1.59)]. The authors suggested that the contradictory findings of neovascularization and IPH could be explained by a blood leakage only present during early IPH in organised stages (Table 2). Nevertheless, this hypothesis was not experimentally tested. Another explanation could be that IPH and thrombosis are hardly discriminable.
IPH histological classifications
None of the previous classification of vulnerable atherosclerotic plaques (62-64) take into account the evolution of IPH, thus several IPH histological classifications were established (70-72). These classifications (71,72) are not commonly used anymore because the classification of Derksen is the most complete and is currently used in anatomopathology. This classification distinguishes four stages of IPH (Table 2) based on histological H&E stain.
According to Derksen’s histological classification, organised IPHs are more vulnerable to breakdown than amorphous and recent IPHs, mainly due to macrophages presence in organised IPHs zones (70). Derksen et al. reported that 81% (over 794 studied) of carotid plaques showed an IPH spreading over an average of 5% of the total plaque volume (70). Among these IPHs, 2% were recent, 11% organised, 75% amorphous, and 9% amorphous with calcifications.
However, so far there is no method that can allow in-vivo diagnosis or follow-up (i.e., imaging) of IPH stages since it is currently only assessable after CEA by histology. Moreover, this criterion is currently not suitable for the clinical decision, because plaque rupture is multifactorial (19,73,74) and other biomarkers need to be taken into account to stratify the ischemic risk (16).
All plaque risk factor of rupture should be evaluated in order to predict future ischemic event, but particular attention should be given in IPH stages assessment especially in the CEA decision for asymptomatic patients.
Imaging
Over the last several years, several imaging techniques have been developed to reliably analyse carotid atherosclerotic plaques composition. In a clinical context, Doppler ultrasonography (75) and computed tomography (CT) (9,10,76) of the supra aortic trunks are commonly used to accurately assess the degree of stenosis. Magnetic resonance angiography (MRA) is being increasingly used (13,77) because it is non-radiant, less nephrotoxic compared to CT, and more accurately examines the cerebral parenchyma.
Ultrasound and contrast enhanced ultrasound
Ultrasounds (US) are the best method to assess the carotid stenosis by imaging (75) but is highly operator dependent. They allow to study the morphologic and hemodynamic features of the plaque by assessing tissues echogenicity. Without contrast medium IPH is hard to discriminate from LRNC only based on carotid plaque echogenicity (78,79). Contrast enhanced US (CEUS) are able to assess neovascularisation in vivo in real time (80). IPH can be indirectly visualised in ultrasonography through the vasa vasorum and neovessels analysis by CEUS (80-82); if results of the sole US examination are inconclusive, presence of neovessels observed with CEUS in the carotid plaque might underline IPH presence. Contrast enhanced microbubbles allow for the visualization of vascularized lesions in hyper echogenicity (82). Results on carotid ultrasound with contrast agent attest that an increased vasa vasorum (83) and microvessels (80) density can enhance the IPH risk. Most of the plaques harbouring an heterogeneous pattern (mixed echoes and anechoic areas) presented IPH at the histological analysis (84).
CT and positron emission tomography (PET) scans
CT is applied in a clinical setting to diagnose morphological abnormalities as stenosis (85), aneurysm or carotid dissection (86) through the tissues density analysis. Computed tomography angiography (CTA) with iodinated contrast medium is required to analyse carotid arteries, but it is also known to underestimates the degree of stenosis (87). However, studies suggest that CTA is able to discriminate IPH parts from lipid-rich and fibrous parts in carotid atherosclerotic plaques (88,89) even if the densities are almost similar (90). Indeed, according to a recent study, IPH can be detected with high sensitivity and specificity by CTA according to attenuation at 25 Hounsfield units (HU) (88). Moreover, calcified rim and soft internal plaques indirectly predict IPH actual presence (91). On symptomatic patients, intraluminal thrombus can be visualized (92). Thus, CT scan is a very specific non-invasive method, but with a limited sensitivity (87). U-King-Im et al. compared CT scans and MRI techniques and found the MRI is a better tool to visualise IPH through plaque ulceration (89). Moreover, CT exposes patients to radiation and thus it is not the first-choice technique to assess IPH.
PET scan is an imaging technique with a high sensitivity, but also exposes patients to radiation (93). PET scan is growing imaging technique that is able to measure metabolic activity in different parts of the body. It allows for the visualisation of angiogenesis and macrophage infiltration, and can discriminate lipid-rich plaque from fibrous plaque (94) but failed so far to identify IPH. Several contrast agents have been developed, but fluorodeoxyglucose (FDG) contrast agents targeting macrophages appear to be the most used; they detect inflammation and present good association with histology on carotid and aorta (94) giving details on plaque metabolism. It remains to be demonstrated that the PET signal can reliably predict future long-term cardiovascular events on carotid imaging. With an FDG contrast agent, it is possible to visualise neovessels into the intima (95). Moreover, 18F-FDG-PET allows the precise visualisation of the plaque anatomy confirmed by guided MRI (96,97). Fluorine F 18-sodium fluoride (18F-NaF) is a radioactive tracer that can be used in PET-MRI, it could be a useful imaging technique to characterise vulnerable plaque; tissue sections with high 18F-NaF uptake demonstrated calcification, macrophage infiltration and cell death (98). New contrasts agents are needed to precisely characterise IPH.
MRI
The MRI-IPH imaging is a direct method based on iron detection with T1 weighted sequences that produces an intraplaque hyperintense signal (99,100), while other carotid components are visualised as isointense or hypointense signals (101,102). The first sequence validated by histology was a T1-weighted sequence called Magnetic Resonance Direct Thrombus Imaging which is able to detect methemoglobin (103). This sequence detects methaemoglobin-rich haemorrhages, with a strong relationship to histologically confirmed complicated plaques (103). Other sequences were developed, such as 3D Magnetization-Prepared Rapid Acquisition Gradient-Echo (104), three-dimensional T1-weighted Turbo-Spin-Echo sequence (105), Simultaneous Non-contrast Angiography and intraPlaque haemorrhage (106), T1 weigh inversion recovery 3D fast field echo sequence (107) or 3D Spoiled gradient recalled echo pulse sequence for Hemorrhage assessment using INversion recovery and multiple Echoes (3D SHINE) sequences (108). The “black blood” sequence needs no contrast agent to discriminate a LRNC from a lipid core with IPH, as well as other plaque components with a good correlation to histology (109,110). These sequences are specific and sensitive to detect IPH, but they failed in the identification of IPH stages (70). Further sequences such as “black blood” and “gradient echo”, “spin echo” or “fast spin echo” sequences are currently investigated to identify IPH (107).
MRI sensitivity can be enhanced with a dynamic contrast agent, via an intravenous bolus (111). Contrast enhanced MR angiography (CE-MRA) shows a higher sensitivity, specificity, positive predictive value, negative predictive value with a shorter acquisition time and less artefacts for IPH detection (100). Gadolinium enhancement is used to visualise fibrous cap integrity, plaque neovascularization (112), and inflammatory infiltration (113) it also helps to confirm IPH visualised in T1 sequences (112,113). Dynamic contrast intensification techniques can better visualise neovascularisation and inflammation (114). New sequences are currently being investigated in order to better characterize IPH dating and at the same time identify other vulnerable plaque features, in order to be used in a clinical setting.
Several studies showed a relationship between histological IPH presence and different stages of IPH assessed by MRI (77,115). Depending on classifications, a T1 hyperintense signal is observed if the IPH is fresh, but this hypersignal weakens over time. Limits of this classification may be correlated to the signal intensity variation, because of the interpersonal chemical composition variation in the plaque. Indeed, throughout the brain T1-weighted sequence application, the haemorrhage signal can evolve from hyperintense to hypointense because of the transformation of methemoglobin into hemosiderin (109). Contrary to this, carotid IPH signal can remain hyperintense for more than 18 months (116). The variability of the signal intensity may help in the detection of plaques involved in ischemic events (117) and can also coincide with the different IPH stages, particularly between amorphous and organised stages (70) according to Derksen’s classification (70). Nevertheless, this hypothesis is still not validated by any study. Methemoglobin signal can mix with other tissues signals, such as calcified tissue or hemosiderin, leading to false negatives (100). On the contrary, perivascular-derived signals of adipocytes can lead to IPH false-positives (100). In order to accurately imaging carotid plaque morphology, histopathological variability should be taken into account (118). Indeed, IPH dating by MRI needs be improved and sequences needs to be standardised before clinical use (24,26).
IPH and clinical outcomes
IPH evaluated by histology and clinical outcomes
Although IPH consideration is increasing in the clinical evaluation of the plaque, it should be analysed together with other interrelated clinical and biological factors (i.e., Inflammation, neovascularisation, fibrous cap thickness, necrotic core volume and composition).
Redgrave et al. demonstrated on 526 symptomatic patients that IPH was associated with a previous stroke, transient ischemic attack (TIA) or amaurosis fugax (67). Another study showed that IPH is associated with cerebrovascular events risk (68) but only in men, which is a well-known cardiovascular risk factor. Few histological studies demonstrate the importance of identifying carotid IPHs on stroke prognosis. A significant study (n=818) by Hellings et al. on symptomatic and asymptomatic stroke patients who underwent CEA (25) found that IPH was present in 69.9% of asymptomatic plaques and 76.3% of symptomatic plaques. Moreover, IPH increases the risk of cardiovascular events from 17.2% to 30.6% on a 3-year period [HR =1.7 (1.2; 2.5)] independent of perioperative events. IPH were also associated with primary outcomes (vascular death, nonfatal stroke, non-fatal myocardial infarction and vascular intervention), stroke and non-stroke vascular events, underlying an overall risk of cardio-vascular events. Other plaque features were not associated with any cardiovascular event thus IPH presence could improve global health care.
Consequently, imaging methods which are able to visualise neo-vessels and IPH should lead to a better understanding of the vulnerable plaque evolution. The challenge of current research is to be able to diagnose in vivo IPH that may cause ischemic events before they happened, in order to improve CEA decision.
Correlation between MRI assessed IPH and clinical events
As previously stated, IPH is an evolving process. The different stages of IPH are unequally involved in plaque vulnerability affecting the subsequent ischemic risk (70). Currently, no imaging technique is precise enough to discriminate Derksen’s IPH stages. As blood components break down over time, they have a different chemical composition and magnetic properties (109) thus MRI might be able to identify Derksen’s IPH stages (70). However, IPH assessed by MRI (MRI-IPH) appears to be a promising tool to predict cerebral ischemic events as stroke, TIA or amaurosis fugax.
Retrospective studies
Several studies have shown that MRI-IPH is an important tool to assess the plaque risk of rupture in symptomatic patients (119). Retrospective studies have established a positive relationship between unstable plaques detected by MRI and the latest neurological symptoms (120). MRI-IPH was also correlated with prior cardiovascular events (29) independent of stenosis degree, age, sex, hypertension, and smoking habit (107). Singh et al. [2013] performed a multivariable analysis and demonstrated that MRI-IPH was associated with the composite cardiovascular event (i.e. angioplasty, stenting or bypass graft) (OR =3.26; 1.14–9.37, P=0.028) (107). A recent study suggests that IPH detected by MPRAGE is a strong indicator of acute focal cerebral infarction (121), moreover IPH increased the risk of acute cerebral ischemic event from 22% to 47% (122). In another study, recent MRI-IPH was associated with ipsilateral stroke and TIA but only for symptomatic patients. This same study concluded that other risks of plaque rupture might be taken into account to predict ipsilateral stroke (15), while another study showed that IPH was associated with stroke (123). Symptomatic patients had a higher prevalence of cerebrovascular events recurrence (107) and an increased risk of subsequent cardiovascular event (117,119). In two studies, all patients with an MRI T1 hyperintense signal had a greater recurrence risk for ischemic ipsilateral events (117,124), as IPH is a risk factor for further carotid IPH (116).
Prospective studies
As some links between cardiovascular, cerebrovascular and IPH were suggested by retrospectives studies (29), prospective studies were conducted. MRI appears to be an interesting tool to assess IPH in asymptomatic patients (16). According to Saam et al., the presence of IPH increases the risk of cerebrovascular events 5.69 times, with an annualised event rate of 17.7% with IPH and 2.4% without IPH (99). Moreover, carotid plaques containing IPH and a ruptured fibrous cap are highly prone to develop ischemic events (99). Thus, this study showed that IPH diagnosed by MRI is a better predictor of stroke than stenosis (99). Other studies showed that IPH, in association with other rupture risk factors (thin fibrous cap, lipid-rich necrotic core), predicts subsequent cerebrovascular events (16) and ischemic events (stroke or TIA) (101). Symptomatic and asymptomatic patients (101,116,125,126) with carotid MRI-IPH, had an increased risk of subsequent cardiovascular events.
As MRI-IPH appears to predict future cerebrovascular ischemic events (68) and is highly discriminative from other plaque components such as necrotic core, macrophages inflammation and fibrous cap (115,127), it could become a valuable target to identify vulnerable carotid atherosclerotic plaques, but it is necessary to standardise the sequences and validate this technique in both genders in order to establish a strong correlation for all populations (68).
Conclusions
Embolism of vulnerable carotid atherosclerotic plaques is a frequent cause of ischemic stroke. Prevention for carotid plaques to become vulnerable is challenging. In vivo tools to assess reliably the factors of carotid plaque evolution are required. It has been demonstrated that IPH is a good predictor of plaque vulnerability and stroke incidence. Several techniques have been developed to assess IPH such as Doppler ultrasonography and CT. Nevertheless, MRI appears to be the most effective way to assess IPH in vivo with reduced risk for the patient. Although MRI may currently be the best way to determine IPH stage, new sequences are required to improve its sensitivity before implementation in clinical practice. Reducing the examination duration, increasing the specificity of the diagnostic alone or in association with other MRI markers of plaque vulnerability could be additional challenges. It also might be relevant to compare in vivo imaging analysis to serum biomarkers (128) to better assess vulnerability of the carotid atherosclerotic plaque. A large-scale cohort study is required to validate the MRI sequences used to diagnose in vivo IPH as a predictor of cerebral ischemic events.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Dr. Kosmas I. Paraskevas) for the series “Carotid Artery Stenosis and Stroke: Prevention and Treatment Part I” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-1974). The series “Carotid Artery Stenosis and Stroke: Prevention and Treatment Part I” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation 2017;135:e146-603. [Crossref] [PubMed]
- Wong KS, Caplan LR, Kim JS. Stroke Mechanisms. Front Neurol Neurosci 2016;40:58-71. [Crossref] [PubMed]
- Naylor AR. Time to rethink management strategies in asymptomatic carotid artery disease. Nat Rev Cardiol 2011;9:116-24. [Crossref] [PubMed]
- Eckstein HH. European Society for Vascular Surgery Guidelines on the Management of Atherosclerotic Carotid and Vertebral Artery Disease. Eur J Vasc Endovasc Surg 2018;55:1-2. [Crossref] [PubMed]
- Moore DJ, Miles RD, Gooley NA, et al. Noninvasive assessment of stroke risk in asymptomatic and nonhemispheric patients with suspected carotid disease. Five-year follow-up of 294 unoperated and 81 operated patients. Ann Surg 1985;202:491-504. [Crossref] [PubMed]
- Abbott AL, Paraskevas KI, Kakkos SK, et al. Systematic Review of Guidelines for the Management of Asymptomatic and Symptomatic Carotid Stenosis. Stroke 2015;46:3288-301. [Crossref] [PubMed]
- Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995;273:1421-8. [Crossref] [PubMed]
- Halliday A, Mansfield A, Marro J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet 2004;363:1491-502. [Crossref] [PubMed]
- Rothwell PM, Gibson R, Warlow CP. Interrelation between plaque surface morphology and degree of stenosis on carotid angiograms and the risk of ischemic stroke in patients with symptomatic carotid stenosis. On behalf of the European Carotid Surgery Trialists’ Collaborative Group. Stroke 2000;31:615-21. [Crossref] [PubMed]
- Barnett HJM, Meldrum HE, Eliasziw M. North American Symptomatic Carotid Endarterectomy Trial (NASCET) collaborators. The appropriate use of carotid endarterectomy. CMAJ 2002;166:1169-79. [PubMed]
- Halliday A, Harrison M, Hayter E, et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet 2010;376:1074-84. [Crossref] [PubMed]
- Naylor AR, Schroeder TV, Sillesen H. Clinical and imaging features associated with an increased risk of late stroke in patients with asymptomatic carotid disease. Eur J Vasc Endovasc Surg 2014;48:633-40. [Crossref] [PubMed]
- Raghavan P, Mukherjee S, Gaughen J, et al. Magnetic resonance angiography of the extracranial carotid system. Top Magn Reson Imaging 2008;19:241-9. [Crossref] [PubMed]
- Zhu C, Tian X, Degnan AJ, et al. Clinical Significance of Intraplaque Hemorrhage in Low- and High-Grade Basilar Artery Stenosis on High-Resolution MRI. AJNR Am J Neuroradiol 2018;39:1286-92. [Crossref] [PubMed]
- Turc G, Oppenheim C, Naggara O, et al. Relationships between recent intraplaque hemorrhage and stroke risk factors in patients with carotid stenosis: the HIRISC study. Arterioscler Thromb Vasc Biol 2012;32:492-9. [Crossref] [PubMed]
- Takaya N, Yuan C, Chu B, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI--initial results. Stroke 2006;37:818-23. [Crossref] [PubMed]
- Yu JH, Kwak HS, Chung GH, et al. Association of Intraplaque Hemorrhage and Acute Infarction in Patients With Basilar Artery Plaque. Stroke 2015;46:2768-72. [Crossref] [PubMed]
- Aboyans V, Ricco JB, Bartelink MEL, et al. Editor’s Choice - 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2018;55:305-68. [Crossref] [PubMed]
- Chistiakov DA, Orekhov AN, Bobryshev YV. Contribution of neovascularization and intraplaque haemorrhage to atherosclerotic plaque progression and instability. Acta Physiol (Oxf) 2015;213:539-53. [Crossref] [PubMed]
- Virmani R, Kolodgie FD, Burke AP, et al. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol 2005;25:2054-61. [Crossref] [PubMed]
- Sluimer JC, Kolodgie FD, Bijnens AP, et al. Thin-walled microvessels in human coronary atherosclerotic plaques show incomplete endothelial junctions relevance of compromised structural integrity for intraplaque microvascular leakage. J Am Coll Cardiol 2009;53:1517-27. [Crossref] [PubMed]
- Mughal MM, Khan MK, DeMarco JK, et al. Symptomatic and asymptomatic carotid artery plaque. Expert Rev Cardiovasc Ther 2011;9:1315-30. [Crossref] [PubMed]
- Brennan ML, Hazen SL. Emerging role of myeloperoxidase and oxidant stress markers in cardiovascular risk assessment. Curr Opin Lipidol 2003;14:353. [Crossref] [PubMed]
- Michel JB, Martin-Ventura JL, Nicoletti A, et al. Pathology of human plaque vulnerability: mechanisms and consequences of intraplaque haemorrhages. Atherosclerosis 2014;234:311-9. [Crossref] [PubMed]
- Hellings WE, Peeters W, Moll FL, et al. Composition of carotid atherosclerotic plaque is associated with cardiovascular outcome: a prognostic study. Circulation 2010;121:1941-50. [Crossref] [PubMed]
- Michel JB, Virmani R, Arbustini E, et al. Intraplaque haemorrhages as the trigger of plaque vulnerability. Eur Heart J 2011;32:1977-85, 1985a, 1985b, 1985c.
- Fryer JA, Myers PC, Appleberg M. Carotid intraplaque hemorrhage: The significance of neovascularity. J Vasc Surg 1987;6:341-9. [Crossref] [PubMed]
- Imparato AM, Riles TS, Mintzer R, et al. The Importance of Hemorrhage in the Relationship Between Gross Morphologic Characteristics and Cerebral Symptoms in 376 Carotid Artery Plaques. Ann Surg 1983;197:195-203. [Crossref] [PubMed]
- Millon A, Mathevet JL, Boussel L, et al. High-resolution magnetic resonance imaging of carotid atherosclerosis identifies vulnerable carotid plaques. J Vasc Surg 2013;57:1046-1051.e2. [Crossref] [PubMed]
- Gao P, Chen Z, Bao Y, et al. Correlation between carotid intraplaque hemorrhage and clinical symptoms: systematic review of observational studies. Stroke 2007;38:2382-90. [Crossref] [PubMed]
- Koutouzis M, Nomikos A, Nikolidakis S, et al. Statin treated patients have reduced intraplaque angiogenesis in carotid endarterectomy specimens. Atherosclerosis 2007;192:457-63. [Crossref] [PubMed]
- Derksen WJM, Peeters W, Tersteeg C, et al. Age and coumarin-type anticoagulation are associated with the occurrence of intraplaque hemorrhage, while statins are associated less with intraplaque hemorrhage: a large histopathological study in carotid and femoral plaques. Atherosclerosis 2011;214:139-43. [Crossref] [PubMed]
- McCarthy MJ, Loftus IM, Thompson MM, et al. Angiogenesis and the atherosclerotic carotid plaque: an association between symptomatology and plaque morphology. J Vasc Surg 1999;30:261-8. [Crossref] [PubMed]
- Sluimer JC, Gasc JM, van Wanroij JL, et al. Hypoxia, hypoxia-inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. J Am Coll Cardiol 2008;51:1258-65. [Crossref] [PubMed]
- Teng Z, He J, Degnan AJ, et al. Critical mechanical conditions around neovessels in carotid atherosclerotic plaque may promote intraplaque hemorrhage. Atherosclerosis 2012;223:321-6. [Crossref] [PubMed]
- Lu J, Duan W, Qiao A. Finite element analysis of mechanics of neovessels with intraplaque hemorrhage in carotid atherosclerosis. Biomed Eng Online 2015;14 Suppl 1:S3. [Crossref] [PubMed]
- Altaf N, Akwei S, Auer DP, et al. Magnetic resonance detected carotid plaque hemorrhage is associated with inflammatory features in symptomatic carotid plaques. Ann Vasc Surg 2013;27:655-61. [Crossref] [PubMed]
- Björkerud S, Björkerud B. Apoptosis is abundant in human atherosclerotic lesions, especially in inflammatory cells (macrophages and T cells), and may contribute to the accumulation of gruel and plaque instability. Am J Pathol 1996;149:367-80. [PubMed]
- Altaf N, Daniels L, Morgan PS, et al. Detection of intraplaque hemorrhage by magnetic resonance imaging in symptomatic patients with mild to moderate carotid stenosis predicts recurrent neurological events. J Vasc Surg 2008;47:337-42. [Crossref] [PubMed]
- Montezano AC, Touyz RM. Oxidative stress, Noxs, and hypertension: experimental evidence and clinical controversies. Ann Med 2012;44 Suppl 1:S2-16. [Crossref] [PubMed]
- Benelli R, Albini A, Noonan D. Neutrophils and Angiogenesis: Potential Initiators of the Angiogenic Cascade. Chem Immunol Allergy 2003;83:167-81. [Crossref] [PubMed]
- Campbell EL. Hypoxia-recruited angiogenic neutrophils. Blood 2015;126:1972-3. [Crossref] [PubMed]
- Ogata A, Kawashima M, Wakamiya T, et al. Carotid artery stenosis with a high-intensity signal plaque on time-of-flight magnetic resonance angiography and association with evidence of intraplaque hypoxia. J Neurosurg 2017;126:1873-8. [Crossref] [PubMed]
- Vita JA, Brennan ML, Gokce N, et al. Serum Myeloperoxidase Levels Independently Predict Endothelial Dysfunction in Humans. Circulation 2004;110:1134-9. [Crossref] [PubMed]
- Tian R, Ding Y, Peng YY, et al. Myeloperoxidase amplified high glucose-induced endothelial dysfunction in vasculature: Role of NADPH oxidase and hypochlorous acid. Biochem Biophys Res Commun 2017;484:572-8. [Crossref] [PubMed]
- Saffarzadeh M, Juenemann C, Queisser MA, et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One 2012;7:e32366. [Crossref] [PubMed]
- Yeagle PL. Cholesterol and the cell membrane. Biochim Biophys Acta 1985;822:267-87. [Crossref] [PubMed]
- Haskard DO, Boyle JJ, Evans PC, et al. Cytoprotective signaling and gene expression in endothelial cells and macrophages-lessons for atherosclerosis. Microcirculation 2013;20:203-16. [Crossref] [PubMed]
- Thrysøe SA, Oikawa M, Yuan C, et al. Longitudinal Distribution of Mechanical Stresses in Carotid Plaques of Symptomatic Patients. Stroke 2010;41:1041-3. [Crossref] [PubMed]
- Jeziorska M, Woolley DE. Local neovascularization and cellular composition within vulnerable regions of atherosclerotic plaques of human carotid arteries. J Pathol 1999;188:189-96. [Crossref] [PubMed]
- Abela GS, Aziz K. Cholesterol crystals rupture biological membranes and human plaques during acute cardiovascular events--a novel insight into plaque rupture by scanning electron microscopy. Scanning 2006;28:1-10. [Crossref] [PubMed]
- Abela GS, Aziz K, Vedre A, et al. Effect of Cholesterol Crystals on Plaques and Intima in Arteries of Patients With Acute Coronary and Cerebrovascular Syndromes. Am J Cardiol 2009;103:959-68. [Crossref] [PubMed]
- Duguid JB. The Thrombogenic Hypothesis and its Implications. Postgrad Med J 1960;36:226-9. [Crossref] [PubMed]
- Daemen MJ, Fergusson MS, Gijsen FJ, et al. Carotid plaque fissure: An underestimated source of intraplaque hemorrhage. Atherosclerosis 2016;254:102-8. [Crossref] [PubMed]
- Chen Y, Yu Q, Xu CB. A convenient method for quantifying collagen fibers in atherosclerotic lesions by ImageJ software. Int J Clin Exp Med 2017;10:14904-10.
- Burt RC. The incidence of acid-fast pigment (ceroid) in aortic atherosclerosis. Am J Clin Pathol 1952;22:135-9. [Crossref] [PubMed]
- Mitchinson MJ, Hothersall DC, Brooks PN, et al. The distribution of ceroid in human atherosclerosis. J Pathol 1985;145:177-83. [Crossref] [PubMed]
- Leclercq A, Houard X, Philippe M, et al. Involvement of intraplaque hemorrhage in atherothrombosis evolution via neutrophil protease enrichment. J Leukoc Biol 2007;82:1420-9. [Crossref] [PubMed]
- Post S, Peeters W, Busser E, et al. Balance between angiopoietin-1 and angiopoietin-2 is in favor of angiopoietin-2 in atherosclerotic plaques with high microvessel density. J Vasc Res 2008;45:244-50. [Crossref] [PubMed]
- Moreno PR, Purushothaman KR, Sirol M, et al. Neovascularization in human atherosclerosis. Circulation 2006;113:2245-52. [Crossref] [PubMed]
- Au-Yeung KK, Woo CW, Sung FL, et al. Hyperhomocysteinemia Activates Nuclear Factor-kappaB in Endothelial Cells via Oxidative Stress. Circ Res 2004;94:28-36. [Crossref] [PubMed]
- Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol 1995;15:1512-31. [Crossref] [PubMed]
- Stary HC, Chandler AB, Glagov S, et al. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1994;89:2462-78. [Crossref] [PubMed]
- Lovett JK, Gallagher PJ, Hands LJ, et al. Histological Correlates of Carotid Plaque Surface Morphology on Lumen Contrast Imaging. Circulation 2004;110:2190-7. [Crossref] [PubMed]
- Kolodgie FD, Petrov A, Virmani R, et al. Targeting of apoptotic macrophages and experimental atheroma with radiolabeled annexin V: a technique with potential for noninvasive imaging of vulnerable plaque. Circulation 2003;108:3134-9. [Crossref] [PubMed]
- Pelisek J, Well G, Reeps C, et al. Neovascularization and angiogenic factors in advanced human carotid artery stenosis. Circ J 2012;76:1274-82. [Crossref] [PubMed]
- Redgrave JNE, Lovett JK, Gallagher PJ, et al. Histological assessment of 526 symptomatic carotid plaques in relation to the nature and timing of ischemic symptoms: the Oxford plaque study. Circulation 2006;113:2320-8. [Crossref] [PubMed]
- Vrijenhoek JEP, Den Ruijter HM, De Borst GJ, et al. Sex is associated with the presence of atherosclerotic plaque hemorrhage and modifies the relation between plaque hemorrhage and cardiovascular outcome. Stroke 2013;44:3318-23. [Crossref] [PubMed]
- Howard DPJ, van Lammeren GW, Rothwell PM, et al. Symptomatic carotid atherosclerotic disease: correlations between plaque composition and ipsilateral stroke risk. Stroke 2015;46:182-9. [Crossref] [PubMed]
- Derksen WJM, Peeters W, van Lammeren GW, et al. Different stages of intraplaque hemorrhage are associated with different plaque phenotypes: a large histopathological study in 794 carotid and 276 femoral endarterectomy specimens. Atherosclerosis 2011;218:369-77. [Crossref] [PubMed]
- Lennihan L, Kupsky WJ, Mohr JP, et al. Lack of association between carotid plaque hematoma and ischemic cerebral symptoms. Stroke 1987;18:879-81. [Crossref] [PubMed]
- Lusby RJ, Ferrell LD, Ehrenfeld WK, et al. Carotid plaque hemorrhage. Its role in production of cerebral ischemia. Arch Surg 1982;117:1479-88. [Crossref] [PubMed]
- Shah PK. Mechanisms of plaque vulnerability and rupture. J Am Coll Cardiol 2003;41:15S-22S. [Crossref] [PubMed]
- Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature 2011;473:317-25. [Crossref] [PubMed]
- Grant EG, Benson CB, Moneta GL, et al. Carotid artery stenosis: gray-scale and Doppler US diagnosis--Society of Radiologists in Ultrasound Consensus Conference. Radiology 2003;229:340-6. [Crossref] [PubMed]
- Ricco JB, Gauthier JB, Richer JP, et al. The evolution of carotid and coronary artery disease after operation for carotid stenosis. Ann Vasc Surg 1992;6:408-12. [Crossref] [PubMed]
- den Hartog AG, Bovens SM, Koning W, et al. Current status of clinical magnetic resonance imaging for plaque characterisation in patients with carotid artery stenosis. Eur J Vasc Endovasc Surg 2013;45:7-21. [Crossref] [PubMed]
- Saba L, Anzidei M, Marincola BC, et al. Imaging of the carotid artery vulnerable plaque. Cardiovasc Intervent Radiol 2014;37:572-85. [Crossref] [PubMed]
- Grønholdt ML, Wiebe BM, Laursen H, et al. Lipid-rich carotid artery plaques appear echolucent on ultrasound B-mode images and may be associated with intraplaque haemorrhage. Eur J Vasc Endovasc Surg 1997;14:439-45. [Crossref] [PubMed]
- Coli S, Magnoni M, Sangiorgi G, et al. Contrast-enhanced ultrasound imaging of intraplaque neovascularization in carotid arteries: correlation with histology and plaque echogenicity. J Am Coll Cardiol 2008;52:223-30. [Crossref] [PubMed]
- Schmidt C, Fischer T, Rückert R-I, et al. Identification of neovascularization by contrast-enhanced ultrasound to detect unstable carotid stenosis. PLoS One 2017;12:e0175331. [Crossref] [PubMed]
- Feinstein SB. Contrast Ultrasound Imaging of the Carotid Artery Vasa Vasorum and Atherosclerotic Plaque Neovascularization. J Am Coll Cardiol 2006;48:236-43. [Crossref] [PubMed]
- Moguillansky D, Leng X, Carson A, et al. Quantification of plaque neovascularization using contrast ultrasound: a histologic validation. Eur Heart J 2011;32:646-53. [Crossref] [PubMed]
- Bluth EI, Kay D, Merritt CR, et al. Sonographic characterization of carotid plaque: detection of hemorrhage. AJR Am J Roentgenol 1986;146:1061-5. [Crossref] [PubMed]
- Korn A, Bender B, Thomas C, et al. Dual energy CTA of the carotid bifurcation: advantage of plaque subtraction for assessment of grade of the stenosis and morphology. Eur J Radiol 2011;80:e120-125. [Crossref] [PubMed]
- Postma AA, Das M, Stadler AAR, et al. Dual-Energy CT: What the Neuroradiologist Should Know. Curr Radiol Rep 2015;3:16. [Crossref] [PubMed]
- Prati F, Pawlowski T, Sommariva L, et al. Intravascular ultrasound and quantitative coronary angiography assessment of late in-stent restenosis: in vivo human correlation and methodological implications. Catheter Cardiovasc Interv 2002;57:155-60. [Crossref] [PubMed]
- Saba L, Francone M, Bassareo PP, et al. CT Attenuation Analysis of Carotid Intraplaque Hemorrhage. AJNR Am J Neuroradiol 2018;39:131-7. [Crossref] [PubMed]
- U-King-Im JM, Fox AJ, Aviv RI, et al. Characterization of carotid plaque hemorrhage: a CT angiography and MR intraplaque hemorrhage study. Stroke 2010;41:1623-9. [Crossref] [PubMed]
- Wintermark M, Jawadi SS, Rapp JH, et al. High-resolution CT imaging of carotid artery atherosclerotic plaques. AJNR Am J Neuroradiol 2008;29:875-82. [Crossref] [PubMed]
- Eisenmenger LB, Aldred BW, Kim SE, et al. Prediction of Carotid Intraplaque Hemorrhage Using Adventitial Calcification and Plaque Thickness on CTA. AJNR Am J Neuroradiol 2016;37:1496-503. [Crossref] [PubMed]
- Eesa M, Hill MD, Al-Khathaami A, et al. Role of CT angiographic plaque morphologic characteristics in addition to stenosis in predicting the symptomatic side in carotid artery disease. AJNR Am J Neuroradiol 2010;31:1254-60. [Crossref] [PubMed]
- Rudd JHF, Myers KS, Bansilal S, et al. Atherosclerosis inflammation imaging with 18F-FDG PET: carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J Nucl Med 2008;49:871-8. [Crossref] [PubMed]
- Menezes LJ, Kotze CW, Agu O, et al. Investigating vulnerable atheroma using combined (18)F-FDG PET/CT angiography of carotid plaque with immunohistochemical validation. J Nucl Med 2011;52:1698-703. [Crossref] [PubMed]
- Calcagno C, Cornily JC, Hyafil F, et al. Detection of neovessels in atherosclerotic plaques of rabbits using dynamic contrast enhanced MRI and 18F-FDG PET. Arterioscler Thromb Vasc Biol 2008;28:1311-7. [Crossref] [PubMed]
- Kwee RM, Teule GJ, van Oostenbrugge RJ, et al. Multimodality Imaging of Carotid Artery Plaques: 18F-fluoro-2-deoxyglucose Positron Emission Tomography, Computed Tomography, and Magnetic Resonance Imaging. Stroke 2009;40:3718-24. [Crossref] [PubMed]
- Izquierdo-Garcia D, Davies JR, Graves MJ, et al. Comparison of Methods for Magnetic Resonance-Guided [18-F]Fluorodeoxyglucose Positron Emission Tomography in Human Carotid Arteries: Reproducibility, Partial Volume Correction, and Correlation Between Methods. Stroke 2009;40:86-93. [Crossref] [PubMed]
- Mechtouff L, Sigovan M, Costes N, et al. 18 F-NaF PET-MRI: an innovative tool to assess carotid artery plaque vulnerability. Eur J Neurol 2018;25:e18-9. [Crossref] [PubMed]
- Saam T, Hetterich H, Hoffmann V, et al. Meta-analysis and systematic review of the predictive value of carotid plaque hemorrhage on cerebrovascular events by magnetic resonance imaging. J Am Coll Cardiol 2013;62:1081-91. [Crossref] [PubMed]
- Qiao Y, Etesami M, Malhotra S, et al. Identification of intraplaque hemorrhage on MR angiography images: a comparison of contrast-enhanced mask and time-of-flight techniques. AJNR Am J Neuroradiol 2011;32:454-9. [Crossref] [PubMed]
- Gupta A, Baradaran H, Schweitzer AD, et al. Carotid plaque MRI and stroke risk: a systematic review and meta-analysis. Stroke 2013;44:3071-7. [Crossref] [PubMed]
- Gupta A, Baradaran H, Kamel H, et al. Intraplaque high-intensity signal on 3D time-of-flight MR angiography is strongly associated with symptomatic carotid artery stenosis. AJNR Am J Neuroradiol 2014;35:557-61. [Crossref] [PubMed]
- Moody AR, Murphy RE, Morgan PS, et al. Characterization of complicated carotid plaque with magnetic resonance direct thrombus imaging in patients with cerebral ischemia. Circulation 2003;107:3047-52. [Crossref] [PubMed]
- Hishikawa T, Iihara K, Yamada N, et al. Assessment of necrotic core with intraplaque hemorrhage in atherosclerotic carotid artery plaque by MR imaging with 3D gradient-echo sequence in patients with high-grade stenosis. Clinical article. J Neurosurg 2010;113:890-6. [Crossref] [PubMed]
- Sigovan M, Bidet C, Bros S, et al. 3D black blood MR angiography of the carotid arteries. A simple sequence for plaque hemorrhage and stenosis evaluation. Magn Reson Imaging 2017;42:95-100. [Crossref] [PubMed]
- Wang J, Börnert P, Zhao H, et al. Simultaneous noncontrast angiography and intraplaque hemorrhage (SNAP) imaging for carotid atherosclerotic disease evaluation. Magn Reson Med 2013;69:337-45. [Crossref] [PubMed]
- Singh N, Moody AR, Rochon-Terry G, et al. Identifying a high risk cardiovascular phenotype by carotid MRI-depicted intraplaque hemorrhage. Int J Cardiovasc Imaging 2013;29:1477-83. [Crossref] [PubMed]
- Zhu DC, Vu AT, Ota H, et al. An optimized 3D spoiled gradient recalled echo pulse sequence for hemorrhage assessment using inversion recovery and multiple echoes (3D SHINE) for carotid plaque imaging. Magn Reson Med 2010;64:1341-51. [Crossref] [PubMed]
- Chu B, Kampschulte A, Ferguson MS, et al. Hemorrhage in the atherosclerotic carotid plaque: a high-resolution MRI study. Stroke 2004;35:1079-84. [Crossref] [PubMed]
- Ota H, Yarnykh VL, Ferguson MS, et al. Carotid Intraplaque Hemorrhage Imaging at 3.0-T MR Imaging: Comparison of the Diagnostic Performance of Three T1-weighted Sequences. Radiology 2010;254:551-63. [Crossref] [PubMed]
- Kerwin W, Hooker A, Spilker M, et al. Quantitative magnetic resonance imaging analysis of neovasculature volume in carotid atherosclerotic plaque. Circulation 2003;107:851-6. [Crossref] [PubMed]
- Cai J, Hatsukami TS, Ferguson MS, et al. In Vivo Quantitative Measurement of Intact Fibrous Cap and Lipid-Rich Necrotic Core Size in Atherosclerotic Carotid Plaque: Comparison of High-Resolution, Contrast-Enhanced Magnetic Resonance Imaging and Histology. Circulation 2005;112:3437-44. [Crossref] [PubMed]
- Tang T, Howarth SPS, Miller SR, et al. Assessment of inflammatory burden contralateral to the symptomatic carotid stenosis using high-resolution ultrasmall, superparamagnetic iron oxide-enhanced MRI. Stroke 2006;37:2266-70. [Crossref] [PubMed]
- Millon A, Boussel L, Brevet M, et al. Clinical and histological significance of gadolinium enhancement in carotid atherosclerotic plaque. Stroke 2012;43:3023-8. [Crossref] [PubMed]
- Puppini G, Furlan F, Cirota N, et al. Characterisation of carotid atherosclerotic plaque: comparison between magnetic resonance imaging and histology. Radiol Med 2006;111:921-30. [Crossref] [PubMed]
- Takaya N, Yuan C, Chu B, et al. Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques: a high-resolution magnetic resonance imaging study. Circulation 2005;111:2768-75. [Crossref] [PubMed]
- Wang X, Sun J, Zhao X, et al. Ipsilateral plaques display higher T1 signals than contralateral plaques in recently symptomatic patients with bilateral carotid intraplaque hemorrhage. Atherosclerosis 2017;257:78-85. [Crossref] [PubMed]
- Lovett JK, Gallagher PJ, Rothwell PM. Reproducibility of histological assessment of carotid plaque: implications for studies of carotid imaging. Cerebrovasc Dis 2004;18:117-23. [Crossref] [PubMed]
- Altaf N, MacSweeney ST, Gladman J, et al. Carotid intraplaque hemorrhage predicts recurrent symptoms in patients with high-grade carotid stenosis. Stroke 2007;38:1633-5. [Crossref] [PubMed]
- Demarco JK, Ota H, Underhill HR, et al. MR Carotid Plaque Imaging and Contrast-Enhanced MR Angiography Identifies Lesions Associated with Recent Ipsilateral Thromboembolic Symptoms: An In Vivo Study at 3T. AJNR Am J Neuroradiol 2010;31:1395-402. [Crossref] [PubMed]
- Kim S, Kwak HS, Chung GH. Carotid intraplaque hemorrhage in patients with greater than fifty percent carotid stenosis was associated an acute focal cerebral infarction. Neurol Asia 2018;23:209-21.
- Ryu HJ, Jeon SJ, Choi SS. Carotid Intraplaque Hemorrhage is Associated with Acute Cerebral Ischemic Events and Progression of Stenosis on Magnetic Resonance Imaging. Investig Magn Reson Imaging 2017;21:242-51. [Crossref]
- Selwaness M, Bos D, van den Bouwhuijsen Q, et al. Carotid Atherosclerotic Plaque Characteristics on Magnetic Resonance Imaging Relate With History of Stroke and Coronary Heart Disease. Stroke 2016;47:1542-7. [Crossref] [PubMed]
- Yamada K, Yoshimura S, Kawasaki M, et al. Preoperative magnetic resonance plaque imaging and carotid artery stenting: a review. Interv Neurol 2012;1:31-8. [Crossref] [PubMed]
- Kurosaki Y, Yoshida K, Fukuda H, et al. Asymptomatic Carotid T1-High-Intense Plaque as a Risk Factor for a Subsequent Cerebrovascular Ischemic Event. Cerebrovasc Dis 2017;43:250-6. [Crossref] [PubMed]
- Hosseini AA, Kandiyil N, Macsweeney STS, et al. Carotid plaque hemorrhage on magnetic resonance imaging strongly predicts recurrent ischemia and stroke. Ann Neurol 2013;73:774-84. [Crossref] [PubMed]
- Yuan C, Mitsumori LM, Beach KW, et al. Carotid atherosclerotic plaque: noninvasive MR characterization and identification of vulnerable lesions. Radiology 2001;221:285-99. [Crossref] [PubMed]
- Matic LP, Iglesias MJ, Vesterlund M, et al. Novel Multiomics Profiling of Human Carotid Atherosclerotic Plaques and Plasma Reveals Biliverdin Reductase B as a Marker of Intraplaque Hemorrhage. JACC Basic Transl Sci 2018;3:464-80. [Crossref] [PubMed]



