Post-stroke cognitive impairment: epidemiology, mechanisms and management
Introduction
Stroke, or cerebrovascular accident (CVA), which is also defined as the dysfunction of brain due to a disturbance of the cerebral blood flow, is the second most common cause of death and adult disability around the world (1). Because of the achievement of the public health and the medicine, the stroke mortality is falling down continuously. In 2008, the stroke death rate of America is 40.6 per 100,000 population, which is three fourths less than its historic 1931 to 1960 norm (2). Following by the decreased stroke death rate, more and more researchers pay attention to the disabilities that stroke survivors suffer from. There are 15 million people worldwide suffering from stroke every year, about 30% of which experience residual disabilities (3). It has been confirmed that stroke could result in the cognitive impairment. However, covered by the severe physical disability, the post-stroke cognitive impairment is likely to be ignored.
In the past, the researchers identified the dementia after stroke as the vascular dementia (4). But not all stroke survivors who suffer from the cognitive decline meet the criteria of the dementia. As a result, the vascular cognitive impairment (VCI) took over the past “vascular dementia”. However, there’s evidence suggesting that the cognitive impairment after stroke is involved in not only the VCI, but also the pathogenesis of Alzheimer’s disease (AD). The clinical study suggested that the pathogenesis of AD make contributions to the 1/3 demented cases after stroke (5). Thus there’s an overlap between VCI and AD. According to the autopsy study, approximately 50% of dementias are attributed to both VCI and AD (6).
According to Nys et al., a high proportion of stroke survivors had met the cognitive impairment within 3 months after stroke (7). Although the prevalence of post stroke cognitive impairment is very high according to the present data, there’s still evidence showing that the present criteria may underestimate the frequency of the dementia and the cognitive decline in stroke survivors (8,9). These patients with the cognitive impairment could be divided according to the degree of the cognitive decline into the mild cognitive impairment and dementia. Interestingly, in different studies, the dementia ratio within 3 months after stroke varies from 6% to 27% (10,11). The variety of the conclusion may be due to the different application of the criteria of the dementia or the cognitive impairment. The present standard criteria of the dementia include the diagnostic and statistical manual of mental disorders IV (DSM IV), international classification of disease-10 (ICD-10) and national institute of neurological and communicative disorders and strokeand the AD and related disorders association (NINCDS-ADRDA) criteria. Besides the demented patients, the degree of the cognitive decline of other cognition-impaired patients who fail to meet the above criteria could be measured by the yardsticks such as the mini-mental state examination (MMSE) score, Montreal cognitive assessment scale (MoCA) score, the abbreviated mental test, AD assessment scale-cognitive (ADAS-Cog) and so on. Of course, there are some other measures which are mainly originated from the above yardsticks. For example, the six-item screener (SIS) is a brief cognitive function test which is derived from the MMSE and designed for either in-person or telephone administration. What’s more, multiple neuropsychological test batteries are used to examine not only the total cognitive function but also the level of impairment on every single cognitive domain like memory, language, visuoconstruction, executive function, calculation, comprehension and judgment.
In this review, we include the new evidence regarding the epidemiology of post-stroke cognitive impairment and discuss its potential risk factors. The mechanisms that could underlie the cognitive impairment after stoke are discussed, including the impaired neuroanatomical structures and the cerebral microbleeds (CMBs) which may result in VCI, and the contribution of stroke to AD. Finally, we critically review the present promising treatment to post-stroke cognitive impairment.
Epidemiology
Prevalence of post-stroke cognitive impairment
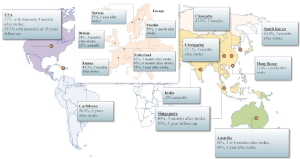
The prevalence studies focus on the whole population who show the cognitive impairment after stroke. Although these studies in community or hospital settings always fail to exclude the patients who have suffered the cognitive decline before the stroke, they have shown the seriousness of the problem. The cross-sectional study widely proceeded in ten countries suggests that about 30% ischemic stroke survivors show a cognitive impairment which is determined by the MMSE score is lower than 27 (12). But the results of the studies vary for the difference between the countries, the races, and the diagnostic criteria. In Europe, such as Britain and Sweden, the prevalence of the cognitive impairment 3 months after stroke ranges from 24% to 39% according to the MMSE, while the prevalence in the same population is up to 96% according to the comprehensive neuropsychological test batteries (13,14). And In Netherland, the Maastricht CODAS which examined the cognitive function of 176 subjects with the first-ever stroke after 6 months by MMSE has suggested that the prevalence of cognitive impairment is up to 70% (15). One study on patients with a first-ever stroke and TIA admitted to the hospital in Norway suggested that 57% stroke patients suffered from the cognitive impairment during the first year after stroke (16). Recently, a study based on the cohort of first-ever stroke patients without pre-stroke dementia in France suggested that the frequency of the cognitive impairment 3-month after stroke was 47.3% (17). In Australia, the studies have shown that cognitive impairment prevalence 3 months after stroke is 50% to 58% according to a series of neuropsychological tests (18,19). What’s more, the study also suggests that the cognitive impairment on the stroke survivor exist on any single domain such as attention, spatial ability, language and executive ability more frequently than the multiple domains (20). In America, the study on 212 subjects from the Framingham Study suggested that 19.3% of cases developed into the dementia in 10 years after stroke (21). One study suggested the prevalence of post stroke cognitive impairment in Mexican Americans was higher than in non-Hispanic whites and about 31% stroke patients of Mexican American would suffer from the post stroke dementia (22), showed that there was a difference between the regions and the races. What’s more, in Caribbean, Chausson et al. examined the cognitive function of 293 stroke patients 5 years after the first-ever stroke from the cohort of ERMANCIA study in Martinique and suggested that 58.9% patients suffered from the cognitive impairment (23). In Asia, the study conducted by Yu et al. in South Korea suggested the highest result of all. Proceeding in 12 hospitals in South Korea which enrolled 620 patients with ischemic stroke, it proposed that the prevalence reached up to 69.8% 3 months after stroke as measured by Korea MMSE (24). The study on 252 Singaporean patients within 6 months post-stroke showed that 44% patients suffered from the cognitive decline, while the prevalence declined to 34% in 1-year follow-up (25). The later prospective study in India showed that the prevalence of cognitive impairment was about 20% in total stroke survivors (26). In China, the study on 179 cases with 3 months after stroke in Hong Kong suggested that the prevalence of cognitive impairment after stroke was 21.8% as measured by MMSE, which would decline to 18% after the removal of previous stroke cases from the sample (27). Zhou et al. examined the cognitive function of 434 patients with stroke by 1-year follow-up in Chongqing. The study suggested a 37.1% of cognitive impairment prevalence 3 months after stroke (28). What’s more, one recent study proceeding in Changsha which included 689 ischemic stroke patients detected that the prevalence of post stroke cognitive impairment was 41.8% (29) (Table 1 and Figure 1).
Full table
The distribution of the post-stroke cognitive impairment.
Risk factors of post-stroke cognitive impairment
The risk of the cognitive impairment after stroke is associated with the overlap of the frequent cerebrovascular disease and the dementia. According to the demography, the age and the education level are related to the post-stroke cognitive impairment risk. The age is the risk factor of not only the stroke but also the cognitive decline. There’s evidence suggesting that the prevalence of the cognitive decline after stroke would increase exponentially as age increases after 65 years old (30). The education level is a conflictive risk factor. It could influence the expression of the cognitive impairment in patients. The cohort study conducted by Elbaz et al. on 4,010 participants suggested the higher education was associated with the better cognitive performances (31). Furthermore, Wu et al. divided 206 patients who suffered from the ischemic stroke into the VCI group and the no-VCI group and examined MoCA score. The result suggested that the sensitivity of MoCA and the number of impaired MoCA factors decreased as the increase of the education level. The score of orientation factor in highest education level patients both with and without VCI is a full score. It seems that the higher education level could increase the tolerance of the cognitive decline (32). However, the education level has no effect on the rate of the aggravation of the cognitive impairment. Singh-Manoux et al. and Zahodne et al. each researched the influence of education on the cognitive impairment with the two samples of 7,454 individuals from the Whitehall II cohort study and 1,023 participants in the Victoria Longitudinal Study. Both studies suggested that there was no significant difference of the rate of decline in cognitive function between each groups of the education level (33,34). Moreover, there’s evidence suggesting that the occupation have effects on the prevalence of the cognitive impairment. Singh-Manoux et al. also suggested that the individuals with high occupations which were defined as the administrative positions had a more obvious cognitive decline than other occupations (33). Another study conducted by Douiri et al. proposed a higher prevalence of cognitive impairment after ischemic stroke in the manual workers (14).
Vascular risk factors such as hypertension, diabetes mellitus, hyperlipidemia, smoking, atrial fibrillation, and smoking increase the risk of both the cognitive impairment due to VCI or AD and the stroke (35). In addition, one recent study suggested that the recurrent stroke or the existing cerebral lesions would increase the prevalence of the cognitive impairment (36). The prevalence of the new-onset dementia in first stroke is about 10%, which in the recurrent stroke is 30%. The study conducted by Sibolt et al. included 486 patients with ischemic stroke, 115 of which met the dementia criteria of the Diagnostic and Statistical Manual of Mental Disorders, 3rd edition criteria. The study showed that patients who had been diagnosed as dementia after stroke would suffer from recurrent stroke earlier than those without dementia, suggesting that the dementia after stroke was associated with increased risk for recurrent stroke (37). It seems that the post-stroke cognitive impairment and the recurrence of stroke could cross as a basis for the aggravation. These evidences support that the vascular therapy would benefit the recovery of the post-stroke impairment.
Mechanisms
The mechanism of post-stroke cognitive impairment remains uncertain. Either VCI or AD promoted by stroke may be the reason of post-stroke cognitive impairment, and evidence suggesting that sometimes they work on the post-stroke cognitive impairment together (Figure 2).
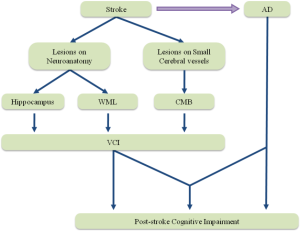
Vascular cognitive impairment (VCI)
Lesions on neuroanatomical structure
Since the past studies, the dementia after stroke has been considered to be based on the neuroanatomical lesions caused by stroke. The study conducted by Tomlinson et al. in 1970 suggested the volume of infarcts was correlated the occurrence and development of cognitive impairment, which would cause the vascular dementia when being higher than 100 mL (38). But the recent study suggested that the total volume of infarcts explained only a small proportion of the variability of cognition in the stroke patients, and supported that infarcts in strategic areas played an important role in the mechanism of cognitive impairment after stroke and were associated with the severity of the dementia (39). It also suggested not the total infarcted volume but the infarcted volume in the strategic areas, such as cortical limbic areas, heteromodal association areas including the frontal cortex and the white matter, explained half of the variability in MMSE and counted for much in the cognitive impairment after stroke.
As the development of studies on the pathogenesis, the lesions on structures such as the hippocampus and entorhinal cortex which were considered to be related only to AD before have been reported to make a difference on the cognitive decline after stroke. The study conducted by Szabo et al. suggested that the lesion on the hippocampus could lead to the impaired persistent memory which was considered as the usual consequence of posterior cerebral artery ischemia (40). By locating the lesions with MRI, the study also showed that the infarct on the left hippocampus would impair verbal long-term memory while that on the right hippocampus may cause the nonverbal long-term memory deficits, suggesting the difference between the bilateral hippocampi. Another study on 658 elderly participants without dementia suggested that brain infarcts are associated with a smaller hippocampus, and that both infarcts and reduced hippocampal volume are independently associated with the memory decline (41). There’s evidence suggesting that the impaired hippocampal neural volume is associated with the post-stroke dementia. The study has demonstrated that the vascular factors such as high cholesterol and diabetes mellitus which are related to the high stroke risk would lead to the atrophy of the hippocapus in the healthy elderly male population (42). And in the further study, Gemmell et al. investigated the hippocampal volume in postmortem samples and suggested that the neuronal volumes of the delayed post-stroke dementia patients were 10-20% smaller in the CA1 and CA2 hippocampal subfields, which were 20% smaller in the CA3 and CA4 hippocampal subfields, compared with elderly controls (43,44). The mechanism of hippocampus lesions related to the post-stroke cognitive impairment remains uncertain. Li et al. conducted the study on middle cerebral artery occlusion model and suggested that an increased GABAergic neurotransmission which and a reduced activity of the extracellular regulated protein kinase (ERK) existed in the bilateral hippocampi and thus contributed to the cognitive impairment after ischemic stroke (45). And Wen et al. proposed that NaHS, the donor of the hydrogen sulfide which was a new type of neurotransmitter and inhibited the hippocampal neuronal damage, was decreased in the rats with the cognitive impairment after ischemic stroke (46). Though this attractive effect appeared on the animal model, it highlighted the role of hippocampus in the VCI and suggested a new view to the treatment of VCI.
The white mater lesions (WMLs) are the common radiological manifestations of sub-clinical ischemic damage of the cerebral parenchyma due to the small cerebrovascular disease. The lacunar stroke is frequently associated with the WMLs and caused by the ischemic damage of the small cerebrovascular disease (47). Both WML and lacunar stroke are predictors of cognitive decline and correlated to the level of cognitive impairment (47). The study on 350 elderly nondementia subjects from a community of Japan detected that the cognitive impairment which was measured by MMSE and Modified Stroop test was present in 15.7% subjects and was associated with the WMLs and remarkable cerebral atrophy (48). The Leukoaraiosis And DISability Study (LADIS) focuses on the relations between WMLs and disability in age from 65 to 84 years (49). Its branch studies provided the evidences for the relation between WMLs and VCI. One study suggested that lacunar infarcts in the thalamus were associated with lower scores of MMSE, which in the putamen/pallidum decreased the memory function (50). Another study suggested that WMLs and lacunar infarcts impaired the cognitive function, especially the psychomotor speed, executive function and global cognitive function (51). And the later study of LADIS on 477 subjects with WMLs in 3 years follow-up also showed that WMLs and brain atrophies such as medial temporal lobe atrophy, subcortical atrophy, and cortical atrophy were independently related to VCI, and the brain atrophy could accelerate the effect of WMLs on VCI (52). And the similar result was proposed by the study on 448 patients with symptomatic atherosclerotic disease from the cohort of SMART-MR in 4 years follow-up, which suggested that the interaction between brain atrophy and WMLs or infarcts could aggravate the cognitive decline (53). The pathogenesis of WMLs in VCI is unclear. The study on 32 nonstroke and nondementia subjects with and without WMLs which were determined by the white matter hyperintensities on MRI explored the mechanism of WMLs resulting in cognitive impairment, and suggested that WMLs may lead to cortical thinning and thus impaired the executive function and verbal fluency (54).
Cerebral microbleeds (CMBs)
CMB is defined as the hemorrhage smaller than 5 mm, which could be detected by the gradient-echo T2*-weighted MRI, and has been recognized as the marker for small vascular diseases such as the subcortical small vascular disease (associated with hypertension) and cerebral amyloid angiopathy (CAA) (55,56). According to the cohort study, the prevalence of CMBs increased with age, from 6.5% in the age 45 to 50 years old to 35.7% in the age older than 80 (57). In the population of stroke survivors, about 35% patients with ischemic stroke and 60% patients with hemorrhage stroke have the CMB (58). The small vascular disease has been reported to be related to the cognitive deficits (59), especially the CAA (60). Thus the role of CMB in the cognitive impairment after stroke has been drawn much attention.
There’s evidence suggesting that CMBs are related to the cognitive impairment. The study conducted by Werring et al. firstly suggested the relation between CMBs and cognitive deficits (61). The patients with CMBs showed an impaired executive dysfunction more frequently than those without CMBs. And the further study has suggested that the CMBs are associated with frontal-executive impairment at follow-up after 5.7 years (62). The existence of CMB may also predict the consequence of the cognitive impairment. The convincing evidences come from two large sample analyses. The AGES-Reykjavik study which included 3,906 older subjects with CMBs suggested that the multiple CMBs located in the deep locations are associated with the lower cognitive function such as slower processing speed and poorer executive function and have a high risk for the vascular dementia (63). And the Rotterdam Scan study which included 3,979 subjects without dementia as measured by the MMSE and neuropsychological batteries suggested that the presence of numerous CMBs especially in a strictly lobar location may be associated with worse performance on cognitive tests in almost all cognitive domains except memory (64).
Furthermore, one study suggested that the absence of the CMBs would contribute to the reversion of the mild VCI to normal cognitive status (65). There’s evidence suggesting that the CMBs may also have effects on the subcortical vascular dementia (66). The patients with CMBs showed the lower total MMSE score and the sub-scores in terms of “attention and calculation” and “orientation” than those without CMBs. It seems that there’s a difference of the impaired cognitive domain between the locations of the CMBs. This idea is supported by the prospective studies. The study on the 439 subjects from the PROSPER study proposed that the infratentorial CMBs may be related to the impaired delayed memory (67). And the study proceeding on 500 nondemented individuals in the RUN-DMC study suggested that the presence and number of the frontal and temporal CMBs were related to the declined cognitive performance as measured by MMSE (68).
Mixed AD with stroke
AD is the most common form of the dementias, which is responsible for about 50-70% of the total demented cases (69) and surpasses the vascular dementia which is the secondary common form and constitutes 15-25% (70). A considerable overlap exists between AD and VCI. According to international working group (IWG) for new research criteria for the diagnosis of AD, the IWG-2 criteria, besides the existence of clinical and biomarker evidences of AD, the mixed AD with stroke should also include the stroke history, or focal neurological features supported by neuroimaging evidences, or both (71). The proportion of patients who suffer from AD with stroke is 56% of all demented cases (6). The CAA, which is a common cause of the stroke especially the hemorrhage stroke, is found in about 90% cases of AD (60,72). Caused by the amyloid deposition on the cerebral vessels, CAA is considered to be related to the pathogenesis of AD (73). Benedictus et al. suggested that the more CMBs may be associated with the higher amyloid burden, which could lead to the CAA (74). And other studies showed that CMBs may have effects on the cognitive decline in the AD patients (75,76). What’s more, the atherosclerosis is also one of the common causes of stroke. The study conducted by Honig et al. suggested that the atherosclerosis may also have effects on the pathogenesis of AD. It showed that the neuritic plaque which was one of the main pathologic manifestations of AD increased as the aggravation of atherosclerosis (77), suggesting the inner correlation between stroke and AD.
Besides that, the risk gene of AD, APOE ε4, is related to the poor cognitive outcome after stroke (78). APOE is a glycoprotein responsible for lipid transport in the brain and circulation, including APOE2, -E3, and -E4 which are encoded by three allele genes ε2, ε3, ε4 on chromosome 19 (79). And APOE ε4 allele is widely recognized as a significant genetic risk factor for sporadic AD (80). The study has shown that the presence of the APOE ε4 allele is associated with the amyloid deposition in the form of neuritic plaques and the increased risk of CAA (81). Moreover, a prospective cohort study on 3,424 elderly individuals suggested that the APOE ε4 carriers had a higher risk of the vascular dementia than the non-carriers (82). The carriers with one ε4 allele had an approximately 1.6-fold greater risk of the vascular dementia, whereas those with two ε4 alleles had a 4.4-fold greater risk. These evidences have shown that the APOE ε4 is associated with the risk of both AD and VCI, and provide a promising genetic therapy target for the cognitive impairment after stroke.
Management
Treatment for cognitive impairment
So far, there’s no unequivocally efficacious treatment to the post-stroke cognitive impairment. Some used in AD have shown some positive effects on post-stroke cognitive impairment. Although studies show that these drugs could make the significant improvement on some cognitive domains like executive function, the uncertainty on the global and daily function makes it difficult to evaluate the worth of the drugs on clinic (30). However, as the possible treatments of post-stroke cognitive impairment, the achievements on trials are still promising.
Cholinesterase inhibitors
Cholinesterase inhibitors, donepezil, galantamine, and rivastigmine have been approved for clinical use in AD (83). Followed by the development of the clinical trials, the cholinesterase inhibitor is confirmed as the promising drug for the treatment of the post-stroke cognitive impairment, among which the donepezil is the most promising one. The studies have suggested a significant for improving the cognitive function and daily living The double-blind, placebo-controlled, randomized clinical trials lasting 24 weeks have suggested that the donepezil has benefits in the cognition, but inconsistent benefits in global cognitive function of the patients with post stroke cognitive impairment (84-87). The recommendation for drug treatments of VCI from American Heart Association/American Stroke Association (AHA/ASA) suggested that donepezil could be effective for cognitive enhancement in patients with VCI and be recommended with the Class IIa; Level A evidence (30). What’s more, one recent trial on patients with right hemisphere stroke who were treated with the donepezil in 4 weeks showed that the significant cognitive improvement existed as measured by MMSE and the increased activation appeared in both prefrontal areas, both inferior frontal lobes, and in the left inferior parietal lobe, which suggested that the effect of donepezil may correlated to the parieto-frontal network in the cognitive impairment after stroke (88). However, the study on the cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL) patients who suffered from the subcortical ischemic vascular dementia showed different results (89). The study tested the effect of donepezil on 168 patients with CADASIL which was measured by vascular ADAS-Cog at 18 weeks. The results revealed that there was no significant difference in the vascular ADAS-cog score between the patients treated with donepezil and controls but an increased executive function existed.
Besides the donepezil, AHA/ASA also proposed the effect of other cholinesterase inhibitors such as galantamine and rivastigmine. One randomized clinical trial on galantamine suggested a significant less decline in cognition, function, and behavior in the patients with vascular dementia mixed with AD (85), while another one suggested that the galantamine could attenuate the cognitive impairment on executive function, but not on daily functions in a sample of patients with VCI (90). However, the meta-analysis on two trails failed to show the efficacy of galantamine but suggested the a higher risk of adverse gastrointestinal side-effects (91). And the trials on rivastigmine suggested the effect on executive function in VCI (92,93).
Memantine
The memantine, a noncompetitive N-methyl-D-aspartate receptor antagonist, which performs the neuroprotective effect by reducing the excitotoxicity, may have effects on both AD and VCI (94). Two randomized clinical trials have shown the memantine improves the cognition as measured by ADAS-cog and behavior as measured by NOSGER disturbed behavior, but not global functioning in patients with mild to moderate vascular dementia (95,96). However, the meta-analysis on the two trials above suggested that the benefits in cognition and behavior were not supported by clinical global measures (97). Interestingly, the effort of the memantine test on animal model is promising. The studies in vivo suggested that memantine could relieve the impaired memory and decrease the neural lesions caused by cerebral ischemia (98-100). The further study on rats with cortex occlusions suggested that the treatment with memantine could reduce the growth of microinfarct and diminish the cognitive deficits (101). Although the benefit of memantine on post-stroke cognitive impairment is still uncertain (30), due to the positive effort on animal models, there’s a good prospect for the future study.
Management of cerebrovascular disease
Treatment on brain lesions
As the post-stroke cognitive impairment is attributed to cerebral lesions due to stroke, it seems that the relief of cerebral lesions could contribute to the cognitive improvement. The citicoline, which is the generic name of cytidine-5’-diphosphocholine (CDP-choline, CDPCho), is widely used for the neuroprotection on clinic (102). The recent studies have shown that the citicoline could prevent the cognitive decline after stroke (103). Two clinic trials were conducted recently. The IDEALE study examined the effectiveness and safety of citicoline on patients with vascular mild cognitive impairment and suggested that the citicoline could improve the post-stroke cognition compared with controls as measured by MMSE after 9-month treatment, but failed to improve the activities of daily living (104). And the other one focused on the effect of citicoline on neurocognitive domains (105). After 12-month treatment, the stroke patients treated with citicoline showed a better functional outcome without statistically significant differences, but the cognitive functions such as the attention-executive functions and temporal orientation in citicoline group was significantly improved compared with controls. Both of the trials proposed that citicoline was a promising agent to improve the impaired cognition after stroke. Ginkgo Biloba, which is a traditional natural herbal product, plays extensive roles on the neural dysfunctions such as the impaired memory, concentration problems, dizziness, headache and so on (106). The study on the vascular dementia rat model has shown the bilobalide which is a extract from Ginkgo Biloba, could protect the learning and memory function by reducing free radical injury and inhibiting the apoptosis of neurons in the brain cortex and hippocampal CA1 region (107). And the recent meta-analyses on the clinical trials of Ginkgo Biloba for dementia suggested a change in cognitive scores in favor of extracts of Ginkgo Biloba compared to placebo (108,109). The clinical trial on the 333 patients with AD and 71 patients with vascular dementia from the GOTADAY study suggested the EGb 761, the extract of Ginkgo Biloba, could improve the cognitive functions and activities of daily living in both AD and vascular dementia groups after 24-week treatment (110). The more recent study on patients with VCI of no-dementia suggested 3-month treatment of Ginkgo Biloba could improve the cognitive function as measured by MoCA scores and the cerebral blood flow (111).
Prevention of vascular risk factors
The increasing evidences demonstrate that the vascular risk factor related to stroke could decrease the risk of post stroke cognitive impairment. The hypertension has been confirmed to be the potential risk factor of post-stroke cognitive impairment. The meta-analysis based on 11 studies suggested that hypertension is a significant risk factor for vascular dementia in the absence of an age difference (112). The study showed that the antihypertensive treatment could reduce the risk of both the stroke and the cognitive impairment, which crossed as a basis for aggregation (113). And a population-based cohort study on 6,249 participants suggested that the use of antihypertensive drug could decrease the risk of dementia with 8% per year of use for people ≤75 years of age (114). The further study revealed that the effects of antihypertensive drugs on vascular dementia varied for the classes of antihypertensive drugs (115). Besides the hypertension, the other vascular risk factors such as diabetes mellitus, hyperlipidemia, smoking, atrial fibrillation, smoking and so on, which have effects on the stroke, could also be the therapy target of post-stroke cognitive impairment (35). The study on 2,932 participants from Coronary Artery Risk Development in Young Adults study suggested that keeping weight, healthful diet, nonsmoking, physical activity, and controlling cholesterol, blood pressure, and fasting glucose were related to the better performance on cognition in later life (116).
Conclusions
The prevalence of post-stroke cognitive impairment in stroke survivors is high and varies for the difference between the countries, the races, and the diagnostic criteria. Both the demographic factors like age, education and occupation and vascular factors count for the high risk of post-stroke cognitive impairment. Post-stroke cognitive impairment could be induced by mechanisms including the VCI due to the neuroanatomical lesions on strategic areas and the CMBs as a result of the small cerebrovascular diseases and mixed AD with stroke. The risk gene APOE ε4 is associated with both AD and VCI. General strategies for managing patients with have been developed. Post-stroke cognitive impairment doesn’t only benefit in the application of anti-dementia treatments, but also the measures focusing on cerebrovascular diseases. Due to the conflictive results of the clinical studies, the further studies still need to determine the efficacy of various therapy strategies.
Acknowledgements
Funding: This work was supported in part by grants from the National Natural Science Foundation of China (81000544, 81171209), and the Shandong Provincial Natural Science Foundation, China (ZR2010HQ004, ZR2011HZ001).
Disclosure: The authors declare no conflict of interest.
References
- Strong K, Mathers C, Bonita R. Preventing stroke: saving lives around the world. Lancet Neurol 2007;6:182-7. [PubMed]
- Towfighi A, Saver JL. Stroke declines from third to fourth leading cause of death in the United States: historical perspective and challenges ahead. Stroke 2011;42:2351-5. [PubMed]
- Mackay J, Mensah GA. eds. The atlas of heart disease and stroke. Geneva: World Health Organization, 2004.
- Erkinjuntti T. Cerebrovascular Dementia: Pathophysiology, Diagnosis and Treatment. CNS Drugs 1999;12:35-48.
- Desmond DW, Moroney JT, Paik MC, et al. Frequency and clinical determinants of dementia after ischemic stroke. Neurology 2000;54:1124-31. [PubMed]
- Jellinger KA. The enigma of mixed dementia. Alzheimers Dement 2007;3:40-53. [PubMed]
- Nys GM, van Zandvoort MJ, de Kort PL, et al. Restrictions of the Mini-Mental State Examination in acute stroke. Arch Clin Neuropsychol 2005;20:623-9. [PubMed]
- Pendlebury ST, Cuthbertson FC, Welch SJ, et al. Underestimation of cognitive impairment by Mini-Mental State Examination versus the Montreal Cognitive Assessment in patients with transient ischemic attack and stroke: a population-based study. Stroke 2010;41:1290-3. [PubMed]
- Bour A, Rasquin S, Boreas A, et al. How predictive is the MMSE for cognitive performance after stroke? J Neurol 2010;257:630-7. [PubMed]
- Zhou DH, Wang JY, Li J, et al. Study on frequency and predictors of dementia after ischemic stroke: the Chongqing stroke study. J Neurol 2004;251:421-7. [PubMed]
- Madureira S, Guerreiro M, Ferro JM. Dementia and cognitive impairment three months after stroke. Eur J Neurol 2001;8:621-7. [PubMed]
- Rist PM, Chalmers J, Arima H, et al. Baseline cognitive function, recurrent stroke, and risk of dementia in patients with stroke. Stroke 2013;44:1790-5. [PubMed]
- Gutiérrez Pérez C, Savborg M, Pahlman U, et al. High frequency of cognitive dysfunction before stroke among older people. Int J Geriatr Psychiatry 2011;26:622-9. [PubMed]
- Douiri A, Rudd AG, Wolfe CD. Prevalence of poststroke cognitive impairment: South London Stroke Register 1995-2010. Stroke 2013;44:138-45. [PubMed]
- Rasquin SM, Verhey FR, van Oostenbrugge RJ, et al. Demographic and CT scan features related to cognitive impairment in the first year after stroke. J Neurol Neurosurg Psychiatry 2004;75:1562-7. [PubMed]
- Ihle-Hansen H, Thommessen B, Wyller TB, et al. Incidence and subtypes of MCI and dementia 1 year after first-ever stroke in patients without pre-existing cognitive impairment. Dement Geriatr Cogn Disord 2011;32:401-7. [PubMed]
- Jacquin A, Binquet C, Rouaud O, et al. Post-stroke cognitive impairment: high prevalence and determining factors in a cohort of mild stroke. J Alzheimers Dis 2014;40:1029-38. [PubMed]
- Sachdev PS, Brodaty H, Valenzuela MJ, et al. Clinical determinants of dementia and mild cognitive impairment following ischaemic stroke: the Sydney Stroke Study. Dement Geriatr Cogn Disord 2006;21:275-83. [PubMed]
- Srikanth VK, Anderson JF, Donnan GA, et al. Progressive dementia after first-ever stroke: a community-based follow-up study. Neurology 2004;63:785-92. [PubMed]
- Srikanth VK, Thrift AG, Saling MM, et al. Increased risk of cognitive impairment 3 months after mild to moderate first-ever stroke: a Community-Based Prospective Study of Nonaphasic English-Speaking Survivors. Stroke 2003;34:1136-43. [PubMed]
- Ivan CS, Seshadri S, Beiser A, et al. Dementia after stroke: the Framingham Study. Stroke 2004;35:1264-8. [PubMed]
- Lisabeth LD, Sanchez BN, Baek J, et al. Neurological, functional, and cognitive stroke outcomes in Mexican Americans. Stroke 2014;45:1096-101. [PubMed]
- Chausson N, Olindo S, Cabre P, et al. Five-year outcome of a stroke cohort in Martinique, French West Indies: Etude Realisee en Martinique et Centree sur l’Incidence des Accidents vasculaires cerebraux, Part 2. Stroke 2010;41:594-9. [PubMed]
- Yu KH, Cho SJ, Oh MS, et al. Cognitive impairment evaluated with Vascular Cognitive Impairment Harmonization Standards in a multicenter prospective stroke cohort in Korea. Stroke 2013;44:786-8. [PubMed]
- Tham W, Auchus AP, Thong M, et al. Progression of cognitive impairment after stroke: one year results from a longitudinal study of Singaporean stroke patients. J Neurol Sci 2002;203-204:49-52. [PubMed]
- Das S, Paul N, Hazra A, et al. Cognitive Dysfunction in Stroke Survivors: A Community-Based Prospective Study from Kolkata, India. J Stroke Cerebrovasc Dis 2013;22:1233-42. [PubMed]
- Tang WK, Chan SS, Chiu HF, et al. Frequency and clinical determinants of poststroke cognitive impairment in nondemented stroke patients. J Geriatr Psychiatry Neurol 2006;19:65-71. [PubMed]
- Zhou DH, Wang JY, Li J, et al. Frequency and risk factors of vascular cognitive impairment three months after ischemic stroke in china: the Chongqing stroke study. Neuroepidemiology 2005;24:87-95. [PubMed]
- Tu Q, Ding B, Yang X, et al. The current situation on vascular cognitive impairment after ischemic stroke in Changsha. Arch Gerontol Geriatr 2014;58:236-47. [PubMed]
- Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011;42:2672-713. [PubMed]
- Elbaz A, Vicente-Vytopilova P, Tavernier B, et al. Motor function in the elderly: evidence for the reserve hypothesis. Neurology 2013;81:417-26. [PubMed]
- Wu Y, Wang M, Ren M, et al. The effects of educational background on Montreal Cognitive Assessment screening for vascular cognitive impairment, no dementia, caused by ischemic stroke. J Clin Neurosci 2013;20:1406-10. [PubMed]
- Singh-Manoux A, Marmot MG, Glymour M, et al. Does cognitive reserve shape cognitive decline? Ann Neurol 2011;70:296-304. [PubMed]
- Zahodne LB, Glymour MM, Sparks C, et al. Education does not slow cognitive decline with aging: 12-year evidence from the victoria longitudinal study. J Int Neuropsychol Soc 2011;17:1039-46. [PubMed]
- Sahathevan R, Brodtmann A, Donnan GA. Dementia, stroke, and vascular risk factors; a review. Int J Stroke 2012;7:61-73. [PubMed]
- Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol 2009;8:1006-18. [PubMed]
- Sibolt G, Curtze S, Melkas S, et al. Poststroke dementia is associated with recurrent ischaemic stroke. J Neurol Neurosurg Psychiatry 2013;84:722-6. [PubMed]
- Tomlinson BE, Blessed G, Roth M. Observations on the brains of demented old people. J Neurol Sci 1970;11:205-42. [PubMed]
- Zekry D, Duyckaerts C, Belmin J, et al. The vascular lesions in vascular and mixed dementia: the weight of functional neuroanatomy. Neurobiol Aging 2003;24:213-9. [PubMed]
- Szabo K, Forster A, Jager T, et al. Hippocampal lesion patterns in acute posterior cerebral artery stroke: clinical and MRI findings. Stroke 2009;40:2042-5. [PubMed]
- Blum S, Luchsinger JA, Manly JJ, et al. Memory after silent stroke: hippocampus and infarcts both matter. Neurology 2012;78:38-46. [PubMed]
- Qiu C, Zhang Y, Bronge L, et al. Medial temporal lobe is vulnerable to vascular risk factors in men: a population-based study. Eur J Neurol 2012;19:876-83. [PubMed]
- Gemmell E, Bosomworth H, Allan L, et al. Hippocampal neuronal atrophy and cognitive function in delayed poststroke and aging-related dementias. Stroke 2012;43:808-14. [PubMed]
- Gemmell E, Tam E, Allan L, et al. Neuron volumes in hippocampal subfields in delayed poststroke and aging-related dementias. J Neuropathol Exp Neurol 2014;73:305-11. [PubMed]
- Li W, Huang R, Shetty RA, et al. Transient focal cerebral ischemia induces long-term cognitive function deficit in an experimental ischemic stroke model. Neurobiol Dis 2013;59:18-25. [PubMed]
- Wen X, Qi D, Sun Y, et al. H2S attenuates cognitive deficits through Akt1/JNK3 signaling pathway in ischemic stroke. Behav Brain Res 2014;269:6-14. [PubMed]
- Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010;9:689-701. [PubMed]
- Koga H, Takashima Y, Murakawa R, et al. Cognitive consequences of multiple lacunes and leukoaraiosis as vascular cognitive impairment in community-dwelling elderly individuals. J Stroke Cerebrovasc Dis 2009;18:32-7. [PubMed]
- LADIS Study Group. 2001-2011: a decade of the LADIS (Leukoaraiosis And DISability) Study: what have we learned about white matter changes and small-vessel disease? Cerebrovasc Dis 2011;32:577-88.
- Benisty S, Gouw AA, Porcher R, et al. Location of lacunar infarcts correlates with cognition in a sample of non-disabled subjects with age-related white-matter changes: the LADIS study. J Neurol Neurosurg Psychiatry 2009;80:478-83. [PubMed]
- Jokinen H, Kalska H, Ylikoski R, et al. Longitudinal cognitive decline in subcortical ischemic vascular disease--the LADIS Study. Cerebrovasc Dis 2009;27:384-91. [PubMed]
- Jokinen H, Lipsanen J, Schmidt R, et al. Brain atrophy accelerates cognitive decline in cerebral small vessel disease: the LADIS study. Neurology 2012;78:1785-92. [PubMed]
- Kooistra M, Geerlings MI, van der Graaf Y, et al. Vascular brain lesions, brain atrophy, and cognitive decline. The Second Manifestations of ARTerial disease--Magnetic Resonance (SMART-MR) study. Neurobiol Aging 2014;35:35-41. [PubMed]
- Zi W, Duan D, Zheng J. Cognitive impairments associated with periventricular white matter hyperintensities are mediated by cortical atrophy. Acta Neurol Scand 2014;130:178-87. [PubMed]
- Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 2009;8:165-74. [PubMed]
- Park JH, Seo SW, Kim C, et al. Pathogenesis of cerebral microbleeds: In vivo imaging of amyloid and subcortical ischemic small vessel disease in 226 individuals with cognitive impairment. Ann Neurol 2013;73:584-593. [PubMed]
- Poels MM, Vernooij MW, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke 2010;41:S103-106. [PubMed]
- Cordonnier C, Al-Shahi Salman R, Wardlaw J. Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain 2007;130:1988-2003. [PubMed]
- Charlton RA, Morris RG, Nitkunan A, et al. The cognitive profiles of CADASIL and sporadic small vessel disease. Neurology 2006;66:1523-6. [PubMed]
- Viswanathan A, Greenberg SM. Cerebral amyloid angiopathy in the elderly. Ann Neurol 2011;70:871-80. [PubMed]
- Werring DJ, Frazer DW, Coward LJ, et al. Cognitive dysfunction in patients with cerebral microbleeds on T2*-weighted gradient-echo MRI. Brain 2004;127:2265-75. [PubMed]
- Gregoire SM, Smith K, Jager HR, et al. Cerebral microbleeds and long-term cognitive outcome: longitudinal cohort study of stroke clinic patients. Cerebrovasc Dis 2012;33:430-5. [PubMed]
- Qiu C, Cotch MF, Sigurdsson S, et al. Cerebral microbleeds, retinopathy, and dementia: the AGES-Reykjavik Study. Neurology 2010;75:2221-8. [PubMed]
- Poels MM, Ikram MA, van der Lugt A, et al. Cerebral microbleeds are associated with worse cognitive function: the Rotterdam Scan Study. Neurology 2012;78:326-33. [PubMed]
- Tang WK, Chen YK, Lu JY, et al. Absence of cerebral microbleeds predicts reversion of vascular ‘cognitive impairment no dementia’ in stroke. Int J Stroke 2011;6:498-505. [PubMed]
- Nardone R, De Blasi P, Seidl M, et al. Cognitive function and cholinergic transmission in patients with subcortical vascular dementia and microbleeds: a TMS study. J Neural Transm 2011;118:1349-58. [PubMed]
- van Es AC, van der Grond J, de Craen AJ, et al. Cerebral microbleeds and cognitive functioning in the PROSPER study. Neurology 2011;77:1446-52. [PubMed]
- van Norden AG, van den Berg HA, de Laat KF, et al. Frontal and temporal microbleeds are related to cognitive function: the Radboud University Nijmegen Diffusion Tensor and Magnetic Resonance Cohort (RUN DMC) Study. Stroke 2011;42:3382-6. [PubMed]
- Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet 2005;366:2112-7. [PubMed]
- Qiu C, De Ronchi D, Fratiglioni L. The epidemiology of the dementias: an update. Curr Opin Psychiatry 2007;20:380-5. [PubMed]
- Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol 2014;13:614-29. [PubMed]
- Jellinger KA. Alzheimer disease and cerebrovascular pathology: an update. J Neural Transm 2002;109:813-36. [PubMed]
- Weller RO, Massey A, Newman TA, et al. Cerebral amyloid angiopathy: amyloid beta accumulates in putative interstitial fluid drainage pathways in Alzheimer’s disease. Am J Pathol 1998;153:725-33. [PubMed]
- Benedictus MR, Goos JD, Binnewijzend MA, et al. Specific risk factors for microbleeds and white matter hyperintensities in Alzheimer’s disease. Neurobiol Aging 2013;34:2488-94. [PubMed]
- Cordonnier C, van der Flier WM. Brain microbleeds and Alzheimer’s disease: innocent observation or key player? Brain 2011;134:335-44. [PubMed]
- Staekenborg SS, Koedam EL, Henneman WJ, et al. Progression of mild cognitive impairment to dementia: contribution of cerebrovascular disease compared with medial temporal lobe atrophy. Stroke 2009;40:1269-74. [PubMed]
- Honig LS, Kukull W, Mayeux R. Atherosclerosis and AD: analysis of data from the US National Alzheimer’s Coordinating Center. Neurology 2005;64:494-500. [PubMed]
- Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol 2011;10:241-52. [PubMed]
- Olaisen B, Teisberg P, Gedde-Dahl T. The locus for apolipoprotein E (apoE) is linked to the complement component C3 (C3) locus on chromosome 19 in man. Hum Genet 1982;62:233-6. [PubMed]
- Sadigh-Eteghad S, Talebi M, Farhoudi M. Association of apolipoprotein E epsilon 4 allele with sporadic late onset Alzheimer’s disease. A meta-analysis. Neurosciences 2012;17:321-6. [PubMed]
- Liu CC, Kanekiyo T, Xu H, et al. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 2013;9:106-18. [PubMed]
- Chuang YF, Hayden KM, Norton MC, et al. Association between APOE epsilon4 allele and vascular dementia: The Cache County study. Dement Geriatr Cogn Disord 2010;29:248-53. [PubMed]
- Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet 2006;368:387-403. [PubMed]
- Wilkinson D, Doody R, Helme R, et al. Donepezil in vascular dementia: a randomized, placebo-controlled study. Neurology 2003;61:479-86. [PubMed]
- Black S, Román GC, Geldmacher DS, et al. Efficacy and tolerability of donepezil in vascular dementia: positive results of a 24-week, multicenter, international, randomized, placebo-controlled clinical trial. Stroke 2003;34:2323-30. [PubMed]
- Román GC, Salloway S, Black SE, et al. Randomized, placebo-controlled, clinical trial of donepezil in vascular dementia: differential effects by hippocampal size. Stroke 2010;41:1213-21. [PubMed]
- Rockwood K, Mitnitski A, Black SE, et al. Cognitive change in donepezil treated patients with vascular or mixed dementia. Can J Neurol Sci 2013;40:564-71. [PubMed]
- Chang WH, Park YH, Ohn SH, et al. Neural correlates of donepezil-induced cognitive improvement in patients with right hemisphere stroke: a pilot study. Neuropsychol Rehabil 2011;21:502-14. [PubMed]
- Dichgans M, Markus HS, Salloway S, et al. Donepezil in patients with subcortical vascular cognitive impairment: a randomised double-blind trial in CADASIL. Lancet Neurol 2008;7:310-8. [PubMed]
- Auchus AP, Brashear HR, Salloway S, et al. Galantamine treatment of vascular dementia: a randomized trial. Neurology 2007;69:448-58. [PubMed]
- Birks J, Craig D. Galantamine for vascular cognitive impairment. Cochrane Database Syst Rev 2013;CD004746. [PubMed]
- Ballard C, Sauter M, Scheltens P, et al. Efficacy, safety and tolerability of rivastigmine capsules in patients with probable vascular dementia: the VantagE study. Curr Med Res Opin 2008;24:2561-74. [PubMed]
- Moretti R, Torre P, Antonello RM, et al. Rivastigmine in subcortical vascular dementia: an open 22-month study. J Neurol Sci 2002;203-204:141-6. [PubMed]
- Wilcock GK. Memantine for the treatment of dementia. Lancet Neurol 2003;2:503-5. [PubMed]
- Wilcock G, Möbius HJ, Stöffler A, et al. A double-blind, placebo-controlled multicentre study of memantine in mild to moderate vascular dementia (MMM500). Int Clin Psychopharmacol 2002;17:297-305. [PubMed]
- Orgogozo JM, Rigaud AS, Stoffler A, et al. Efficacy and safety of memantine in patients with mild to moderate vascular dementia: a randomized, placebo-controlled trial (MMM 300). Stroke 2002;33:1834-9. [PubMed]
- Areosa SA, Sherriff F, McShane R. Memantine for dementia. Cochrane Database Syst Rev 2005;CD003154. [PubMed]
- Kilic U, Yilmaz B, Reiter RJ, et al. Effects of memantine and melatonin on signal transduction pathways vascular leakage and brain injury after focal cerebral ischemia in mice. Neuroscience 2013;237:268-76. [PubMed]
- Babu CS, Ramanathan M. Post-ischemic administration of nimodipine following focal cerebral ischemic-reperfusion injury in rats alleviated excitotoxicity, neurobehavioural alterations and partially the bioenergetics. Int J Dev Neurosci 2011;29:93-105. [PubMed]
- Watanabe T, Iwasaki K, Takasaki K, et al. Dynamin 1 depletion and memory deficits in rats treated with Abeta and cerebral ischemia. J Neurosci Res 2010;88:1908-17. [PubMed]
- Shih AY, Blinder P, Tsai PS, et al. The smallest stroke: occlusion of one penetrating vessel leads to infarction and a cognitive deficit. Nat Neurosci 2013;16:55-63. [PubMed]
- Grieb P. Neuroprotective properties of citicoline: facts, doubts and unresolved issues. CNS Drugs 2014;28:185-93. [PubMed]
- Alvarez-Sabín J, Roman GC. Citicoline in vascular cognitive impairment and vascular dementia after stroke. Stroke 2011;42:S40-43. [PubMed]
- Cotroneo AM, Castagna A, Putignano S, et al. Effectiveness and safety of citicoline in mild vascular cognitive impairment: the IDEALE study. Clin Interv Aging 2013;8:131-7. [PubMed]
- Alvarez-Sabín J, Ortega G, Jacas C, et al. Long-term treatment with citicoline may improve poststroke vascular cognitive impairment. Cerebrovasc Dis 2013;35:146-54. [PubMed]
- Baskys A, Cheng JX. Pharmacological prevention and treatment of vascular dementia: approaches and perspectives. Exp Gerontol 2012;47:887-91. [PubMed]
- Li WZ, Wu WY, Huang H, et al. Protective effect of bilobalide on learning and memory impairment in rats with vascular dementia. Mol Med Rep 2013;8:935-41. [PubMed]
- Wang BS, Wang H, Song YY, et al. Effectiveness of standardized ginkgo biloba extract on cognitive symptoms of dementia with a six-month treatment: a bivariate random effect meta-analysis. Pharmacopsychiatry 2010;43:86-91. [PubMed]
- Weinmann S, Roll S, Schwarzbach C, et al. Effects of Ginkgo biloba in dementia: systematic review and meta-analysis. BMC Geriatr 2010;10:14. [PubMed]
- Ihl R, Tribanek M, Bachinskaya N, et al. Efficacy and tolerability of a once daily formulation of Ginkgo biloba extract EGb 761(R) in Alzheimer's disease and vascular dementia: results from a randomised controlled trial. Pharmacopsychiatry 2012;45:41-6. [PubMed]
- Zhang SJ, Xue ZY. Effect of Western medicine therapy assisted by Ginkgo biloba tablet on vascular cognitive impairment of none dementia. Asian Pac J Trop Med 2012;5:661-4. [PubMed]
- Sharp SI, Aarsland D, Day S, et al. Hypertension is a potential risk factor for vascular dementia: systematic review. Int J Geriatr Psychiatry 2011;26:661-9. [PubMed]
- Sörös P, Whitehead S, Spence JD, et al. Antihypertensive treatment can prevent stroke and cognitive decline. Nat Rev Neurol 2013;9:174-8. [PubMed]
- Haag MD, Hofman A, Koudstaal PJ, et al. Duration of antihypertensive drug use and risk of dementia: A prospective cohort study. Neurology 2009;72:1727-34. [PubMed]
- Coca A. Hypertension and vascular dementia in the elderly: the potential role of anti-hypertensive agents. Curr Med Res Opin 2013;29:1045-54. [PubMed]
- Reis JP, Loria CM, Launer LJ, et al. Cardiovascular health through young adulthood and cognitive functioning in midlife. Ann Neurol 2013;73:170-9. [PubMed]