The conundrum of asymptomatic carotid stenosis—determinants of decision and evidence
Introduction
Conundrum: a problem that is difficult to deal with (1) is probably an accurate description of the present controversies and lack of consensus on the management of asymptomatic carotid artery disease.
The definition of asymptomatic carotid stenosis (ACS) adopted as criteria for inclusion on prospective natural history studies and controlled trials (RCT’s) encompasses the absence of ipsilateral appropriate ocular and/or hemispheric symptoms—transient ischemic attacks (TIA) and stroke—for at least a 6-month period preceding diagnosis; the presence of contralateral symptoms or ipsilateral appropriate symptoms before that 6-month interval are not considered (2).
Atherosclerosis is the most common cause of ACS. Its prevalence increases from the 5th decade, being higher in patients older than 70 years, more common in men than women. It is not only associated to increased risk of ischemic stroke in the appropriate carotid territory, but also to a higher incidence of acute coronary events and vascular death (3).
The mechanisms underlying the ocular and hemispheric symptoms are more dependent on distal embolization from atherothrombosis in the carotid bifurcation rather than flow restriction, which is generally compensated through collateral circulation and functional integrity of the circle of Willis, even in the presence of concomitant intracranial occlusive disease.
Asymptomatic carotid stenosis and risk of stroke and mortality
Stroke is a leading cause of death in western world and a significant cause of disability and loss of quality of life. In the USA around 600,000 first-ever strokes occur each year (4) and in Europe it accounts for 1.1 million deaths per year, being the second leading cause of death (5). More than 50% of the survivors remain with some form of dependency for their daily activities (6). Its financial impact is very significant making stroke prevention a major goal for health policies.
Approximately 35% of all ischemic strokes are associated to carotid bifurcation stenosis, 25% of being related to intracranial disease (lacunar infarcts) and 20% approximately from cardioembolic nature. It is estimated that in 11% (out of those 35% ischemic strokes associated with carotid stenosis) stroke is the first-ever neurological event associated to a stenosis >50% of the internal carotid artery (ICA) (7). These represent an important number of patients where those first-ever strokes could be prevented or reduced by early diagnosis of a significant carotid lesion and prompt intervention, medical and/or surgical.
Durable benefit of carotid endarterectomy (CEA) for ICA 70–99% stenosis with a 50% reduction on stroke-risk from 12% to 6% at 5-year follow-up and a 4.6% absolute risk reduction at 10 years when compared with state-of-the-art best medical treatment (BMT), was clearly demonstrated irrespective of higher mortality mainly due to cardiac causes (2,8,9).
These observations were the basis for recommendations and Guidelines for the treatment of ACS, which should include control of modifiable risk factors and life-style changes for all patients plus invasive CEA for selected 70–99% stenosis in patients with acceptable life expectancy and provided the interventional risk would be <3% (3,10-13). Based on CREST trial results carotid artery stenting (CAS) was endorsed by the AHA/ACC guidelines as an alternative to CEA for ACS (11) but not on more recent Guidelines (13).
Critical evaluation of ACST results demonstrated a 4.6% relative risk reduction offered by CEA + BMT vs. BMT alone, which means that a significant number of procedures 950/1,000 could be ultimately considered unnecessary. Or that only 5% of all CEA’s—1 for every 20 CEAs (or only about 50 for every 1,000 CEAs—would be clinically effective in reducing stroke risk, meaning that 95% of all invasive procedures performed were probably unnecessary and potentially harmful, as there is a low but definitive risk associated to CEA (6).
Several other observations fueled the controversy on management of ACS drawing attention to the importance of medical, nonsurgical, intervention, based on effective reduction of stroke and death risks achieved with medial therapy in stenosis >50–60% and also on the fact that the majority of patients in randomized trials were not having proper and full medical intervention (14). Long-term mortality rates are associated to risk factors for coronary artery disease often present in patients with ACS such as age >70 years, male sex, smoking, high circulating cholesterol levels not properly controlled, arterial hypertension, diabetes, chronic renal dysfunction, which could explain the higher incidence of vascular events and coronary death overcoming the risk of cerebral infarction and stroke and raising questions on appropriateness of carotid interventions, particularly in older patients (15-20). Second, the effect of contemporary BMT including treatment of modifiable risk factors, smoking restriction (reinforced by legislation in some countries), active reduction of LDL cholesterol tailored to the stratification of cardiovascular risk by generalized use of statins and other lowering-lipid drugs, use of aspirin plus appropriate treatment of arterial hypertension (21), which has contributed to significantly reduce stroke-risk associated with moderate to severe ACS thus indirectly suggesting that medical management would be sufficient in patients with 50–99% ACS (22-24).
Similar effect of BMT was reported on the reduction of myocardial infarct rates (24) and in patients with intracranial stenosis, BMT alone offering better protection against stroke incidence than intervention with stenting (25). These observations were also echoed in Guidelines recommending that BMT should be the preferred option also for patients with high surgical risk for CEA (13).
Reduction in carotid plaque size with aggressive lipid-lowering treatment was documented, but progression of disease with increase on plaque size was noted in some patients, suggesting a non-uniform response of all stenosis to the same medical treatment (26,27). Rather than a size reduction in the plaques, aggressive lipid-lowering treatment as statins has shown an anti-inflammatory effect in the plaques, also decreasing their thrombogenicity and propensity for rupture (28).
Criticism for the isolated policy of BMT for all patients with 70–99% stenosis resulted from the fact that studies confirming reduction of stroke risk with BMT have included patients with moderate non-surgical 50–70% stenosis with a lower stroke-risk and also from published reports of insufficient patient compliance with medical treatment (7,29).
Improvement on the selection of ACS that would benefit from invasive treatment, either CEA or CAS, became a priority with a two-fold aim: (I) to reduce the excessive number of carotid interventions being performed, restricting its indications to subgroups of patients with higher risk of neurological events (II) to minimize variability in worldwide practice, ranging from the USA where almost 90% of all CEA have been performed for asymptomatic disease, comparing to 15–20% only in the north European countries, 30% in Australia, 40% in Hungary and Switzerland and 70% in Italy (30,31).
Definition of BMT as previously mentioned is multifactorial: life-style modifications with healthy habits such as exercise, Mediterranean diet, active smoking cessation, plus control of associated disorders as diabetes, arterial hypertension and renal dysfunction, reduction of LDL-C levels by use of appropriate lipid-lowering treatments and anti-platelet therapy. These are essential tools for proper management of atherosclerosis, and they should be pursued in all patients with atherosclerotic arterial disease, whatever location on carotid, coronary, aorto-iliac and peripheral segments, ultimately preventing cardiovascular events (32). The active reduction of LDL-C levels to <70 mg/dL for the high-risk group and <55 mg/dL for those in the very high-risk group by statins and other lowering-lipid drugs should be pursued to achieve maximal benefit on the reduction of vascular events and also to achieve stabilization of the carotid stenosis (33-35).
Is there strong evidence that isolated BMT will be sufficient to prevent strokes in ACS?
The suggestion that BMT treatment would not be enough for all patients with ACS—one fits all—has been recently clearly discussed (36) where not only reduction on overall death and stroke risk should be compared with long-term benefit of CEA and not only to 30-day outcome of the carotid intervention, but the very concept of truly asymptomatic patient is challenged. Some patients may have suffered neurological symptoms when asleep and the presence of silent brain infarcts in the carotid territory.
Progression of baseline 50–69% asymptomatic carotid stenosis was reported in 29.1% and 24.7% of patients, it was associated with an annual stroke rate ranging from 2.1% to 7.7% during an average follow-up of 3.5 years compared to 0.4% annually in those with stable plaques (37). Data from the ACSRS, a natural history study involving 1,121 patients with 50–99% asymptomatic carotid stenosis under medical treatment, demonstrated plaque progression in 19,8% during a mean follow-up of 4.0 years (38) associated to an annual risk of stroke of 2.0%, but included only 32% of the total number of strokes occurring during the same period. For more severe stenosis 80–99%, progression had an annual stroke-risk of 3.1%, suggesting that baseline severity of stenosis and its progression were independent predictors of stroke-risk, but not sufficient to identify all patients who developed strokes. More recent data from ACST1 confirmed that a two-level progression increased significantly the risk of stroke (39) and for very severe stenosis >90% a higher rate of ipsilateral neurological events and death was recognized without beneficial effect from medical treatment (40).
Therefore, the quest for the identification of subgroups of patients with increased risk of neurological dysfunction—vulnerable patient—or the recognition of active plaques more prone to disruption and brain embolization—vulnerable plaque—became a major goal-holy grail. Detecting vulnerable patients and/or vulnerable plaques underlying asymptomatic carotid stenosis would culminate in appropriated beneficial invasive treatment as well as in the reduction of the excessive number of unnecessary carotid procedures.
Not all ACS are equal—what is the profile of the patient at risk?
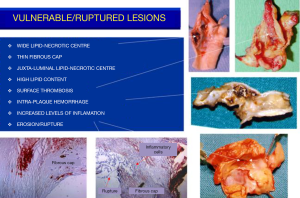
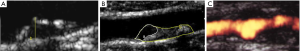
The concept of vulnerable plaque was derived from autopsy studies providing evidence than two-thirds to three-fourths of fatal acute myocardial infarctions were associated to rupture of the plaque’s fibrous cap leading to local thrombosis with or without coronary occlusion and distal embolization of atherothrombotic material. These observations provided better understanding of the pathophysiology of acute coronary syndromes attributed to active inflammation leading to rupture of coronary plaques loaded with lipids, covered by thin fibrous cap prone to rupture exposing the subendothelial contents to blood components, platelet adhesion, local thrombosis plus distal embolization (41-46). Identification of ulceration and/or thin-capped atheromatous plaques covering areas of lipid deposits or hemorrhagic components became target for imaging arteries in atherosclerosis. Pathology observations of specimens of carotid endarterectomies in symptomatic patients confirmed similar process due to intense inflammation, plaque rupture and ulceration, thrombogenic calcified noduli near the lumen, extrusion of subendothelial components (47,48), or from the core containing lipids and/or blood derivates (49) located close to the lumen of the artery (Figure 1). Recognition of pathological markers of plaque activity were obtained from histological detailed studies of carotid specimens (50,51) providing guidance for a better understanding of the requirements for carotid plaque imaging with new available non-invasive technologies in order to identify potential markers of plaque vulnerability (Figure 2). During the last 20 years various studies and a remarkable bulk of information emerged for the assessment of patients with ACS aiming to identify those subgroups at a higher risk to develop neurological symptoms, including plaque-related features (52)—vulnerable plaques—and patient-related parameters- vulnerable patient.
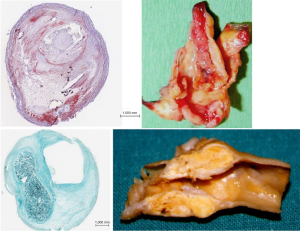
Despite all these advances, the degree of stenosis is still the most relevant factor for the clinical decision as suggested by ACAS and ACST trials (2,8,9) considering that more severe stenotic lesions are associated with higher neurological risk (53,54). Carotid plaques with specific morphological features predisposing to local thrombosis and distal brain embolization would possibly respond differently to adequate medical treatment (BMT) and different neurological risk was based on clinical data and macroscopic and histologic observations from endarterectomy specimens. A stable carotid plaque, with low risk of complications would have homogenous structure, regular non-eroded thick fibrous cap overlying a hard-central core with scarce inflammatory activity. In contrast vulnerable plaques may have heterogenous structure, ulcerated surface, soft lipid-rich or hemorrhagic core covered by a thin fibrous cap rich in inflammatory cells, prone to rupture, leading to embolization of thrombus or debris causing neurologic events (Figure 3).
Intensive research aiming to identify this subgroup of vulnerable plaques related to increased risk (prone to rupture or erosion) was conducted using ultrasonography (50-56), magnetic resonance imaging (MRI) (57-60), computed tomography angiography (CTA) (61), isolated or combined with positron emission tomography (PET-CTA), optical coherence tomography and contrast-enhanced ultrasound (CEUS) to detect increased neo-vascularization (62-64).
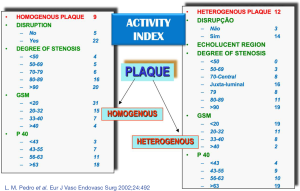
Our group studied the significance of plaque structure using advanced high-definition ultrasound technology (HDU) and provided evidence that heterogenous and echolucent plaques with gray-median scale (GSM) <25 as previously suggested (65) plus the juxta-luminal location of echolucent areas in heterogenous lesions, with evidence of surface disruption and higher degrees of stenosis were common in specimens obtained from CEA in symptomatic patients (66-69) (Figure 4) and its biochemical composition suggested increased plaque biological activity (70-74). In another study we showed that plaque heretogeneity assessed by HDU with the statistical geometric feature reflected plaque inflammation and a vulnerable plaque phenotype (75,76). By assessing the relative importance of all these factors an objective measurement of plaque activity was derived—Activity Index (AI)—in order to identify asymptomatic lesions prone to develop symptoms (Figure 5). Using advanced image analysis (77-79), its diagnostic accuracy was improved providing higher accuracy (78-80) to identify those asymptomatic lesions that developed appropriate neurological symptoms during a 4-year follow-up period (Figure 6). Many approaches have also been attempted by several groups interested in ultrasound. We have also successfully designed novel radiofrequency algorithms based on the center frequency shifts, that detected vulnerable plaques in vivo (confirmed by histology) with an accuracy of 78% (81).
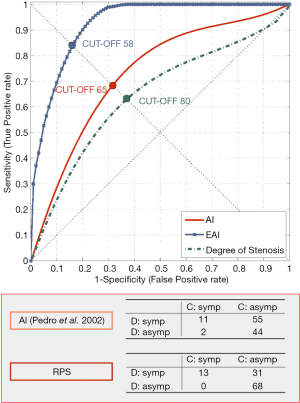
The Asymptomatic Carotid Stenosis and Risk of Stroke (ACSRS) trial, mentioned above, a comprehensive natural history study involving 1,121 patients with asymptomatic 50–99% stenosis combining clinical parameters and computer-assisted plaque analysis based on HDU, identified a high-risk group with an annual rate of stroke of 5.3% (37) and confirmed the value of our previous observation of the significance of the juxta-luminal echolucent (black) area (JLBA) (67,82,83).
Studies with Transcranial Doppler (TCD) with detection of HITS on the middle cerebral artery provided recognition of embolization activity from the carotid plaques which is associated to an increased risk of stroke (84,85). Also, intensive medical therapy achieving low LDL levels has been demonstrated to effectively reduce the number of HITS detected in the middle cerebral artery with TCD and 1-year risk of stroke in a group of asymptomatic patients with stenosis >60% (86,87). In those patients without TCD HITS the 1-year risk of stroke was 1.3%, lower than potential risk of intervention.
MRI, CT and PET (88,89) offered a more objective, reproducible and both qualitative and quantitative characterization of the plaque structure, its components, and also the identification of functional activity like inflammation and thrombosis thus contributing to detect the more vulnerable carotid lesions. However, its widespread use in a busy clinical setting is probably not optimal neither cost-effective.
Studies with contrast-enhanced ultrasound (CEUS) provided evidence of increased vascularization within the plaque structure in patients with appropriate neurological syndromes (88) but its advantage to identify vulnerable asymptomatic plaques in comparison with easier and less-expensive HDU studies was not tested in more extensive prospective studies.
The vulnerable-patient concept emerged and it also of high interest. A comprehensive profile of the high risk patient emerged from the ACSRS study: (I) presence of 80–99% stenosis (II) high systolic blood pressure (III) smoking history of >10 pack-years (IV) contralateral TIA’s or stroke (V) plaque echolucency (Gray Scale Median-GSM) (VI) plaque type and area (VII) plaque structural heterogeneity evidenced by Discrete White Areas (DWA) (VIII) juxta-luminal black area >8 mm2 (JLBA) and (IX) progression of stenosis (18,38,52,83) (see appropriate chapter). In other studies, various clinical and brain imaging parameters were shown to be associated to a higher stroke-risk in ACS patients too namely (I) presence of contralateral neurological symptoms, either TIA’s and/or stroke (90-92) (II) contralateral internal carotid occlusion (90,93) (III) multivessel occlusive disease associated with exhausted vasomotor cerebral autoregulation (94) (IV) presence of clinically silent brain infarctions in the ipsilateral brain hemisphere to carotid stenosis (95).
The presence of active atherosclerotic disease in other territories with evidence of systemic inflammation, a known factor for progression and rupture of atherosclerotic plaques, and presence of hypercoagulation status may be associated to an increased stroke-risk in patients with ACS, but no definitive evidence has been yet produced.
Decline in cognitive function as a result of the presence of ACS has been extensively discussed. Improvement on cognitive function recognized after CEA and CAS was noted, but further studies may be required before indicating CEA in asymptomatic patients with cognitive impairment (36).
In Table 1 established markers of both, plaque and patient vulnerability with established evidence of an increased neurological risk are represented.

Full table
For practical purposes in a busy clinical set-up is difficult to use all these tests to identify the subgroup of asymptomatic patients with higher stroke-risk potentially benefiting from an appropriate prophylactic carotid intervention.
Therefore, based on current evidence we suggest the following strategy to assess patients with asymptomatic carotid 70–99% stenosis:
- Clinical and neurological assessment to identify modifiable and controllable risk factors for atherosclerosis and previous history of contralateral neurological symptoms.
- CT or MR brain imaging to identify ipsilateral silent brain infarcts.
- HD-Ultrasound studies to assess severity of stenosis and associated plaque features such as ulceration, significant JLBA, plaque echolucency and heterogeneity.
Why and when to intervene? CEA or CAS?
Management of ACS patients has two main objectives. First, to treat atherosclerosis aiming to control its progression, minimize ischemic events and reduce cardiovascular mortality through appropriate medical treatment (BMT) as previously mentioned. Second, is to prevent ipsilateral stroke as the first-ever neurological event.
The mechanisms leading to stroke associated in ACS are (I) embolization of thrombotic material and/or plaque debris from the carotid lesion to the brain circulation, (II) reduction in brain perfusion usually in patients with severe occlusive disease and multi-vessel involvement and (III) progression to complete occlusion which has been associated to ipsilateral strokes as demonstrated in ACST (8,9).
Acute internal carotid occlusion can be associated to major strokes in one third of the patients, to minor deficit or transient symptoms in another third or to be completely asymptomatic. Nevertheless at least in two thirds of the patients it may cause a disabling event. Also, patients with chronic internal carotid artery occlusion are at increased risk of stroke during follow-up (96,97).
Identification of a subgroup of patients with ACS with increased risk of stroke is mandatory and requires comprehensive clinical assessment beyond severity of stenosis as previously stated.
Based on current evidence, the management of ACS should begin with established medical treatment (BMT) for all patients immediately upon diagnosis and should include:
- Lifestyle modification with appropriate diet and physical exercise;
- Cessation of smoking habits;
- Control of diabetes, hypertension and renal dysfunction;
- Anti-platelet therapy;
- Active management of dyslipidemia with lipid-lowering medication.
Statins should be started immediately to achieve lipid targets and LDL-C levels according to cardiovascular risk stratification and complementary use of other drugs like ezetimibe or PCSK9 receptor inhibitors—proprotein convertase subtilisin-kexin type 9, a hepatic protease that attaches and internalizes LDL receptors into lysosomes, hence promoting their destruction. By preventing LDL receptor destruction, LDL-C levels can be lowered 50%-60% above that achieved by statin therapy alone (98). It should be used in patients with persistent high LDL-C levels not responding to treatment.
Active monitoring of the efficacy of BMT should be pursued and include: (I) assessment of patient compliance to medication (II) control of LDL-C and triglyceride levels and (III) carotid stenosis evaluation with ultrasound to monitor progression of disease and stabilization of the carotid plaque characteristics.
Immediate carotid intervention should only be considered in the following subgroups as also suggested in the recent ESVS Guidelines:
- Severely stenotic lesions (>90%) with or without contralateral occlusion.
- Plaque features such as (I) heterogenous echolucent plaques, with juxta-luminal location of the echolucent region (II) thin-capped lesion with erosion with mobile flaps and ulceration assessed by HDU as mentioned in the previous section.
- Homogeneous and very echolucent plaques assessed by GSM <25, with erosion or ulceration.
- Multivessel extracranial severe occlusive disease including contralateral ICA occlusion.
- Silent brain infarctions in appropriate location suggesting embolization from the carotid lesion, particularly in the absence of other cardioembolic sources.
- Evidence of embolic episodes during TCD monitorization.
Structural changes in carotid plaques due to widespread use of statins has been objectively documented by increased echogenicity, thickening of the echogenic cap and reduction of hypoechoic areas within the plaque area on high-definition ultrasonography (HDU) and reduction on plaque volume (26), evidence of histological modifications with increased fibrous proliferation and reduction of core lipid deposits on carotid plaque specimens have been documented following CEA in recently symptomatic patients (99). Data from virtual histology analysis based on IVUS observations conducted in natural history studies (100) from coronary arteries did suggest a change of paradigm from morphological features of plaque vulnerability from thin-capped lesions with a lipid-necrotic core leading to ulceration and thromboembolic coronary events, which were only responsible for a small percentage of patients developing acute coronary events during a 3.4-year follow-up study (42,45,46,101,102), to hemodynamic factors (103) leading to plaque erosion and exposure to blood components such as fibrinogen, endogenous inhibitors of fibrinolysis and to pro-coagulant microparticles (104-106) have challenged the true relevance of the concept of vulnerable plaque (102,106).
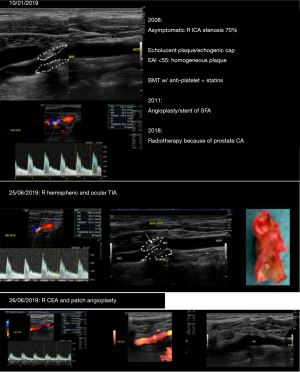
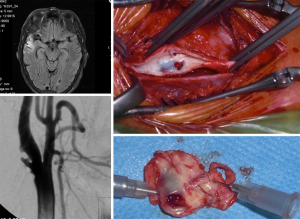
As an illustration, in Figure 7 it is shown a recent case of a patient with a 70% right ICA stenosis followed conservatively for 15 years on BMT and adequate triglyceride and LDL-C levels with clear stabilization of the plaque, increasing GSM and thickening of the echogenic cap. This patient suffered a TIA event and high definition ultrasonography revealed sudden progression of the plaque with erosion and a mobile flap and was successfully treated by CEA.
Stenosis severity and its hemodynamic effect leading to lesion erosion gained relevance again, especially in patients with endothelial dysfunction and reduced regenerative potential within the atheromatous plaques (105,106) in diabetes and renal dysfunction, and also drawing new attention to procoagulant components, which can predispose to local thrombosis at the plaque surface and distal embolization (106).
Monitoring the evolution of 70–90% asymptomatic carotid stenosis under correctly controlled BMT and objective assessment of the impact of hematological factors that may signal systemic inflammation and increased pro-thrombotic activity in the blood will be interesting research areas for the near future.
Which carotid intervention? CEA or CAS?
The gold standard technique has been CEA and its contemporary results showed a significant reduction on the stroke and mortality rates to around 1% (107), which is substantially lower than historical series (108).
CAS was introduced as a less invasive technique, not associated to peripheral nerve palsy, but its early results were disappointing. Technical evolution from materials to technology, as well as the increasing experience of the operators, confirmed its feasibility with relatively low risk, especially in asymptomatic patients.
The IKAROS study (65) suggested a higher risk for neurological complications, stroke and TIA’s, in echolucent lesions with GSM <25 despite the use of brain protection devices.
Five modern RCT's were conducted to compare CAS with CEA (EVA-3S, SPACE, ICSS, CREST, ACT-1 (109-113). The first four of these trials included symptomatic patients and showed that CAS was consistently associated to a higher peri-procedural neurological risk when compared to CEA and in the ICSS trial there was clear evidence of a significantly higher peri-procedural new brain infarcts after CAS when compared to CEA (114) Meta-analysis and reviews of these studies confirmed the non-superiority of CAS over CEA (115,116).
Could data from symptomatic patients be extrapolated to the management of ACS?
Two big modern RCT's included asymptomatic patients-CREST (112) and ACT-1 (113).
Nearly half (47%) of the CREST patients were asymptomatic and the results were not different in both groups regarding the primary endpoints (stroke and acute myocardial infarction). Nevertheless, if only stroke is considered, the risk of CAS was 2.5% and CEA was 1.4% (not statistically significant). In CREST trial the overall risk for both techniques were similar, most of the peri-operative strokes were minor, without significant disability, and small, silent myocardial infarctions detected by troponin elevation were markers of cardiac risk and increased future mortality. However, treatment of carotid stenosis is geared to prevent stroke and myocardial infarction is not a stroke equivalent. If silent myocardial infarction are to be included, then silent brain infarctions should also be searched as they are also markers of reduced life-expectancy and loss of brain mass with the associated cognitive impairment.
The ACT 1 published in 2016 was designed for asymptomatic patients. Its primary endpoints, as well as the overall results, were similar to the asymptomatic group of CREST and the peri-operative stroke rates were 2.8% for CAS and 1.4% for CEA patients (not statistically significant).
Comparison of CAS and CEA in contemporary administrative registries provides information from the “real-world” even if corresponding to a lower level of evidence; the risk of CAS was reported to be prohibitively high, and far higher than the accepted threshold from the AHA (111) in 47% of the CAS registries and only in 5% of the CEA registries (117).
Long-term outcomes and durability of both techniques are similar, according to data providing from CREST (118), ACT-1 (113) and ICSS (111) (symptomatic patients) trials. CAS techniques performed by very experienced operators with large caseloads (119,120), the use of reversed-flow protection and transcarotid revascularization (TCAR) (121-123) might be safer but require further evaluations.
In summary, when compared to CEA, CAS seems to be associated to higher peri-procedural stroke but overcoming this hurdle, long-term durability is equivalent to endarterectomy. CEA continues as the gold standard for intervention in patients with ACS and high risk for stroke as stated in Guidelines (13).
In our experience CAS has been reserved for non-atherosclerotic lesions, proximal (supra-aortic) or distal ICA stenosis, as well as for post-irradiation disease or hostile necks and in a very limited subgroup of patients who may have experienced vocal cord paralysis from a previous contralateral carotid or neck surgery.
Further research is required to clarify the cellular and biochemical mechanisms responsible for plaque progression and regression, the healing potential of superficial erosions and ulcerations with medical treatment in severe stenosis and also the effect of variations on flow dynamics and shear-stress forces on sudden destabilization of carotid plaques in patients on long-term best medical treatment. Proper surveillance will be required for those patients with 70–90% ACS on long-term medical management and delayed carotid intervention.
Acknowledgments
Funding: IG has been supported by the Swedish Research Council, Strategic Research Area Exodiab, Dnr 2009-1039, the Swedish Heart and Lung Foundation, the Skåne University Hospital Funds and the Swedish Stroke National Association.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Dr. Kosmas I. Paraskevas) for the series “Carotid Artery Stenosis and Stroke: Prevention and Treatment Part I” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-2020-cass-12). The series “Carotid Artery Stenosis and Stroke: Prevention and Treatment Part I” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cambridge Advanced Learner’s Dictionary and Thesaurus. Cambridge University Press.
- Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA 1995;273:1421-8. [Crossref] [PubMed]
- Goldstein LB, Bushnell CD, Adams RJ, et al. Guidelines for the primary prevention of stroke. A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:517-84. [Crossref] [PubMed]
- Nichols M, Townsend N, Luengo-Fernandez R, et al. European cardiovascular disease statistics 2012. European Heart Network, Brussels, European Society of Cardiology, Sophia Antipolis. Available online: www.escardio.org/about/what/../EuroHeart/../2012-CVD-statistics.aspx
- Public Report for England, Wales and Northern Ireland. Prepared on behalf of the Intercollegiate Stroke Working Party. Royal College of Physicians. National Sentinel stroke clinical audit 2010 round 7. May 2011. p.43.
- Brown DL, Boden-Albala B, Langa KM, et al. Projected costs of ischemic stroke in the United States. Neurology 2006;67:1390-5. [Crossref] [PubMed]
- Naylor AR. Why is the management of carotid disease so controversial? Surgeon 2015;13:34-43. [Crossref] [PubMed]
- Asymptomatic Carotid Surgery Trial Collaborators. The MRC Asymptomatic Carotid Surgery Trial (ACST): carotid endarterectomy prevents disabling and fatal carotid territory strokes. Lancet 2004;363:1491-502.
- Halliday A, Harrison M, Hayter E, et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet 2010;376:1074-84. [Crossref] [PubMed]
- Liapis CD, Bell PF, Mikhailidis D, et al. ESVS Guidelines. Invasive treatment for carotid stenosis: indications, techniques. Eur J Vasc Endovasc Surg 2009;37:1-19. [Crossref] [PubMed]
- Brott TG, Halperin JL, Abbara S, et al. ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/ SAIP/SCAI/SIR/SNIS/SVM/SVS. Guideline on management of patients with extracranial carotid and vertebral artery disease. Circulation 2011;124:54-130.
- Bladin C, Chambers B, Crimmins D, et al. Guidelines for patient selection and performance of carotid-artery-stenting: Inter-Collegiate Committee of the RACP/RACS/RANZCR. Intern Med J 2011;41:344-7. [Crossref]
- Naylor AR, Ricco JB, de Borst GJ, et al. Management of Atherosclerotic Carotid and Vertebral Artery Disease: 2017 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2018;55:3-81. [Crossref] [PubMed]
- Abbott AL. Medical (Nonsurgical) Intervention Alone Is Now Best for Prevention of Stroke Associated with Asymptomatic Severe Carotid Stenosis. Results of a Systematic Review and Analysis. Stroke 2009;40:e573-83. [Crossref] [PubMed]
- Marquardt L, Geraghty GC, Mehta Z, et al. Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment: a prospective, population-based study. Stroke 2010;41:e11-7. [Crossref] [PubMed]
- Raman G, Moorthy D, Hadar N, et al. Management strategies for asymptomatic carotid stenosis: a systematic review and meta-analysis. Ann Intern Med 2013;158:676-85. [Crossref] [PubMed]
- Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation 2014;129:S1-45. [Crossref] [PubMed]
- Kakkos SK, Nicolaides A, Griffin M, et al. Asymptomatic Carotid Stenosis and Risk of Stroke (ACSRS) Study Group. Factors associated with mortality in patients with asymptomatic carotid stenosis: results from the ACSRS Study. Int Angiol 2005;24:221-30. [PubMed]
- Nicolaides AN. Screening for cardiovascular risk. Br J Cardiol 2010;17:105-7.
- Giannopoulos A, Kakkos S, Abbott A. Long-term Mortality in Patients with Asymptomatic Carotid Stenosis: Implications for Statin Therapy. Eur J Vasc Endovasc Surg 2015;50:573-82. [Crossref] [PubMed]
- Tendera M, Abioyans V, Bartelink ML, et al. ESC guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology. Eur Heart J 2011;32:2851-906. [Crossref] [PubMed]
- Goessens BMB, Visseren FLJ, Kappelle LJ, et al. Asymptomatic carotid artery stenosis and the risk of new vascular events in patients with manifest arterial disease: the SMART Study. Stroke 2007;38:1470-5. [Crossref] [PubMed]
- Brott TG, Halperin JL, Abbara S, et al. ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/ SAIP/SCAI/SIR/SNIS/SVM/SVS. Guideline on management of patients with extracranial carotid and vertebral artery disease. Circulation 2011;124:489-532. [Crossref] [PubMed]
- Myerson M, Coady S, Taylor H, et al. Declining severity of myocardial infarction from 1987 to 2002: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation 2009;119:503-14. [Crossref] [PubMed]
- Derdeyn CP, Chimowitz MI, Lynn M, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet 2014;383:333-41. [Crossref] [PubMed]
- Spence JD, Eliasziw M, DiCicco M, et al. Carotid plaque area: a tool for targeting and evaluating vascular preventive therapy. Stroke 2002;33:2916-22. [Crossref] [PubMed]
- Spence JD, Hackam DG. Treating arteries instead of risk factors: a paradigm change in management of atherosclerosis. Stroke 2010;41:1193-9. [Crossref] [PubMed]
- Crisby M, Nordin-Fredriksson G, et al. Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: implications for plaque. Circulation 2001;103:926-33. [Crossref] [PubMed]
- Schneider PA, Naylor AR. Transatlantic debate: asymptomatic carotid artery stenosis: medical therapy alone versus medical therapy plus carotid endarterectomy or stenting? Eur J Vasc Endovasc Surg 2010;40:274-81. [Crossref] [PubMed]
- McPhee JT, Schanzer A, Messina LM, et al. Carotid artery stenting has increased rates of post-procedure stroke, death and resource utilization than does carotid endarterectomy in the United States, 2005. J Vasc Surg 2008;48:1442-50. [Crossref] [PubMed]
- Vikatmaa P, Mitchell D, Jensen LP, et al. Variation in clinical practice in carotid surgery in nine countries 2005-2010: lessons from VASCUNET and recommendations for the future of national clinical audit. Eur J Vasc Endovasc Surg 2012;44:11-7. [Crossref] [PubMed]
- The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111-88. [Crossref]
- Spence JD. Cerebrovascular disease: identifying high-risk patients from carotid plaque composition. Nat Rev Cardiol 2010;7:426-8. [Crossref] [PubMed]
- Spence JD, Pelz D, Veith FJ. Asymptomatic carotid stenosis. Identifying patients at high enough risk to warrant endarterectomy or stenting. Stroke 2014;45:655-7. [Crossref] [PubMed]
- Taylor F, Ward K, Moore TH, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013.CD004816. [PubMed]
- Paraskevas KI, Veith FJ, Ricco JB. Best medical treatment alone may not be adequate for all patients with asymptomatic carotid artery stenosis J Vasc Surg 2018;68:572-5. [Crossref] [PubMed]
- Nicolaides AN, Kakkos SK, Kyriacou E, et al. Asymptomatic internal carotid artery stenosis and cerebrovascular risk stratification. J Vasc Surg 2010;52:1486-96.e1. [Crossref] [PubMed]
- Kakkos SK, Nicolaides AN, Charalambous I, et al. Predictors and clinical significance of progression or regression of asymptomatic carotid stenosis. J Vasc Surg 2014;59:956-67.e1. [Crossref] [PubMed]
- Hirt LS. Progression rate and ipsilateral neurological events in asymptomatic carotid stenosis. Stroke 2014;45:702-6. [Crossref] [PubMed]
- Conrad MF, Michaczyk BS, Opalacz A, et al. The natural history of asymptomatic severe carotid artery stenosis. J Vasc Surg 2014;60:1218-26. [Crossref] [PubMed]
- Davies MJ. Stability and instability: the two faces of coronary atherosclerosis. The Paul Dudley White Lecture,1995. Circulation 1996;94:2013-20. [Crossref] [PubMed]
- Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation 1995;92:657-71. [Crossref] [PubMed]
- Falk E, Nakano M, Bentzon JF, et al. Update on acute coronary syndromes: the pathologists’ view. Eur Heart J 2013;34:719-28. [Crossref] [PubMed]
- van der Wal AC, Becker AE, van der Loos CM, et al. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 1994;89:36-44. [Crossref] [PubMed]
- Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation 2003;108:1664-72. [Crossref] [PubMed]
- Bentzon JF, Otsuka F, Virmani R, et al. Mechanisms of plaque formation and rupture. Circ Res 2014;114:1852-66. [Crossref] [PubMed]
- Lusby RJ, Ferrell LD, Ehrenfeld WF, et al. Carotid plaque hemorrhage: its role in the production of cerebral ischemia. Arch Surg 1982;117:1479-88. [Crossref] [PubMed]
- Imparato AM, Riles TS, Mintzer RM, et al. The importance of hemorrhage in the relationship between gross morphologic characteristics and cerebral symptoms in 376 carotid arteries. Ann Surg 1983;197:195-203. [Crossref] [PubMed]
- Bassiouny HS, Davis H, Massawa N, et al. Critical carotid stenosis: Morphologic and chemical similarity between symptomatic and asymptomatic plaques. J Vasc Surg 1989;9:202-12. [Crossref] [PubMed]
- Spanos K, Tzorbatzoglou I, Lazari P, et al. Carotid artery plaque echomorphology and its association with histopathologic characteristics. J Vasc Surg 2018;68:1772-80. [Crossref] [PubMed]
- Salem MK, Bown MJ, Sayers RD, et al. Identification of Patients with a Histologically Unstable Carotid Plaque Using Ultrasonic Plaque Image Analysis. Eur J Vasc Endovasc Surg 2014;48:118-25. [Crossref] [PubMed]
- Nicolaides AN, Kakkos SK, Griffin M, et al. Severity of asymptomatic carotid stenosis and risk of ipsilateral hemispheric ischaemic events: results from ACSRS. Eur J Vasc Endovasc Surg 2005;30:275-84. [Crossref] [PubMed]
- Lal BK, Hobson RW 2nd, Pappas PJ, et al. Pixel distribution analysis of B-mode ultrasound scan images predicts histologic features of atherosclerotic carotid plaques. J Vasc Surg 2002;35:1210-7. [Crossref] [PubMed]
- el-Barghouty N, Geroulakos G, Nicolaides A, et al. Computer-assisted carotid plaque characterisation. Eur J Vasc Endovasc Surg 1995;9:389-93. [Crossref] [PubMed]
- Pedro LM. A window to atherosclerosis: high definition ultrasonography in the study of the arterial wall (PhD thesis). University of Lisbon, Portugal, 2003.
- Mitchell CC, Stein JH, Cook TD, et al. Histopathologic Validation of grayscale carotid plaque characteristics related to plaque vulnerability. Ultrasound Med Biol 2017;43:129-37. [Crossref] [PubMed]
- Cai J, Hatsukami TS, Ferguson MS, et al. In vivo quantitative measurement of intact fibrous cap and lipid rich necrotic core size in atherosclerotic carotid plaque: comparison of high-resolution, contrast enhanced magnetic resonance imaging and histology. Circulation 2005;112:3437-44. [Crossref] [PubMed]
- Saam T, Hetterich H, Hoffmann V, et al. Metanalysis and systematic review of the predictive value of carotid plaque hemorrhage on cerebrovascular events by magnetic resonance imaging. J Am Coll Cardiol 2013;62:1081-91. [Crossref] [PubMed]
- Selwaness M, Bos D, van den Bouwhuijsen Q, et al. Carotid atherosclerotic plaque characteristics on magnetic resonance imaging relate with history of stroke and coronary heart disease. Stroke 2016;47:1542-7. [Crossref] [PubMed]
- Sun J, Balu N, Hippe D, et al. Subclinical carotid atherosclerosis: short term natural history of lipid rich necrotic core- a multicenter study with MR imaging. Radiology 2013;268:61-8. [Crossref] [PubMed]
- Saba L, Caddeo G, Sanfilippo R, et al. CT and ultrasound in the study of ulcerated carotid plaque compared with surgical results: potentialities and advantages of multidetector row CT angiography. AJNR Am J Neuroradiol 2007;28:1061-6. [Crossref] [PubMed]
- Cocker MS, Spence JD, Hammond R, et al. for the Canadian Atherosclerosis Imaging Network (CAIN)- Project II. [18F]-Fluorodeoxyglucose PET/CT imaging as a marker of carotid plaque inflammation: Comparison to immunohistology and relationship to acuity of events. International Journal of Cardiology 2018;271:378-86. [Crossref] [PubMed]
- Truijman MT, Kwee RM, van Hoof RH, et al. Combined 18F-FDG PET-CT and DCE¬MRI to assess inflammation and microvascularization in atherosclerotic plaques. Stroke 2013;44:3568-70. [Crossref] [PubMed]
- Saba L, Saam T, Jäger HR, et al. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol 2019;18:559-72. [Crossref] [PubMed]
- Biasi GM, Froio A, Diethrich EB, et al. Carotid plaque echolucency increases the risk of stroke in carotid stentint: the Imaging in Carotid Angioplasty and Risk of Stroke [ICAROS] Study. Circulation 2004;110:756-62. [Crossref] [PubMed]
- Pedro LM, Pedro MM, Gonçalves I, et al. Computer-assisted carotid plaque analysis: Characteristics of plaques associated with cerebrovascular symptoms and cerebral infarction. Eur J Vasc Endovasc Surg 2000;19:118-23. [Crossref] [PubMed]
- Pedro LM, Fernandes e Fernandes J, Pedro MM, et al. Ultrasonographic Risk Score of Carotid Plaques. Eur J Vasc Endovasc Surg 2002;24:492-8. [Crossref] [PubMed]
- Pedro LM, Gonçalves I, Fernandes e Fernandes R, et al. Activity Index: a tool to identify active carotid plaques. In: Sanches J, Laine A, Suri JS (Editors). Ultrasound Imaging: Advances and Applications. Springer 2012:163-75.
- Pedro LM, Fernandes R, Silvestre L, et al. Ultrasonographic quantification of carotid stenosis: a reappraisal using a new gold standard. In: Saba L, Sanches J, Pedro LM, et al. (Editors). Multi-Modality Atherosclerosis Imaging, Diagnosis and Treatment. Springer, 2014.
- Gonçalves I, Moses J, Dias N, et al. Changes related to age and cerebrovascular symptoms in the extracellular matrix of human carotid plaques. Stroke 2003;34:616-22. [Crossref] [PubMed]
- Gonçalves I, Moses J, Pedro LM, et al. Echolucency of carotid plaques correlates with plaque cellularity. Eur J Vasc Endovasc Surg 2003;26:32-8. [Crossref] [PubMed]
- Gonçalves I, Lindholm MW, Pedro LM, et al. Elastin and calcium rather than collagen or lipid content are associated with the echogenicity of human carotid plaques. Stroke 2004;35:2795-800. [Crossref] [PubMed]
- Gonçalves I, Ares MP, Moberg A, et al. Elastin and collagen-rich human carotid plaques have increased levels of the cysteine protease inhibitor cystatin C. J Vasc Res 2008;45:395-401. [Crossref] [PubMed]
- Gonçalves I, Nitulescu M, Saido TC, et al. Activation of calpain-1 in human carotid artery atherosclerotic lesions. BMC Cardiovasc Disord 2009;9:26. [Crossref] [PubMed]
- Halak S, Ostling G, Edsfeldt A, et al. Spotty Carotid Plaques Are Associated with Inflammation and the Occurrence of Cerebrovascular Symptoms. Cerebrovasc Dis Extra 2018;8:16-25. [Crossref] [PubMed]
- Acharya UR, Mookiah M, Sree SV, et al. Atherosclerotic plaque tissue characterization in 2D ultrasound longitudinal carotid scans for automated classification: a paradigm for stroke risk assessment. Med Biol Eng Comput 2013;51:513-23. [Crossref] [PubMed]
- Seabra J, Sanches J, Pedro LM, et al. Ultrasound plaque Enhanced Activity Index for predicting neurological symptoms. Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics). In: Proceedings of IbPRIA 2011:6669:184-91.
- Seabra J, Pedro LM, Fernandes e Fernandes J, et al. Ultrasound profile of carotid plaque: a new approach towards stroke prediction. In: Saba L, Sanches J, Pedro LM, Suri J (eds.). Multi-Modality Atherosclerosis Imaging and Diagnosis. Springer, 2014:173-85.
- Pedro LM, Sanches J, Seabra J, et al. Asymptomatic Carotid Disease: A New Tool for Assessing Neurological Risk. Echocardiography 2014;31:353-61. [Crossref] [PubMed]
- Afonso D, Seabra J, Pedro LM, et al. An Ultrasonographic Risk Score for Detecting Symptomatic Carotid Atherosclerotic Plaques. IEEE J Biomed Health Inform 2015;19:1505-13. [Crossref] [PubMed]
- Pedro LM, Fernandes e Fernandes J, Lopes R, et al. The Activity Index as a tool for the identification of active carotid plaques: a double-blind study. Int Angiol 2006.25. Abstract 144.
- Erlöv T, Cinthio M, Edsfeldt A, et al. Determining carotid plaque vulnerability using ultrasound center frequency shifts. Atherosclerosis 2016;246:293-300. [Crossref] [PubMed]
- Kakkos SK, Griffin MB, Nicolaides AN, et al. The size of the juxta-luminal hypoechoic area in ultrasound images of asymptomatic carotid plaques predicts the occurrence of stroke. J Vasc Surg 2013;57:609-18.e1. [Crossref] [PubMed]
- Markus HS, King A, Shipley M, et al. Asymptomatic embolisation for prediction of stroke in the asymptomatic carotid emboli study: a prospective observational study. Lancet Neurol 2010;9:663-71. [Crossref] [PubMed]
- Topakian R, King A, Kwon U, et al. Ultrasonic plaque echolucency and emboli signals predict stroke in asymptomatic carotid stenosis. Neurology 2011;77:751-8. [Crossref] [PubMed]
- Spence JD. Technology Insight: ultrasound measurement of carotid plaque– patient management, genetic research, and therapy evaluation. Nat Clin Pract Neurol 2006;2:611-9. [Crossref] [PubMed]
- Spence JD, Coates V, Li H, et al. Effects of Intensive Medical Therapy on Microemboli and Cardiovascular Risk in Asymptomatic Carotid Stenosis. Arch Neurol 2010;67:180-6. [Crossref] [PubMed]
- Saha SA, Gourineni V, Feinstein SB. The use of contrast¬ enhanced ultrasonography for imaging of carotid atherosclerotic plaques: current evidence, future directions. Neuroimaging Clin N Am 2016;26:81-96. [Crossref] [PubMed]
- Etesami M, Hoi Y, Steinman DA, et al. Comparison of carotid plaque ulcer detection using contrast enhanced and time of flight MRA techniques. Am J Neuroradiol 2013;34:177-84. [Crossref] [PubMed]
- The European Carotid Surgery Trialists Collaborative Group. Risk of stroke in the distribution of an asymptomatic carotid artery. Lancet 1995;345:209-12. [Crossref] [PubMed]
- North American Symptomatic Carotid Endarterectomy Trial Collaborators, Barnett HJM, Taylor DW, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445-53. [Crossref] [PubMed]
- Kanber B, Hartshorne TC, Horsfield MA, et al. A novel ultrasound-based carotid plaque risk index associated with the presence of cerebrovascular symptoms. Ultraschall Med 2015;36:480-6. [PubMed]
- Flaherty ML, Flemming K, McClelland R, et al. Population-based study of symptomatic internal carotid artery occlusion: incidence and long-term follow-up. Stroke 2004;35:e349-52. [Crossref] [PubMed]
- King A, Serena J, Bornstein NM, et al. Does impaired cerebrovascular reactivity predict stroke risk in asymptomatic carotid stenosis: a prospective substudy of the asymptomatic carotid emboli study. Stroke 2011;42:1550-5. [Crossref] [PubMed]
- Kakkos SK, Sabetai M, Tegos T, et al. Silent embolic infarcts on computed tomography brain scans and risk of ipsilateral hemispheric events in patients with asymptomatic internal carotid artery stenosis. J Vasc Surg 2009;49:902-9. [Crossref] [PubMed]
- Janko M, Moore R, Kim AH, et al. Carotid occlusion is associated with more frequent neurovascular events than moderately severe carotid stenosis. J Vasc Surg 2017;66:1445-9. [Crossref] [PubMed]
- Bryan DS, Carson J, Hall H, et al. Natural History of Carotid Artery Occlusion. Ann Vasc Surg 2013;27:186-93. [Crossref] [PubMed]
- Oh M, Kim H, Shin EW, et al. Effects of ezetimibe/simvastatin 10/10 mg versus Rosuvastatin 10 mg on carotid atherosclerotic plaque inflammation. BMC Cardiovasc Disord 2019;19:201. [Crossref] [PubMed]
- van Lammeren GW, den Ruijter HM, Vrijenhoek JE, et al. Time-dependent changes in atherosclerotic plaque composition in patients undergoing carotid surgery. Circulation 2014;129:2269-76. [Crossref] [PubMed]
- Stone GW, Maehara A, Lansky AJ. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011;364:226-35. [Crossref] [PubMed]
- Buffon A, Biasucci LM, Liuzzo G, et al. Widespread coronary inflammation in unstable angina. N Engl J Med 2002;347:5-12. [Crossref] [PubMed]
- Libby P, Pasterkamp G. Requiem for the vulnerable plaque. Eur Heart J 2015;36:2984-7. [PubMed]
- Zarins CK, Giddens DP, Bharadvaj BK, et al. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res 1983;53:502-14. [Crossref] [PubMed]
- Scalone G, Niccoli G, Refaat H, et al. Not all plaque ruptures are born equal: an optical coherence tomography study. Eur Heart J Cardiovasc Imaging 2017;18:1271-7. [Crossref] [PubMed]
- Edsfeldt A, Goncalves I, Grufman H, et al. Impaired fibrous repair: a possible contributor to atherosclerotic plaque vulnerability in patients with type II diabetes. Arterioscler Thromb Vasc Biol 2014;34:2143-50. [Crossref] [PubMed]
- Nilsson J. Atherosclerotic plaque vulnerability in the statin era. European Heart Journal 2017;38:1638-44. [Crossref] [PubMed]
- Pothof AB, Zwanenburg ES, Deery SE, et al. An update on the incidence of perioperative outcomes after carotid endarterectomy, stratified by type of preprocedural neurologic symptom. J Vasc Surg 2018;67:785-92. [Crossref] [PubMed]
- Flanigan DP, Flanigan ME, Dorne AL, et al. Long-term results of 442 consecutive, standardized carotid endarterectomy procedures in standard-risk and high-risk patients. J Vasc Surg 2007;46:876-82. [Crossref] [PubMed]
- Mas JL, Chatellier G, Beyssen B, et al. Endarterectomy versus Stenting in Patients with Symptomatic Severe Carotid Stenosis. N Engl J Med 2006;355:1660-71. [Crossref] [PubMed]
- Eckstein HH, Ringleb P, Allenberg JR, et al. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol 2008;7:893-902. [Crossref] [PubMed]
- Bonati LH, Dobson J, Featherstone RL, et al. Long-term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: the International Carotid Stenting Study (ICSS) randomised trial. Lancet 2015;385:529-38. [Crossref] [PubMed]
- Brott TG, Hobson RW II, Howard G, et al. Stenting versus Endarterectomy for Treatment of Carotid-Artery Stenosis. N Engl J Med 2010;363:11-23. [Crossref] [PubMed]
- Rosenfield K, Matsumura JS, Chaturvedi S, et al. Randomized Trial of Stent versus Surgery for Asymptomatic Carotid Stenosis. N Engl J Med 2016;374:1011-20. [Crossref] [PubMed]
- Altinbas A, Algra A, Bonati LH, et al. for ICSS Investigators. Periprocedural hemodynamic depression is associated with a higher number of new ischemic brain lesions after stenting in the International Carotid Stenting Study-MRI Substudy. Stroke 2014;45:146-51. [Crossref] [PubMed]
- Murad MH, Shahrour A, Shah ND, et al. A systematic review and meta-analysis of randomized trials of carotid endarterectomy vs stenting. J Vasc Surg 2011;53:792-7. [Crossref] [PubMed]
- Moore WS, Barnett HJM, Beebe HG, et al. Guidelines for Carotid Endarterectomy. A multidisciplinary consensus statemen from the Ad Hoc Committee, American Heart Association. Stroke 1995;26:188-201. [Crossref] [PubMed]
- Paraskevas KI, Kalmykov EL, Naylor AR. Stroke/Death Rates Following Carotid Artery Stenting and Carotid Endarterectomy in Contemporary Administrative Dataset Registries: A Systematic Review. Eur J Vasc Endovasc Surg 2016;51:3-12. [Crossref] [PubMed]
- Brott TG, Howard G, Roubin GS, et al. Long-Term Results of Stenting versus Endarterectomy for Carotid-Artery Stenosis. N Engl J Med 2016;374:1021-31. [Crossref] [PubMed]
- Gray WA, Rosenfield KA, Jaff MR, et al. Influence of site and operator characteristics on carotid artery stent outcomes: analysis of the CAPTURE 2 (Carotid ACCULINK/ACCUNET Post Approval Trial to Uncover Rare Events) clinical study. JACC Cardiovasc Interv 2011;4:235-46. [Crossref] [PubMed]
- Lal BK, Roubin GS, Rosenfield K, et al. Quality Assurance for Carotid Stenting in the CREST-2 Registry. J Am Coll Cardiol 2019;74:3071-9. [Crossref] [PubMed]
- King AH, Norman MS, Kumins H, et al. The learning curve of transcarotid artery revascularization. J Vasc Surg 2019;70:516-21. [Crossref] [PubMed]
- Schermerhorn ML, Liang P, Eldrup-Jorgensen L, et al. Association of Transcarotid Artery Revascularization vs Transfemoral Carotid Artery Stenting with Stroke or Death Among Patients with Carotid Artery Stenosis. JAMA 2019;322:2313-22. [Crossref] [PubMed]
- Schermerhorn ML, Liang P, Dakour-Aridi H, et al. In-hospital outcomes of transcarotid artery revascularization and carotid endarterectomy in the Society for Vascular Surgery Vascular Quality Initiative. J Vasc Surg 2020;71:87-95. [Crossref] [PubMed]