Molecular biology of BPIFB1 and its advances in disease
Bactericidal/permeability-increasing (BPI)-fold-containing family B member 1 (BPIFB1), also known as long-palate lung and nasal epithelium clone 1 (LPLUNC1), belongs to the BPI-fold-containing family (1). These proteins have largely been considered to be specifically expressed in the respiratory tract (2,3). However, recent studies have found that abnormal expression of BPIFB1 is associated with a variety of diseases, such as severe pulmonary disease caused by cystic fibrosis (CF), infection with vibrio cholerae, non-obese diabetes, and tumors. Therefore, to promote the development of treatment, this study reviewed the role of BPIFB1 in different diseases and related therapeutic targets.
Molecular biology of BPIFB1
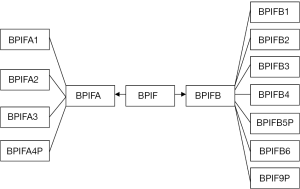
In 1999, Weston was first to discover BPIF expression in rat embryonic nasal mucosa epithelium and adult rat bronchial and pulmonary mucosa (4). Later, another team cloned the gene in human respiratory and nasopharyngeal epithelial tissue (5). Homology analysis showed that the BPIF was highly conserved in human, pig, mouse, rat, ox, and other higher organisms, with 99% homology. It was found that the gene family was located at 20q11, and encoded 8 functional proteins (6,7). The classification of the human BPI family is shown in Figure 1. Notably, members of this family all contain domains structurally similar to lipopolysaccharide (LPS)-binding protein and BPI (8). Crystal diffraction analysis showed that the BPI domain was a hydrophobic barrel-shaped structure, which could specifically bind to LPS of the cell wall in Gram-negative microflora and perform antibacterial function (9,10). Thus, this family constitutes the “epithelial frontier,” owing to its host defense and innate immune properties. However, it has been observed that BPIF does not have an obvious bacteriostatic or bactericidal effect in vitro. The most likely explanation is that BPI/LBP is combined with LPS in the cell wall of Gram-negative bacteria to exert its biological effect, while BPIF protein is a selective inhibitor of oropharyngeal, respiratory, and other intracellular pathogens (11). BPIF protein can also serve as a marker for airway inflammatory response after being chemically stimulated. According to the number of functional domains, BPIF can be divided into BPIFA (protein containing a single domain) and BPIFB (protein containing two domains) (7). Among family members, the size and interval of introns are highly conserved, suggesting that they co-evolved during some past genetic events. BPIF, although highly glycosylated, is expressed differently in different species or at different locations within the same species. Through electronic hybridization, high-throughput tissue chip, or multi-tissue cDNA microarray detection, tissue specific expression of BPIF has been demonstrated. It is expressed centrally in the nasopharynx, mouth, nasal cavity, respiratory tract, and digestive tract (12,13). Previous studies have shown that BPIFA1, BPI, and other genes among the family members are associated with nasopharyngeal carcinoma (NPC), non-small cell lung cancer, and other diseases.
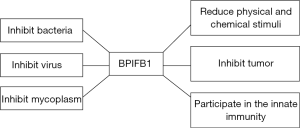
The gene of BPIFB1 is located at chromosome 20q11.21–20q11.22, and contains 16 exons and 15 introns, encoding 484 amino acids. The 5' terminal of the BPIFB1 protein has a signal peptide sequence composed of 19 amino acids (14). BPIFB1 protein is most highly expressed in the trachea, followed by the lung, and weakly expressed in salivary glands, the duodenum, and the stomach. Although the biology of this secreted protein is poorly understood, multiple array-based studies have suggested that some are differentially expressed in a variety of diseases (Figure 2).
Biological effects of BPIFB1 in tumors
Inflammation is a defensive response of the body to pathogenic infection and various tissue damage. In this process, by affecting the interaction of various cells and factors in the microenvironment, it regulates the balance of physiological and pathological signal networks. Epidemiological studies have shown that patients with chronic inflammation are susceptible to a variety of tumors (15-17). The estimated 15–20% of tumor is due to chronic infections and inflammation caused by factors such as the environment (18). Recent studies have found that BPIFB1 appears abnormal in multiple types of tumor tissue, suggesting that it may play a role in the development of tumors.
NPC
NPC is a cancer arising from the nasopharynx epithelium and has a very unique pattern of geographical distribution. Human nasopharyngeal carcinoma mucosa maintains a warm humid environment which is suitable for all kinds of microbial growth. Unlike other head and neck cancers, the occurrence and development of NPC is believed to be mainly due to Epstein-Barr (EB) virus, which is considered to be an important factor of tumorigenesis and is closely related to the occurrence of NPC. Long-term exposure to various chemicals and external stimulation also lead to damage of the nasopharyngeal mucosa inflammatory response, which stimulate proliferation of nasopharyngeal epithelia, eventually evolving into NPC. BPIFB1 is highly expressed in normal adult and fetal nasopharyngeal epithelial tissue, and is especially abundant in nasopharyngeal and respiratory secretions, lavage fluid, sputum, and media of bronchial epithelial cells (19). As a newly cloned gene, BPIFB1 was found down-expressed in NPC biopsies which indicates that it may play an important role in the tumorigenesis of NPC (20). It has the function of host defense, along with bactericidal and anti-inflammatory effects through the protein domain. Meanwhile, research has proven that BPIFB1 can also kill bacteria and inhibit tumor cell growth and metastasis. BPIFB1 protein can inhibit the tumorigenicity of respiratory epithelia and the proliferation rate of the EB virus, accelerate the apoptosis of B cells, and hinder the occurrence and development of NPC. BPIFB1 can significantly alter the distribution of the NPC cells cycle in the following manner: cells significantly increase in the G1 phase and decrease in the S, G2, and M phase. This indicates that BPIFB1 controls the occurrence and development of NPC by inhibiting the G1–S phase of cell cycle of NPC cells. Further studies have shown that BPIFB1 can regulate the expression of IL-6, cyclinD1, and BLC-2 by inhibiting STAT3 activation (21). Further research shows that BPIFB1 can inhibit the proliferation of nasal tumor cells and promote the apoptosis of nasal tumor cells through signal transduction pathways such as mitogen-activated protein kinase (MAPK) and mi r-141-PTEN-AKT (22). Moreover, recently studies show that BPIFB1 markedly inhibited NPC cell migration, invasion, and lung-metastatic abilities. And it is also involved in the modulation of radiosensitivity in NPC. In particular, BPIFB1 negatively regulates its interactor vitronectin (VTN), thereby inhibiting VTN-induced proliferation, anti-apoptotic effects, G2/M phase arrest, DSB repair, and the activation of the ATM-Chk2 and ATR-Chk1 pathways after irradiation; this ultimately improved NPC cell radiosensitivity (1,23).
Gastric cancer
Gastric cancer is a common malignant tumor of the digestive system, being the second most common cancer and the second leading cause of cancer death. Despite a decline in incidence and mortality, the outlook of metastatic gastric cancer cases remains poor. The median survival usually does not exceed one year when treated with systematic chemotherapy in metastatic settings (24). Helicobacter pylori (Hp) infection is the most important factor for gastric cancer (25). Studies have confirmed that Hp damages the gastric mucosal barrier by secreting urease (26). In addition, Hp can produce LPS which inhibits the binding of laminin to receptors. The vacuolar toxin gene can also cause gastric mucosa erosion or ulcers. BPIFB1 plays a certain role in the occurrence and development of gastric cancer. BPIFB1 is highly expressed in gastric mucosal surface epithelia and submucosal glands, while it is absent or down-regulated in gastric cancer tissues. Further studies have revealed a specific molecular mechanism in the pathogenesis of gastric cancer. BPIFB1 protein is structurally similar to LPS-binding protein and bactericidal/permeable protein, and plays an important role in innate immunity. The LBP domain of BPIFB1 can bind to LPS, causing a killing effect of Gram-negative bacteria, which plays an important role in resistance to helicobacter pylori infection (27).
Other tumors
In addition, BPIFB1 has been found to be associated with salivary gland tumors and lung cancer. Vargas et al. detected BPIFA1, BPIFA2, and BPIFB1 in salivary gland tumors by immunohistochemistry, which were strongly expressed in mucus cells and mucus plugs. The mutation of the BPIFB1 gene is also associated with the risk of lung cancer and the down-regulation of expression directly leads to poor prognosis in lung cancer patients (28,29).
Biological effect of BPIFB1 in respiratory disease
The respiratory system includes the nose, pharynx, larynx, trachea, bronchus, lung, pleura, etc., and is in direct contact with the outside world. As air passes into the respiratory tract, pathogenic microorganisms and harmful substances can provoke the development of respiratory diseases. Innate immunity is the body’s first defense against the invasion of pathogenic microorganisms. BPIFB1 is considered to contribute to innate immunity through its structural similarity with BPI protein and LPS-binding protein, both of which are innate immune molecules with recognized roles in sensing and responding to Gram-negative bacteria.
CF
CF is a common and fatal autosomal inherited disease. CF is caused by mutations of CF transmembrane conductance regulator (CFTR), leading to exocrine dysfunction involving the lungs, pancreas, liver, reproductive system, and other organs, with the most prominent lesions being found in the respiratory system. In lungs, thick secretions clog bronchi, causing repeated bronchial infections and airway obstruction. The activity of mucosal epithelial cilia is inhibited, and poor mucus drainage gradually leads to bronchiectasis and respiratory failure. Some studies demonstrated that genetic variants in the BPIFA1/BPIFB1 region are associated with decreased gene expression and increased lung disease severity in cystic fibrosis (CF). This suggests that decreased BPIFA1 and/or BPIFB1 expression may be detrimental to CF lung function. For example, proteomic analysis of nasal epithelial cells from CF patients has demonstrated that BPIFA1 and BPIFB1 are increased in the sputum (30). BPIFA1 has been shown to have surfactant-like functions and to be involved in regulating the amiloride-sensitive epidermal sodium channel, which is an important innate defense mechanism for the lungs (31,32). BPIFA1 and BPIFB1 were also both able to reduce IL-8 production in response to P. aeruginosa infection and RNA-Seq data indicated that both molecules modulate the function of CF airway epithelia cells (33).
Chronic obstructive pulmonary disease (COPD)
COPD involves the chronic emphysema of airway obstruction. Excessive airway mucus secretion is one of the important characteristics of COPD, and can promote the rapid decline of lung function. Under physiological conditions, airway mucus can provide effective protection for the lungs against pathogens, harmful airborne particles, and toxic chemicals, playing a key role in innate immune response (34,35). However, high concentrations of airway mucus lead to impaired mucociliary clearance and mucous adhesion, resulting in airway obstruction and bacterial infection. Some studies have shown that mycoplasma pneumoniae (MP) infection is associated with a variety of respiratory diseases and can participate in the development of COPD. In addition, excessive airway mucus adhesion to the airway wall through hypoxic epithelial necrosis and potential immune regulation mechanisms has become a key factor in chronic airway inflammation and lung injury (36). BPIFB1 exhibits limited expression in healthy airways and is detected in the submucosal glands of larger airways (37). Compared with the conflicting data from BPIFA1 findings, BPIFB1 seems to be consistently upregulated in several respiratory diseases and serves as a defensive protein (38). BPIFB1 mRNA expression and protein level were found to be significantly increased in COPD patients when compared to non-COPD subjects (39). Furthermore, Ghafouri et al. found that BPIFB1 expression was significantly higher in COPD patients, suggesting that it is involved in the inflammatory response of the respiratory tract (40). In COPD patients, extended epithelial remodeling with goblet cell metaplasia and inflammation takes place, and the observed increase in BPIFB1 levels in COPD patients could be a direct consequence of this. This idea is strengthened by the observation that the number of goblet cells correlates very strongly with the BPIFB1 protein levels. It is possible that BPIFB1, similarly to its family member BPIFA1, also plays a role in airway protection, but the way that BPIFB1 is able to perform this function requires additional functional analysis (37).
Asthma
Asthma is a chronic respiratory disease characterized by chronic airway inflammation and wheezing, and is influenced by a variety of factors. The pathogenesis of asthma is associated with certain genes, allergies, infections, and other causes. Recurrent respiratory tract infection is often an important factor in the development and progression of asthma. Studies have shown that 37% patients with asthma attacks have a recent history of respiratory infections (41). In 1994, Yano first reported that MP infection was associated with asthma (42). Clinical studies have confirmed that MP infection can increase the expression of BPIFB1, but in the mouse model, allergic airway inflammation of mycoplasma decreased BPIFB1 protein expression, eventually leading to the long-term settling of a variety of bacteria in the airway (43). Since this discovery, a number of studies have reported that asthma is accompanied by acute infections of mycoplasma pneumonia (44-46). BPIFB1, which is primarily produced by airway epithelia, has been reported to exert anti-inflammatory properties during microbial infections and Toll-like receptor agonist stimulation. Clinical studies have confirmed that BPIFB1 is significantly expressed in sputum, and can discriminate asthma inflammatory phenotypes (47). It has been reported that MP infection can increase BPIFB1 expression and that BPIFB1 level is higher in bronchoalveolar lavage (BAL) fluid of asthma patients after segmental allergen challenge (48).
Summary
Although the role of BPIFB1 in certain diseases is poorly understood, the function of innate immunity has been confirmed. The different expressions in various tissues of the body determine their different functions, and the regulation in tissues is also quite different. The efficacy of BPIFB1 in anti-bacterial activity, tumor inhibition, and respiratory disease is increasingly evident, but the specifics behind its regulation mechanism are not yet clear and thus warrant further exploration and research.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (81770028), the Natural Science Foundation of Guangdong (2018A030310011), the Key Laboratory of Shenzhen Respiratory Disease (ZDSYS201504301616234), the Project of Shenzhen Basic Research Plan (JCYJ20170307095633450), and the Clinical Research of Shenzhen Municipal Health and Family Planning Commission (SZLY2017024). This work also was supported by special funding for high-level disciplines from the Shenzhen Institute of Respiratory Diseases.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-3462). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wei F, Tang L, He Y, et al. BPIFB1 (LPLUNC1) inhibits radioresistance in nasopharyngeal carcinoma by inhibiting VTN expression. Cell Death Dis 2018;9:432. [Crossref] [PubMed]
- Bingle CD, Wilson K, Lunn H, et al. Human LPLUNC1 is a secreted product of goblet cells and minor glands of the respiratory and upper aerodigestive tracts. Histochem Cell Biol 2010;133:505-15. [Crossref] [PubMed]
- Liao Q, Zeng Z, Guo X, et al. LPLUNC1 suppresses IL-6-induced nasopharyngeal carcinoma cell proliferation via inhibiting the Stat3 activation. Oncogene 2014;33:2098-109. [Crossref] [PubMed]
- Alves DB, Bingle L, Bingle CD, et al. BPI-fold (BPIF) containing/plunc protein expression in human fetal major and minor salivary glands. Braz Oral Res 2017;31:e6. [Crossref] [PubMed]
- Bingle CD, Bingle L. Characterisation of the human plunc gene, a gene product with an upper airways and nasopharyngeal restricted expression pattern. Biochim Biophys Acta 2000;1493:363-7. [Crossref] [PubMed]
- Musa M, Wilson K, Sun L, et al. Differential localisation of BPIFA1 (SPLUNC1) and BPIFB1 (LPLUNC1) in the nasal and oral cavities of mice. Cell Tissue Res 2012;350:455-64. [Crossref] [PubMed]
- Britto CJ, Cohn L. Bactericidal/Permeability-increasing protein fold-containing family member A1 in airway host protection and respiratory disease. Am J Respir Cell Mol Biol 2015;52:525-34. [Crossref] [PubMed]
- Prokopovic V, Popovic M, Andjelkovic U, et al. Isolation, biochemical characterization and anti-bacterial activity of BPIFA2 protein. Arch Oral Biol 2014;59:302-9. [Crossref] [PubMed]
- Shao Y, Li C, Che Z, et al. Cloning and characterization of two lipopolysaccharide-binding protein/bactericidal permeability-increasing protein (LBP/BPI) genes from the sea cucumber Apostichopus japonicus with diversified function in modulating ROS production. Dev Comp Immunol 2015;52:88-97. [Crossref] [PubMed]
- Nam BH, Moon JY, Park EH, et al. Antimicrobial activity of peptides derived from olive flounder lipopolysaccharide binding protein/bactericidal permeability-increasing protein (LBP/BPI). Mar Drugs 2014;12:5240-57. [Crossref] [PubMed]
- Haigh B, Hood K, Broadhurst M, et al. The bovine salivary proteins BSP30a and BSP30b are independently expressed BPI-like proteins with anti-Pseudomonas activity. Mol Immunol 2008;45:1944-51. [Crossref] [PubMed]
- Tsou YA, Peng MT, Wu YF, et al. Decreased PLUNC expression in nasal polyps is associated with multibacterial colonization in chronic rhinosinusitis patients. Eur Arch Otorhinolaryngol 2014;271:299-304. [Crossref] [PubMed]
- Seshadri S, Lin DC, Rosati M, et al. Reduced expression of antimicrobial PLUNC proteins in nasal polyp tissues of patients with chronic rhinosinusitis. Allergy 2012;67:920-8. [Crossref] [PubMed]
- Bingle CD, Craven CJ. PLUNC: a novel family of candidate host defence proteins expressed in the upper airways and nasopharynx. Hum Mol Genet 2002;11:937-43. [Crossref] [PubMed]
- Bremnes RM, Al-Shibli K, Donnem T, et al. The role of tumor-infiltrating immune cells and chronic inflammation at the tumor site on cancer development, progression, and prognosis: emphasis on non-small cell lung cancer. J Thorac Oncol 2011;6:824-33. [Crossref] [PubMed]
- Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology 2011;140:1807-16. [Crossref] [PubMed]
- Moore MM, Chua W, Charles KA, et al. Inflammation and cancer: causes and consequences. Clin Pharmacol Ther 2010;87:504-8. [Crossref] [PubMed]
- Anand P, Kunnumakkara AB, Sundaram C, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res 2008;25:2097-116. [Crossref] [PubMed]
- Xu Y, Tao Z, Jiang Y, et al. Overexpression of BPIFB1 promotes apoptosis and inhibits proliferation via the MEK/ERK signal pathway in nasopharyngeal carcinoma. Int J Clin Exp Pathol 2019;12:356-64. [PubMed]
- Zhang B, Nie X, Xiao B, et al. Identification of tissue-specific genes in nasopharyngeal epithelial tissue and differentially expressed genes in nasopharyngeal carcinoma by suppression subtractive hybridization and cDNA microarray. Genes Chromosomes Cancer 2003;38:80-90. [Crossref] [PubMed]
- Xiong F, Deng S, Huang HB, et al. Effects and mechanisms of innate immune molecules on inhibiting nasopharyngeal carcinoma. Chin Med J (Engl) 2019;132:749-52. [Crossref] [PubMed]
- Boon K, Bailey NW, Yang J, et al. Molecular phenotypes distinguish patients with relatively stable from progressive idiopathic pulmonary fibrosis (IPF). PloS One 2009;4:e5134. [Crossref] [PubMed]
- Wei F, Wu Y, Tang L, et al. BPIFB1 (LPLUNC1) inhibits migration and invasion of nasopharyngeal carcinoma by interacting with VTN and VIM. Br J Cancer 2018;118:233-247. [Crossref] [PubMed]
- Zhou S, Xu B, Qi L, et al. Next-generation sequencing reveals mutational accordance between cell-free DNA from plasma, malignant pleural effusion and ascites and directs targeted therapy in a gastric cancer patient. Cancer Biol Ther 2019;20:15-20. [Crossref] [PubMed]
- Wroblewski LE, Peek RM Jr, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev 2010;23:713-39. [Crossref] [PubMed]
- Ji R, Wang P, Kou GJ, et al. Impaired gastric mucosal integrity identified by confocal endomicroscopy in Helicobacter pylori-negative functional dyspepsia. Neurogastroenterol Motil 2020;32:e13719. [Crossref] [PubMed]
- Lozano-Pope I, Sharma A, Matthias M, et al. Effect of myeloid differentiation primary response gene 88 on expression profiles of genes during the development and progression of Helicobacter-induced gastric cancer. BMC Cancer 2017;17:133. [Crossref] [PubMed]
- Vargas PA, Speight PM, Bingle CD, et al. Expression of PLUNC family members in benign and malignant salivary gland tumours. Oral Dis 2008;14:613-9. [Crossref] [PubMed]
- Jin G, Zhu M, Yin R, et al. Low-frequency coding variants at 6p21.33 and 20q11.21 are associated with lung cancer risk in Chinese populations. Am J Hum Genet 2015;96:832-40. [Crossref] [PubMed]
- Bingle L, Wilson K, Musa M, et al. BPIFB1 (LPLUNC1) is upregulated in cystic fibrosis lung disease. Histochem Cell Biol 2012;138:749-58. [Crossref] [PubMed]
- Gakhar L, Bartlett JA, Penterman J, et al. PLUNC is a novel airway surfactant protein with anti-biofilm activity. PloS One 2010;5:e9098. [Crossref] [PubMed]
- Garcia-Caballero A, Rasmussen JE, Gaillard E, et al. SPLUNC1 regulates airway surface liquid volume by protecting ENaC from proteolytic cleavage. Proc Natl Acad Sci U S A 2009;106:11412-7. [Crossref] [PubMed]
- Saferali A, Tang AC, Strug LJ, et al. Immunomodulatory function of the cystic fibrosis modifier gene BPIFA1. PLoS One 2020;15:e0227067. [Crossref] [PubMed]
- Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med 2010;363:2233-47. [Crossref] [PubMed]
- Filho MM, Aguiar PN Jr, de Mello RA. Chronic obstructive pulmonary disease alters immune cell composition and immune checkpoint inhibitor efficacy in non-small cell lung cancer. Ann Transl Med 2019;7:S42. [Crossref] [PubMed]
- Zhou-Suckow Z, Duerr J, Hagner M, et al. Airway mucus, inflammation and remodeling: emerging links in the pathogenesis of chronic lung diseases. Cell Tissue Res 2017;367:537-50. [Crossref] [PubMed]
- De Smet EG, Seys LJ, Verhamme FM, et al. Association of innate defense proteins BPIFA1 and BPIFB1 with disease severity in COPD. Int J Chron Obstruct Pulmon Dis 2017;13:11-27. [Crossref] [PubMed]
- Ghosh N, Dutta M, Singh B, et al. Transcriptomics, proteomics and metabolomics driven biomarker discovery in COPD: an update. Expert Rev Mol Diagn 2016;16:897-913. [Crossref] [PubMed]
- Titz B, Sewer A, Schneider T, et al. Alterations in the sputum proteome and transcriptome in smokers and early-stage COPD subjects. J Proteomics 2015;128:306-20. [Crossref] [PubMed]
- Ghafouri B, Stahlbom B, Tagesson C, et al. Newly identified proteins in human nasal lavage fluid from non-smokers and smokers using two-dimensional gel electrophoresis and peptide mass fingerprinting. Proteomics 2002;2:112-20. [Crossref] [PubMed]
- Teichtahl H, Buckmaster N, Pertnikovs E. The incidence of respiratory tract infection in adults requiring hospitalization for asthma. Chest 1997;112:591-6. [Crossref] [PubMed]
- Yano T, Ichikawa Y, Komatu S, et al. Association of Mycoplasma pneumoniae antigen with initial onset of bronchial asthma. Am J Respir Crit Care Med 1994;149:1348-53. [Crossref] [PubMed]
- Schröder NW, Schumann RR. Non-LPS targets and actions of LPS binding protein (LBP). J Endotoxin Res 2005;11:237-42. [Crossref] [PubMed]
- Wood PR, Hill VL, Burks ML, et al. Mycoplasma pneumoniae in children with acute and refractory asthma. Ann Allergy Asthma Immunol 2013;110:328-34.e1. [Crossref] [PubMed]
- Peters J, Singh H, Brooks EG, et al. Persistence of community-acquired respiratory distress syndrome toxin-producing Mycoplasma pneumoniae in refractory asthma. Chest 2011;140:401-7. [Crossref] [PubMed]
- Hong SJ. The Role of Mycoplasma pneumoniae Infection in Asthma. Allergy Asthma Immunol Res 2012;4:59-61. [Crossref] [PubMed]
- Baines KJ, Simpson JL, Wood LG, et al. Sputum gene expression signature of 6 biomarkers discriminates asthma inflammatory phenotypes. J Allergy Clin Immunol 2014;133:997-1007. [Crossref] [PubMed]
- Fujii K, Nakamura H, Nishimura T. Recent mass spectrometry-based proteomics for biomarker discovery in lung cancer, COPD, and asthma. Expert Rev Proteomics 2017;14:373-86. [Crossref] [PubMed]