
A novel supplemental maneuver to predict fluid responsiveness in critically ill patients: blood pump-out test performed before renal replacement therapy
Introduction
The concept of fluid resuscitation is highlighted in the guidelines of the Surviving Sepsis Campaign (1). In the early stage, the fluid resuscitation is an indispensable and important treatment for patients with septic shock (2,3). Reasonable volume therapy can increase the preload of the heart by overfilling the fluid, thereby increase the cardiac output, improve the hemodynamic state of the patients, optimize the heart function and improve the tissue perfusion. However, the expansion in volume results in an increase of preload, not elevation of cardiac output, even sometimes increasing the burden of cardiopulmonary capacity. After volume therapy, only 50% of patients with unstable hemodynamics had increased cardiac output. Therefore, it is particularly important to predict the patient’s response to fluid responsiveness before volume therapy, that is, to accurately evaluate the patient's fluid responsiveness before volume therapy (4,5).
There are several strategies that can be used to predict fluid responsiveness and the most used two methods are fluid challenge and the passive leg raising (PLR) maneuver (6,7). The first strategy is based on titration and monitoring of the effects of volume expansion. This protocol is recommended by the National Institute for Clinical Excellence (8). This strategy is associated with good outcomes. However, it may also result in repeated ineffective fluid boluses. Indeed, fluid overload and positive fluid balance are associated with poor prognosis (9,10). PLR is an easy-to-perform and reliable method to assess fluid responsiveness. Even when many other dynamic predictors are inconsistent, it maintains excellent performance and avoids unnecessary fluid management. Importantly, its prediction remains valuable in patients with cardiac arrhythmias or spontaneous breathing activity (11,12). PLR has been demonstrated to produce changes in preload, increasing stroke volume (SV) significantly in patients who meet at the responder part of the ventricular function curve of Frank-Starling. Likewise, this is considered a reversible filling volume test as its effect on SV disappears after the patient returns to the supine position (13-15). The PLR test has been included in the last update of the recommendations of the Surviving Sepsis Campaign (1) and in a consensus conference of the European Society of Intensive Care Medicine (16). However, clinically, not all severe cases can be successfully implemented PLR (17,18).
AKI occurs in 5–45% of critically ill patients, and renal replacement therapy (RRT) is the main treatment of critically ill patients with severe AKI (19,20). Fluid management plays a critical role in AKI patients. But is there a suitable, and easy but long-term neglected way to evaluate volumetric reactivity in this particular population? According to our pilot study, there is about 210 mL blood drained from the body at the start of RRT. The procedure of blood drainage, named by blood pump-out test (BPT), is inverse to the autologous bloodletting from the PLR test, which may make patients with insufficient effective circulating blood volume have decreased CO, while patients with blood volume overload or normal changes in CO may have a variety of possibilities. Little studies focus on BPT and we hypothesized that BPT could serve as a supplement maneuver in predicting fluid responsiveness in patients with AKI underwent RRT.
Methods
This single-center, real-world, prospective clinical study (ChiCTR-DDD-17010534) was conducted from June 2016 to August 2018 at Guangdong Provincial People’s Hospital and approved by the hospital’s Ethical Committee (No. GDREC2016313H) and all patients enrolled were informed about the clinical trial and accepted to participate.
Patients
Inclusion criteria were as follows: (I) age ≥18 years old; (II) renal replacement therapy was needed. Exclusion criteria were as follows: (I) age <18 years old; (II) pregnant women or patients with end-stage malignant tumors; (III) patients who do not need blood purification therapy or PiCCO monitoring can not be performed; (IV) no informed consent.
All enrolled patients must have undergone RRT who had a transpulmonary thermodilution device already in place (PiCCO device, Pulsion Medical Systems, Munich, Germany).
Study design
PLR process (Figure 1) and determination of parameters
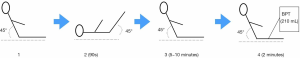
- Patients took a 45° semi-recumbent position, and had PLR when the blood purification was about to the blood drainage. After each position change and after PLR test, we recorded hemodynamic parameters accordingly. The pulse contour-derived cardiac output was calibrated by transpulmonary thermodilution at baseline, and then when the CO value is stable, we will take the corresponding value as the baseline CO value measured by pulse contour-derived cardiac output.
- In the timeframe of PLR effects, the maximum value of CO will appear during 30-90 s after the onset of the PLR test, which was measured by real-time pulse contour-derived cardiac output monitoring. Fluid responders was defined with an increase in the pulse contour analysis-derived ∆CO ≥10% during PLR, if ∆CO <10%, then it was non-responders. ∆COPLR = (COmaximum − CObaseline)/CObaseline, CObaseline refers to the baseline value of CO in a stable state before PLR, and COmaximum will appear during 30–90 s after the onset of the PLR test.
Determination of the parameters during BPT in the blood purification pipelines (Figure 2)
After PLR, patients returned to the 45° semi-recumbent position for 5–10 minutes. Re-assess CO in the semi-recumbent position (should return to baseline), it usually took five to ten minutes, then BPT in the RRT pipelines would begin. The speed of blood drainage was approximately 100 mL/minute, and the whole process of blood drainage would last for about 2 minutes. During the process of arterial end blood drainage, the venous end blood return pipeline is temporarily closed to ensure that no additional fluid enters the body during the process of blood drainage. Within the 0.5–2 minutes during blood drainage, CO minimum was taken by pulse contour-derived CO. During the whole process, intravenous rehydration stopped, except the vasoactive drugs that pumped into the veins at a constant speed. ∆COBPT = (CObaseline − COminimum)/CObaseline, CObaseline refers to the baseline value of CO in a stable state before BPT, and COminimum will appear during 120 s after the onset of the BPT test. The CRRT machine is made in Germany by Fesenius, and the blood filter model is Ultraflux AV1000S.
Statistical analysis
The normality of data was tested by the Kolmogorov-Smirnov normality test. Continuous variables were expressed as median as mean ± standard deviation (SD). The sample size was based on the assumption of finding 92.5% sensitivity in pre-experiment and the intention to obtain a significant of α=0.05, allowing an error of δ=0.08. We calculated that 65 patients needed to be included in the study. Comparisons of variables between cases with vs. cases without fluid responsiveness were assessed by a two-tailed Student’s t test or a Mann-Whitney U test, as appropriate. A receiver-operating characteristic (ROC) curve was constructed to test the ability of the LPLR-induced changes in the CO to predict a fluid responsiveness. Sensitivities, specificities and areas under (AUROCs) the ROC curve are expressed as mean (95% CI). The diagnostic cut off was determined by the best Youden index value. Since some patients underwent several BPTs, each BPT was considered as a “case”, and all cases were included in the primary analysis. SPSS19.0 software was used to analyse the data. A P≤0.05 was considered statically significant.
Results
Patient characteristics
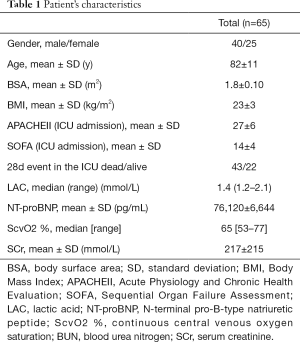
Sixty-five patients met inclusion criteria and enrolled in this study. Their characteristics were reported in Table 1.
Full table
Responders vs. non-responders identified by ∆CO PLR ≥10% during PLR
There were 31 responders vs. 34 non-responders during PLR with ∆COPLR ≥10%. Compared with non-responders, responders had lower MAP (73±11 vs. 84±14 mmHg, P=0.005), CVP (7±3 vs. 10±5 cmH2O, P=0.043) as well as SI (29.37±11.94 vs. 41.25±16.44 mL/m2, P=0.008) while the former had lower CO (4.64±1.34 vs. 6.40±3.89 mL/m2, P=0.072) but with no significant statistical difference (Table 2).

Full table
Changes of hemodynamic variables during BPT
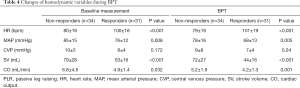
Based on the increase of ∆CO BPT during PLR (golden standard), responders and non-responders had the contrary hemodynamic changes during BPT compared with those during PLR test (Table 3). Responders showed higher HR (94.5 vs. 79.9/min) and lower CO (4.0 vs. 5.4) compared with non-responders (Table 4).

Full table
Full table
Prediction of CO changes to fluid responsiveness during BPT
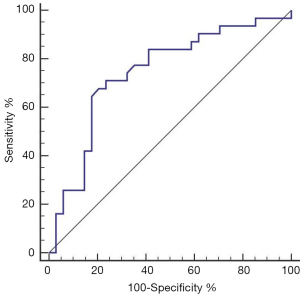
The aera under the ROC curve was 0.74 (95% CI: 0.62–0.84) (Figure 2). The positive and negative predictive values were 0.709 and 0.765, respectively. Its Youden index was 0.474, and the positive and negative likelihood ratios were 2.19 and 0.42, respectively (Table 5).
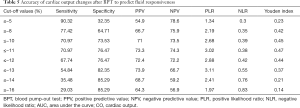
Full table
Discussion
To our knowledge, this was the first study to access the value of BPT in predicting fluid responsiveness based on a standard PLR test in critically ill patients. Interestingly, we found that BPT is a supplemental maneuver to PLR based on blood drainage in CRRT, which might supply a novel way to guide fluid management in the next steps in AKI patients, like limitation of unnecessary fluid infusion or expansion if CO decreases more than 11% compared with baseline during BPT.
Fluid therapy is the key treatment of critical patients. Insufficient volume affects the perfusion of important organs, exacerbates the ischemia and hypoxia of tissues and organs, and even makes organ damage irreversible. At the same time, the critically ill patients are often accompanied by increasing age, complicated with many basic diseases, and the damage of important organs etc. (21). The excessive volume load will also aggravate the injury of tissues and organs. More importantly, capacity states are not equal to fluid responsiveness (22). According to the Frank-Staring curve, at its ascending branch, the SV will increase obviously before the increase of negative load, but it will be harmful to increase the rehydration during its plateau period. Therefore, it is very important to determine the position of the patient on the curve, that is to say, the accurate judgment of the patient's volumetric reactivity is the key factor of the critical patient's fluid therapy (23).
The fluid challenge and the PLR maneuver are two techniques which are widely used, practical, easy to perform, and physiologically based, which can be used to predict critically ill patients’ fluid responsiveness with a high degree of accuracy (24,25). Both PLR and fluid challenge are based on the cardiac function curve of Frank-Starling. However, fluid administration does not always lead to increase CO (13). Is it possible to know if our patient will respond to fluids without administration, avoiding the negative consequences of excessive volume? (26). PLR induces a translocation of venous blood from the legs and the splanchnic compartment toward the cardiac chambers (6,27,28). Fluid management influences ICU patients’ outcomes. Both overhydration and conservative fluid therapy can lead to complications. Inappropriate fluid management in the treatment of critically ill patients can increase morbidity and mortality (29,30). Invasive static measurements have been used to evaluate volaemia, such as central venous pressure or pulmonary capillary wedge pressure. It has been demonstrated that these parameters are bad indicators of volaemia and are not useful as predictors of an adequate response to fluid therapy, including when, how much fluid to administer, as only half of critically ill patients respond to fluid loading with an increase in CO called “fluid responsiveness”. Traditionally, clinical symptoms, volaemic status has been evaluated using MAP, HR, it is known that MAP and HR cannot be used reliably to measure changes in central blood volume. Based on the above indicators, no appropriate treatment can be given clinically (31,32). Based on the hemodynamic consequences of the heart-lung interactions, the use of dynamic indices of preload that result from respiratory variations is well-accepted point-of-care predicting parameters of fluid responsiveness (33). The use of stroke volume variation (SVV) and pulse pressure variation (PPV) to accurately predict a positive response to fluid administration, however, may be restricted to mechanical ventilation condition and normal rhythm (34).
Our research differs from previous research in many ways. RRT is the main treatment of critically ill patients with severe AKI (19,20). When it comes to the beginning of RRT, based on our previous experimental findings, about 210 mL blood drained off body circulation within a short time (appropriately 2 min). The process of blood drainage before RRT simulates the effects of autologous bloodletting, without changing patients’ positions. Hence, we can infer that its value in evaluating fluid responsiveness is inversed to the effects of PLR test. The main interest of BPT is to limit unnecessary fluid infusion. Since the effects of BPT are inversed to the autologous bloodletting from the PLR test, patients with insufficient effective circulating blood volume may have a decrease in CO, while patients with blood volume overload or normal changes in CO may have a variety of possibilities. We hypothesized that blood drainage before RRT can help in predicting fluid responsiveness. In our study, we have showed that BPT was a good predictor of fluid responsiveness for critically ill patients. A decrease of CO greater than 11.0% after blood drainage maneuver predicts a fluid responsiveness with 70.9% sensitivity and 76.5% specificity, and with highest AUROC (0.74±0.06; 95% CI: 0.62 to 0.84).
The results of our clinical trial provide a method for predicting fluid responsiveness with moderate specificity and sensitivity. However, our clinical trial has several limitations. Firstly, using PLR in the context of weaning is that it requires a technique to measure cardiac output. Which restricted the patients we included, required a high level of medical equipment and doctors’ clinical experience and the results of the study cannot be applied to primary hospitals. Secondly, we also did not specifically investigate some other conditions that could be associated with weaning-induced cardiac dysfunction, such as hypertrophic obstructive cardiomyopathy. Thirdly, the use of saline is unavoidable in PICCO monitoring, and the effect of this fluid on CO cannot be eliminated. Finally, PLR cannot be used to specific situations like intra-abdominal hypertension, amputation of both legs and so on (17,18), as we mentioned in the background part, which limited the correlation study between PLR and BPT to predict fluid responsiveness in the above groups of patients. And in our study, the most patients were with septic shock, acute heart failure and pulmonary infection.
Conclusions
Our study found that BPT could serve as a supplemental maneuver to assess fluid responsiveness in critically ill patients with AKI, which was likely to direct the future fluid management without extra fluid expansion.
Acknowledgments
Funding: This work was supported by the grant from Medical Scientific Research Foundation of Guangdong Province, People’s Republic of China (Grant number: A2018064), and National Clinical Key Specialty Construction Project of China (2012-649, 2013-544).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Glenn Hernández and Guo-Wei Tu) for the series “Hemodynamic monitoring in critically ill patients” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.04.56). The series “Hemodynamic Monitoring in Critically Ill Patients” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by Ethical Committee of Guangdong Provincial People’s Hospital (No. GDREC2016313H). Informed consent was taken from all individual participants. This clinical trial has been registered at Chictr.org.cn as ChiCTR-DDD-17010534.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017;43:304-77. [Crossref] [PubMed]
- Thacker JK, Mountford WK, Ernst FR, et al. Perioperative Fluid Utilization Variability and Association With Outcomes: Considerations for Enhanced Recovery Efforts in Sample US Surgical Populations. Ann Surg 2016;263:502-10. [Crossref] [PubMed]
- Stein A, de Souza LV, Belettini CR, et al. Fluid overload and changes in serum creatinine after cardiac surgery: predictors of mortality and longer intensive care stay. A prospective cohort study. Crit Care 2012;16:R99. [Crossref] [PubMed]
- Neyra JA, Li X, Canepa-Escaro F, et al. Cumulative Fluid Balance and Mortality in Septic Patients With or Without Acute Kidney Injury and Chronic Kidney Disease. Crit Care Med 2016;44:1891-900. [Crossref] [PubMed]
- Claure-Del Granado R, Mehta RL. Fluid overload in the ICU: evaluation and management. BMC Nephrol 2016;17:109. [Crossref] [PubMed]
- Biais M, Ouattara A, Janvier G, et al. Case scenario: respiratory variations in arterial pressure for guiding fluid management in mechanically ventilated patients. Anesthesiology 2012;116:1354-61. [Crossref] [PubMed]
- De Backer D, Heenen S, Piagnerelli M, et al. Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med 2005;31:517-23. [Crossref] [PubMed]
- Vallet B, Blanloeil Y, Cholley B, et al. Guidelines for perioperative haemodynamic optimization. Ann Fr Anesth Reanim 2013;32:e151-8. [Crossref] [PubMed]
- Biais M, de Courson H, Lanchon R, et al. Mini-fluid Challenge of 100 ml of Crystalloid Predicts Fluid Responsiveness in the Operating Room. Anesthesiology 2017;127:450-6. [Crossref] [PubMed]
- Brandstrup B, Tonnesen H, Beier-Holgersen R, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg 2003;238:641-8. [Crossref] [PubMed]
- Girotto V, Teboul JL, Beurton A, et al. Carotid and femoral Doppler do not allow the assessment of passive leg raising effects. Ann Intensive Care 2018;8:67. [Crossref] [PubMed]
- Mesquida J, Gruartmoner G, Ferrer R. Passive leg raising for assessment of volume responsiveness: a review. Curr Opin Crit Care 2017;23:237-43. [Crossref] [PubMed]
- Wiersema UF, Bihari S. The Frank-Starling Curve Is Not Equivalent to the Fluid Responsiveness Curve. Crit Care Med 2017;45:e335-6. [Crossref] [PubMed]
- Monnet X, Cipriani F, Camous L, et al. The passive leg raising test to guide fluid removal in critically ill patients. Ann Intensive Care 2016;6:46. [Crossref] [PubMed]
- Marik PE. Fluid Responsiveness and the Six Guiding Principles of Fluid Resuscitation. Crit Care Med 2016;44:1920-2. [Crossref] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Monnet X, Marik PE, Teboul JL. Prediction of fluid responsiveness: an update. Ann Intensive Care 2016;6:111. [Crossref] [PubMed]
- Mahjoub Y, Touzeau J, Airapetian N, et al. The passive leg-raising maneuver cannot accurately predict fluid responsiveness in patients with intra-abdominal hypertension. Crit Care Med 2010;38:1824-9. [Crossref] [PubMed]
- Legrand M, Darmon M, Joannidis M, et al. Management of renal replacement therapy in ICU patients: an international survey. Intensive Care Med 2013;39:101-8. [Crossref] [PubMed]
- Joannidis M, Forni LG. Clinical review: timing of renal replacement therapy. Crit Care 2011;15:223. [Crossref] [PubMed]
- Chang L, Cheng MF, Jou ST, et al. Search of Unknown Fever Focus Using PET in Critically Ill Children With Complicated Underlying Diseases. Pediatr Crit Care Med 2016;17:e58-65. [Crossref] [PubMed]
- Weiss E, Paugam-Burtz C, Jaber S. Shock Etiologies and Fluid Management in Liver Failure. Semin Respir Crit Care Med 2018;39:538-45. [Crossref] [PubMed]
- Perner A, Prowle J, Joannidis M, et al. Fluid management in acute kidney injury. Intensive Care Med 2017;43:807-15. [Crossref] [PubMed]
- Casey JD, Brown RM, Semler MW. Resuscitation fluids. Curr Opin Crit Care 2018;24:512-8. [Crossref] [PubMed]
- Monnet X, Bleibtreu A, Ferre A, et al. Passive leg-raising and end-expiratory occlusion tests perform better than pulse pressure variation in patients with low respiratory system compliance. Crit Care Med 2012;40:152-7. [Crossref] [PubMed]
- Monnet X, Osman D, Ridel C, et al. Predicting volume responsiveness by using the end-expiratory occlusion in mechanically ventilated intensive care unit patients. Crit Care Med 2009;37:951-6. [Crossref] [PubMed]
- Galarza L, Mercado P, Teboul JL, et al. Estimating the rapid haemodynamic effects of passive leg raising in critically ill patients using bioreactance. Br J Anaesth 2018;121:567-73. [Crossref] [PubMed]
- Barjaktarevic I, Toppen WE, Hu S, et al. Ultrasound Assessment of the Change in Carotid Corrected Flow Time in Fluid Responsiveness in Undifferentiated Shock. Crit Care Med 2018;46:e1040-6. [Crossref] [PubMed]
- Monnet X, Marik P, Teboul JL. Passive leg raising for predicting fluid responsiveness: a systematic review and meta-analysis. Intensive Care Med 2016;42:1935-47. [Crossref] [PubMed]
- Monnet X, Pinsky MR. Predicting the determinants of volume responsiveness. Intensive Care Med 2015;41:354-6. [Crossref] [PubMed]
- Deng QW, Tan WC, Zhao BC, et al. Is goal-directed fluid therapy based on dynamic variables alone sufficient to improve clinical outcomes among patients undergoing surgery? A meta-analysis. Crit Care 2018;22:298. [Crossref] [PubMed]
- Bentzer P, Griesdale DE, Boyd J, et al. Will This Hemodynamically Unstable Patient Respond to a Bolus of Intravenous Fluids? JAMA 2016;316:1298-309. [Crossref] [PubMed]
- Myatra SN, Monnet X, Teboul JL. Use of 'tidal volume challenge' to improve the reliability of pulse pressure variation. Crit Care 2017;21:60. [Crossref] [PubMed]
- Mesquida J, Kim HK, Pinsky MR. Effect of tidal volume, intrathoracic pressure, and cardiac contractility on variations in pulse pressure, stroke volume, and intrathoracic blood volume. Intensive Care Med 2011;37:1672-9. [Crossref] [PubMed]


