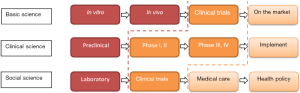
Health Services & Policy Research in translational medicine
Translational medicine is often defined through its process. Researchers from different disciplines may see the process differently (Figure 1) (1). Basic scientists and clinicians may describe it as the “bench to bedside” approach of medical research (2). Only a few institutes of translational medicine realize that Health Services & Policy Research (HSPR) is an important component in translational medicine (3,4). Health services & policy researchers can bridge laboratory research, clinical studies, community practice, and health policy to improve public health.
The goal of translational medicine is to translate academic research into population health (5). US National Health Institute launched the Clinical and Translational Science Awards (CTSA) program in 2006 to strengthen the full spectrum of translational research. In 2012, NIH published a summary report based on the stakeholders’ feedback about the CTSA program. Only two vague long-term outcomes were proposed to measure the value added by the CTSA program: increased utilization of new treatments in the community and community health indicators improvement (6). However, the CTSA program has realized that NIH should engage with Center of Medicare and Medicaid Services (CMS) and Agency of Healthcare Research and Quality (AHRQ) to promote HSPR such as comparative effectiveness research and patient centered outcomes research. Basic scientists and clinicians need to work synergistically with health services & policy researchers in the translational process.
HSPR in translational medicine is different from the biostatistical support in the current CTSA program. HSPR evaluates effectiveness and cost-effectiveness of the new technology or treatment with observational data and survey data when the new treatment expands discovery to larger patient populations. HSPR employs comparative effectiveness research to determine if a certain treatment or technology works in a real-world setting when the practice-oriented stage of translational research is implemented and find out subgroups of patients who benefit the most (or the least) from the new therapy. If everything was successful, we need to evaluate the current healthcare policy and delivery system to identify barriers to disseminate and implement the new therapy and propose certain policy changes to efficiently reach clinicians and patients. In summary, translational medicine is not just bench to bedside, prototype to device, or beaker to pill. HSPR is an essential component of translational medicine.
It is a critical move for Annals of Translational Medicine to create a new HSPR column. This new column will provide a forum for the researches that bridge academic research, health policy, and medical practice. The articles appearing in this column will be authored by scholars from universities, government, private research organizations, and industry. We welcome all submissions that examine how and whether new therapies and research knowledge reach the intended people and are implemented efficiently. The publications from the HSPR column will strengthen communication with practitioners and policymakers and influence medical practice and health policy at all administrative levels.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Woolf SH. The meaning of translational research and why it matters. JAMA 2008;299:211-3. [PubMed]
- Reflections on Translational Medicine [Internet]. 2011[cited 2014 Jun 20]. Available online: http://www.youtube.com/watch?v=w9C9TisSiY4&feature=youtube_gdata_player
- Institute for Translational Medicine at the University of Chicago: What is translational medicine? [Internet], 2014[cited 2014 Jun 20]. Available online: http://itm.uchicago.edu/about-3/what-is-translational-medicine-2/
- UCSF Clinical & Translational Science Institute: Community Engagement & Health Policy [Internet], 2014[cited 2014 Jun 27]. Available online: http://ctsi.ucsf.edu/about-us/programs/community-engagement-health-policy
- Zerhouni EA. Translational and clinical science--time for a new vision. N Engl J Med 2005;353:1621-3. [PubMed]
- National Center for Advancing Translational Sciences: Request for Information: Enhancing the Clinical and Translational Science Awards Program [Internet], 2012[cited 2014 Jun 27]. Available online: http://www.ncats.nih.gov/files/report-ctsa-rfi.pdf