
Mechanical ventilation during thoracic surgery: towards individualized medicine
Lung protective ventilation during the intraoperative period has been associated with controversial clinical benefits in patients undergoing general anesthesia (1,2). While there is unity on the application of physiological tidal volumes (VT 6–8 mL/kg of predicted body weight), at least for double lung ventilation, there is a lack of consensus on how to set positive end-expiratory pressure (PEEP) intraoperatively. Individual characteristics, such as body mass index, chest wall dimensions, preoperative lung condition, and pleural pressures, make it impossible for anesthesiologists to choose the optimal PEEP level without additional monitoring.
Some authors advocate personalized PEEP titration based on several techniques, while others suggest that this fine-tuning is clinically irrelevant and recommend the use of low and fixed PEEP levels to all patients. Too low PEEP levels, however, can lead to atelectasis and lung heterogeneity, which can increase driving pressure (ΔP), a variable associated with the development of postoperative pulmonary complications (PPC), including ARDS (3,4). To complicate things further, the development of atelectasis depends not only on the intraoperative PEEP, but also on the type of surgery, the use of recruitment maneuvers, the postoperative ventilator weaning process, and respiratory therapy.
With a much higher incidence after thoracic than after abdominal surgeries, PPC are associated with increased hospital mortality, ICU admission, and hospital length of stay (5). Thoracic surgery is particularly risky in elderly patients (6), in whom details can make a difference. In these patients, a personalized perioperative approach to reduce the risk of hypoxemia, lung injury, and other pulmonary complications seems justified. One should pay especial attention to chest wall and thoracic spine deformities, respiratory muscle weakness, increased alveolar dead space, and diminished ventilatory response to hypoxia and hypercapnia. This individualized care gives more emphasis on physiology and functional status than on chronologic age (6).
Unsafe ventilator settings, mainly when applied on top of atelectatic lungs, as well as atelectasis per se, are factors associated with PPC. Adequate management of these two modifiable factors during the perioperative period can lead to improved outcomes. For instance, a postoperative intensive alveolar recruitment strategy in the ICU to reduce atelectasis in hypoxemic patients after cardiac surgery resulted in less severe pulmonary complications and reduced hospital and ICU length of stay (7). Intraoperative unsafe ventilator settings not always cause clinically relevant lung injury and PPC. One can ventilate healthy lungs with low PEEP values and moderately high driving pressures without producing significant damage if the duration of ventilation is short, and the surgery does not trigger a systemic inflammatory process that facilitates lung injury (8,9). The combination of unsafe ventilator settings and high-risk surgeries, nevertheless, increases the risk of PPC.
In most clinical trials, it is hard to distinguish the role of lung injury and postoperative atelectasis in the development of lung complications. In thoracic surgeries with one-lung ventilation (OLV), differentiating these modifiable factors seems to be a more significant challenge. In this scenario, there is an increased risk of lung injury due to considerable lung stress and strain in addition to the presence of atelectasis of at least one whole lung. Moreover, the damage to the lungs can be aggravated by the inflammation induced by this type of surgery (10). Hence, one should not overlook the importance of adequate ventilator settings in mitigating lung injury.
In OLV, however, there is no solid consensus on how to set the ventilator (11). A Canadian multi-institutional survey on the use of lung-protective strategies during OLV, published in 2018 (12), concluded that most anesthesiologists defined low peak airway pressure as the primary target of lung-protective ventilation. Furthermore, in this survey (12), only 64% of the respondents actively tried to minimize VT, a variable associated with a lower incidence of PPC when accompanied by adequate PEEP (13). Previous studies on protective ventilation during intraoperative OLV showed the benefits of associating physiological VT and PEEP (14-18). Three clinical trials compared low VT (<8 mL/kg) with high VT (≥8 mL/kg) (14,15,17), and another study randomized patients to an individualized PEEP which produced the lowest driving pressure or to a PEEP of 5 cmH2O (16). All of them (13,15-17) pointed to an association of high VT and high driving pressure with pulmonary complications. Finally, an additional risk for PPC following thoracic surgery is the use of high FIO2, which is often necessary to maintain oxygenation with insufficient PEEP levels (19).
In this issue of Annals of Translational Medicine, Liu et al. (20) performed a randomized clinical trial to evaluate the influence of PEEP on oxygenation and lung mechanics in elderly patients undergoing elective thoracoscopic surgery. The authors randomly allocated 100 patients aged 65 years or more into one of two groups: (I) PEEP of 5 cmH2O (PEEP5) or (II) PEEP titrated according to electrical impedance tomography (PEEPEIT). During a decremental PEEP titration from 15 to 1 cmH2O, the authors defined PEEPEIT as the intercept point between overdistention and collapse after a recruitment maneuver. Other ventilatory parameters were the same during double-lung ventilation and OLV. They found that PEEPEIT was significantly higher than PEEP5; furthermore, PaO2/FIO2 and dynamic compliance (Cdyn) were higher while driving pressure was lower in PEEPEIT compared to PEEP5. After surgery, all patients were extubated with PEEP of 5 cmH2O. There were no differences in the use of vasopressors, PPC or hospital length of stay.
During OLV, the authors did find better oxygenation and lower driving pressures in the PEEPEIT group compared to the PEEP5 group. These findings suggest that their recruitment maneuver and PEEP choice were able to achieve their goal of avoiding atelectasis in the ventilated lung. They, however, did not take advantage of the improved oxygenation by lowering FIO2 to the lowest safe level possible, which could have further avoided absorption atelectasis and possibly lung injury. Moreover, at the end of the surgery, after lung recruitment, all patients resumed double lung ventilation with PEEP of 5 cmH2O, neutralizing the beneficial effects of PEEP individualization on respiratory system compliance and driving pressure. A significant difference in oxygenation persisted, though (20). These results suggest that the postoperative recruitment maneuver followed by PEEP of 5 cmH2O was enough to equal the protection of an individualized PEEP selection in terms of postoperative atelectasis and PPC. In other words, the additional lung protection gained with lower driving pressures and better compliance did not translate into a clinical benefit in the postoperative period. This finding is indicative that the difference in lung injury between groups was mild at most, perhaps because of the short duration of the procedures. However, it is impossible to know from the data at hand if atelectasis and PPC would have been lower if patients from the PEEPEIT group had been maintained with the titrated PEEP until liberation from mechanical ventilation.
By protocol, the benefits of PEEP individualization during the intraoperative period did not extend to the postoperative period. This choice brings to attention the weaning phase, another critical component of the ventilatory management during the perioperative period. Before extubation, anesthesiologists usually wean patients on spontaneous breathing, without PEEP and on high FIO2, a practice that can favor lung collapse. The use of positive pressure (CPAP) and low FIO2 during weaning can preserve, at least partially, the lung recruitment reached during surgery (21). For example, Pereira et al. (22) had patients during the weaning period under pressure-support mode, keeping FIO2 at 50% and maintaining PEEP according to randomization to mitigate atelectasis formation. Such a strategy most likely contributed to the difference in postoperative collapsed lung tissue evaluated by whole-lung computed tomography between groups: 6.4%±4.1% in PEEPEIT-arm vs. 10.8%±7.1% in PEEP4-arm.
In an attempt to provide the best compromise between collapsed and overdistended lung, Liu et al. (20) titrated PEEP according to EIT. They chose the nearest PEEP above the intersection of the curves representing collapse and overdistension, as previously reported (22). This titration criterion usually results in PEEP levels equal to or lower than the PEEP of best compliance and can reduce the incidence of postoperative atelectasis following abdominal surgeries (22). In Liu’s study, PEEP varied from 9 to 13 cmH2O, values similar to those reported in a Spanish study in which PEEP was titrated, after a recruitment maneuver, according to the best compliance in 690 patients on OLV (23). Other authors have used different PEEP titration methods, such as the incremental PEEP selection targeting the lowest driving pressure (16). Incremental PEEP titrations, however, might result in overdistention without lung recruitment. Not always surpassing opening pressures, this method does not focus on avoidance of atelectasis and results in small differences between the groups during the intraoperative period (16).
We want to call attention to yet another aspect of the ventilation protocol: recruitment maneuvers. Both groups, PEEPEIT and PEEP5, were submitted to a bag-squeezing recruitment maneuver of 35 cmH2O for 15 seconds at the restart of double lung ventilation. For healthy nonobese patients, inspiratory pressure less than 40 cmH2O might not be enough to open all the atelectatic areas in all patients (24). Besides, the bag-squeezing recruitment maneuver often leads to depressurization when switching from manual to controlled ventilation (Figure 1A), resulting in partial lung collapse (25). Conversely, recruitment maneuvers using controlled ventilatory modes are safer against derecruitment (Figure 1B).
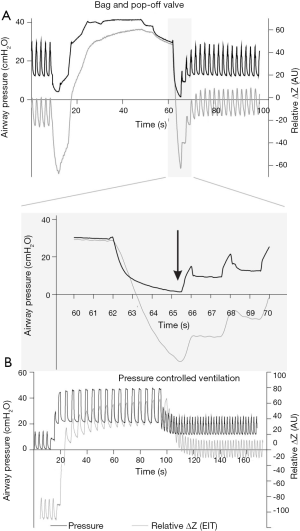
The study of Liu et al. shed light on relevant aspects of lung protection in elderly patients undergoing OLV for thoracic surgery. The association of recruitment maneuvers, PEEP titration, and physiological VT led to better physiological respiratory variables, some of them directly related to better postoperative outcomes in other studies. Perhaps even more important than the answers they provided were the questions their results provoked. Why did better intraoperative settings fail to improve outcomes? Was it a matter of time? Was the thoracic surgery too mild an inflammatory first hit? Is the weaning phase a more critical determinant of postoperative outcomes? Negative but carefully performed studies can challenge our current knowledge and push science further. “The only true wisdom is in knowing you know nothing” (Socrates).
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-2005). ELVC reports personal fees from Timpel SA, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Futier E, Constantin JM, Paugam-Burtz C, et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med 2013;369:428-37. [Crossref] [PubMed]
- PROVE Network Investigators for the Clinical Trial Network of the European Society of Anaesthesiology, Hemmes SN, Gama de Abreu M, et al. High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet 2014;384:495-503. [Crossref] [PubMed]
- Ladha K, Vidal Melo MF, McLean DJ, et al. Intraoperative protective mechanical ventilation and risk of postoperative respiratory complications: hospital based registry study. BMJ 2015;351:h3646. [Crossref] [PubMed]
- Neto AS, Hemmes SN, Barbas CS, et al. Association between driving pressure and development of postoperative pulmonary complications in patients undergoing mechanical ventilation for general anaesthesia: a meta-analysis of individual patient data. Lancet Respir Med 2016;4:272-80. [Crossref] [PubMed]
- García-Miguel FJ, Serrano-Aguilar PG, López-Bastida J. Preoperative assessment. Lancet 2003;362:1749-57. [Crossref] [PubMed]
- Wang S, Li X, Li Y, et al. The long-term impact of postoperative pulmonary complications after video-assisted thoracic surgery lobectomy for lung cancer. J Thorac Dis 2017;9:5143-52. [Crossref] [PubMed]
- Costa Leme A, Hajjar LA, Volpe MS, et al. Effect of intensive vs moderate alveolar recruitment strategies added to lung-protective ventilation on postoperative pulmonary complications: a randomized clinical trial. JAMA 2017;317:1422-32. [Crossref] [PubMed]
- Wellman TJ, Winkler T, Costa EL, et al. Effect of local tidal lung strain on inflammation in normal and lipopolysaccharide-exposed sheep*. Crit Care Med 2014;42:e491-500. [Crossref] [PubMed]
- Tucci MR, Costa EL, Wellman TJ, et al. Regional lung derecruitment and inflammation during 16 hours of mechanical ventilation in supine healthy sheep. Anesthesiology 2013;119:156-65. [Crossref] [PubMed]
- de la Gala F, Piñeiro P, Garutti I, et al. Systemic and alveolar inflammatory response in the dependent and nondependent lung in patients undergoing lung resection surgery: a prospective observational study. Eur J Anaesthesiol 2015;32:872-80. [PubMed]
- Bignami E, Saglietti F, Di Lullo A. Mechanical ventilation management during cardiothoracic surgery: an open challenge. Ann Transl Med 2018;6:380. [Crossref] [PubMed]
- Kidane B, Choi S, Fortin D, et al. Use of lung-protective strategies during one-lung ventilation surgery: a multi-institutional survey. Ann Transl Med 2018;6:269. [Crossref] [PubMed]
- Blank RS, Colquhoun DA, Durieux ME, et al. Management of one-lung ventilation: impact of tidal volume on complications after thoracic surgery. Anesthesiology 2016;124:1286-95. [Crossref] [PubMed]
- Yang M, Ahn HJ, Kim K, et al. Does a protective ventilation strategy reduce the risk of pulmonary complications after lung cancer surgery?: a randomized controlled trial. Chest 2011;139:530-7. [Crossref] [PubMed]
- Marret E, Cinotti R, Berard L, et al. Protective ventilation during anaesthesia reduces major postoperative complications after lung cancer surgery: a double-blind randomised controlled trial. Eur J Anaesthesiol 2018;35:727-35. [Crossref] [PubMed]
- Park M, Ahn HJ, Kim JA, et al. Driving pressure during thoracic surgery: a randomized clinical trial. Anesthesiology 2019;130:385-93. [Crossref] [PubMed]
- Zhang BJ, Tian HT, Li HO, et al. The effects of one-lung ventilation mode on lung function in elderly patients undergoing esophageal cancer surgery. Medicine (Baltimore) 2018;97:e9500. [Crossref] [PubMed]
- El Tahan MR, Pasin L, Marczin N, et al. Impact of low tidal volumes during one-lung ventilation. a meta-analysis of randomized controlled trials. J Cardiothorac Vasc Anesth 2017;31:1767-73. [Crossref] [PubMed]
- Okahara S, Shimizu K, Suzuki S, et al. Associations between intraoperative ventilator settings during one-lung ventilation and postoperative pulmonary complications: a prospective observational study. BMC Anesthesiol 2018;18:13. [Crossref] [PubMed]
- Liu K, Huang C, Xu M, et al. PEEP guided by electrical impedance tomography during one-lung ventilation in elderly patients undergoing thoracoscopic surgery. Ann Transl Med 2019;7:757. [Crossref] [PubMed]
- Kostic P, LoMauro A, Larsson A, et al. Specific anesthesia-induced lung volume changes from induction to emergence: a pilot study. Acta Anaesthesiol Scand 2018;62:282-92. [Crossref] [PubMed]
- Pereira SM, Tucci MR, Morais CCA, et al. Individual positive end-expiratory pressure settings optimize intraoperative mechanical ventilation and reduce postoperative atelectasis. Anesthesiology 2018;129:1070-81. [Crossref] [PubMed]
- iPROVE Network investigators, Belda J, Ferrando C, et al. The effects of an open-lung approach during one-lung ventilation on postoperative pulmonary complications and driving pressure: a descriptive, multicenter national study. J Cardiothorac Vasc Anesth 2018;32:2665-72.
- Rothen HU, Sporre B, Engberg G, et al. Re-expansion of atelectasis during general anaesthesia: a computed tomography study. Br J Anaesth 1993;71:788-95. [Crossref] [PubMed]
- Young CC, Harris EM, Vacchiano C, et al. Lung-protective ventilation for the surgical patient: international expert panel-based consensus recommendations. Br J Anaesth 2019;123:898-913. [Crossref] [PubMed]


