
Vasopressors in septic shock: which, when, and how much?
Introduction
Septic shock, which is characterized by severe hemodynamic failure, remains a major challenge associated with 30% to 40% hospital mortality, even though important therapeutic advances have been made over the past decades (1). Fluid administration is the first-line therapy, which aims at correcting hypotension and low blood flow related to both relative and absolute hypovolemia (2). However, as hypotension is also induced by sepsis-related systemic vasodilatation, vasopressor therapy is fundamental in septic shock, aiming at correcting the vascular tone depression and then at improving organ perfusion pressure (2).
In spite of recently published expert consensus statements on the use of vasopressors in septic shock (3), controversies still exist on some issues (4) such as, whether very early use of norepinephrine (NE) could improve outcome, whether individualized target of mean arterial pressure (MAP) should be applied, whether vasopressin should be added to NE in the case of refractory shock and whether novels agents such as angiotensin II (AT-II), could become of interest. The aim of this review is to address these questions with reference to recent literature.
Which vasopressor should be considered in septic shock?
A large variety of vasopressors acting on different vascular receptors are available at the bedside (Table 1). Among them, NE remains the most commonly used vasopressor and is recommended as the first-line agent by the Surviving Sepsis Campaign (SSC) experts (2). As a strong α-adrenergic agonist, NE increases blood pressure primarily through its vasoconstrictive properties with little effect on heart rate.
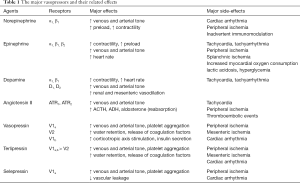
Full table
Vasopressin is recommended as a second-line vasopressor by the SSC (2), despite the absence of proven outcome benefits in large randomized controlled trials (RCTs) comparing vasopressin with NE (5,6). A post-hoc analysis of the VASST trial (5) found that vasopressin was more effective in less severe shock, where adding vasopressin to NE might help reach the initial MAP target faster. The SSC has suggested adding vasopressin to NE (weak recommendation, low quality of evidence) with the intent to rising MAP to target or to decrease NE dosage (2). This could prevent the deleterious consequences of an excessive adrenergic load. A meta-analysis of studies performed in patients with distributive shock showed a lower incidence of atrial fibrillation when vasopressin was added to NE compared to NE alone (7). However, this result was driven by one study performed in post-cardiac surgery (8). When only studies in sepsis were analyzed, no difference in the incidence of atrial fibrillation was found (7). Nevertheless, an individual patient data meta-analysis of four RCTs including 1,453 patients with septic shock showed fewer episodes of atrial fibrillation but more digital ischemia when vasopressin was added to NE compared to NE alone (9). This meta-analysis, which also showed fewer requirements for renal replacement therapy, confirmed the absence of benefit in terms of mortality (9). More recently, a RCT conducted in cancer patients with septic shock, comparing vasopressin to NE as the first-line vasopressor therapy, showed no difference in both cardiac arrhythmia and mortality rate (10). Since the response of adding vasopressin is difficult to predict in terms of potential benefits and toxicity (11), agents that have selective effects on vascular receptors such as terlipressin (12) and selepressin (13) have been evaluated. A large RCT conducted by Liu et al. comparing terlipressin to NE showed no difference in mortality, but terlipressin had more adverse events (12). It is noteworthy that the long half-life of this drug makes it difficult to be used in practice. Selepressin is a highly selective vasopressin V1a receptor agonist. Animal studies using experimental models of septic shock showed that selepressin may improve several hemodynamic variables such as MAP, cardiac output, blood lactate level, fluid volume, and fluid balance, and may even reduce mortality (14). However, in a recent large randomized, double-blind, placebo-controlled, multi-center clinical trial (SEPSIS-ACT), performed in patients with septic shock receiving NE, administration of selepressin, compared with placebo, did not increase vasopressor-free days and ventilator-free days within 30 days (15).
Epinephrine is another second-line vasopressor (2). The SSC experts suggested adding epinephrine to NE (weak recommendation, low quality of evidence), aiming to target MAP and reduce NE requirements. Annane et al. comparing two vasopressor strategies (NE + dobutamine vs. epinephrine) in patients with septic shock reported no differences in both efficacy and safety (16). A recent meta-analysis of 12 RCTs confirmed the equivalence effect between the epinephrine and NE + dobutamine (17). Due to its potent β1-adrenergic effect, epinephrine is more indicated in the presence of cardiac dysfunction than in its absence. Nevertheless, epinephrine may have serious side effects such as tachycardia, tachyarrhythmias and increased blood lactate levels (17), which might be a confounding factor when interpreting lactate as a marker of tissue hypoxia. It has to be noted that in the context of cardiogenic shock, epinephrine was shown to be associated with increased mortality (18).
AT-II is a non-adrenergic vasoconstrictor that is the product of the renin-angiotensin-aldosterone system. A recent RCT (ATHOS-3 trial) showed that AT-II (compared with placebo) effectively increased blood pressure in patients with vasodilatory shock that did not respond to high doses of conventional vasopressors (19). Moreover, a NE sparing-effect was observed for AT-II compared to placebo (19). It is noteworthy that in the ATHOS-3 trial, patients who required less than 5 ng/kg/min to achieve the MAP target had lower levels of endogenous baseline AT-II than their counterparts in the >5 ng/kg/min AT-II subgroup (20). It has been hypothesized that patients sensitive to low levels of AT-II (≤5 ng/kg/min) are more likely to have an AT-II insufficiency (20). Additionally, patients who required ≤5 ng/kg/min AT-II had less severe shock (higher baseline MAP) and lower baseline NE-equivalent doses than those who required >5 ng/kg/min AT-II (20). Accordingly, the beneficial effect seen in these patients may support the concept of using AT-II earlier in the course of disease (20). A recent literature review including 24 studies confirmed the effectiveness of AT-II at increasing blood pressure in all types of shock (21). However, the ATHOS-3 trial was not designed to detect a survival benefit from AT-II, and concerns exist on its safety profile (22). Thus, further large studies are still warranted to clarify those issues.
Dopamine was used in the past as the first-line vasopressor in septic shock. However, observational studies showed an increased risk of tachyarrhythmias and mortality rate (23,24). A large RCT confirmed that dopamine compared with NE was associated with more frequent adverse events (especially tachyarrhythmias) even though no significant difference in mortality was observed (25). Furthermore, a meta-analysis including both randomized and observational trials concluded that dopamine is associated with an increased risk of death compared with NE (26). The latest SSC guidelines recommended dopamine only in the case of bradycardia (2).
Taken all these evidence based on RCTs (NE vs. dopamine, NE + dobutamine vs. epinephrine, NE vs. early vasopressin), NE remains the first-choice vasopressor in patients with septic shock. Vasopressin and epinephrine represent second-line vasopressor therapies and dopamine should be avoided. AT-II might be an alternative in patients with refractory shock, however, safety issues still needed to be clarified in the future.
When to use vasopressors? The earlier, the better
In their recent one-hour bundle publication (27), the SSC recommends applying vasopressors within the first hour when fluid administration is not sufficient to achieve the hemodynamic resuscitation goals. Recently, 34 experts from the European Society of Intensive Care Medicine (ESICM) have recommended starting vasopressors early, before full completion of fluid resuscitation (3). Such a practice is still struggling to be implemented as the majority of intensivists start vasopressors only after complete fluid resuscitation or after checking that preload-independency has been achieved (3).
There are at least five major arguments in favor of the early use of NE.
Firstly, early NE administration could correct hypotension faster and then prevent prolonged severe hypotension. Retrospective data showed that not only the degree but also the duration of hypotension in the initial phase of septic shock are key determinants of patients’ outcome (28,29). A recent retrospective study suggests that the time to achieve a MAP 65 mmHg is shorter when NE is initiated within the first 6 hours of resuscitation compared to a more delayed initiation (30). A recent single-center RCT in septic shock showed that the time to achieve MAP 65 mmHg was significantly shorter when NE was initiated together with fluid infusion compared to when NE was initiated only if 30 mL/kg crystalloids failed to achieve the target MAP (31).
Secondly, early NE infusion could increase cardiac output through several mechanisms. One of them is that NE could increase cardiac preload and reduce preload dependency (32,33) at the early phase of septic shock, by increasing the mean systemic filling pressure and redistributes blood from the abnormally increased unstressed volume to the stressed volume through α-adrenergic-mediated reduction of venous capacitance (34). Importantly, NE could be used to exert a synergistic effect along with fluid infusion and thus enhances the effectiveness of resuscitation. Additionally, NE could increase cardiac output by increasing cardiac contractility (35). In patients with septic shock who have already received adequate fluid administration, Hamzaoui et al. found that early NE administration could increase the left ventricular ejection fraction and other indices of left and right systolic function (35). Two mechanisms can be responsible for this effect: (I) improvement in the coronary perfusion pressure through an increase in the diastolic arterial pressure (DAP), and (II) β1-adrenergic stimulation of the cardiomyocytes since at the early phase of septic shock, the β1-adrenergic receptors are not yet down-regulated (35).
Thirdly, early NE administration may recruit microvessels and improve microcirculation in cases of severe hypotension through an increase in organ perfusion pressure. Accordingly, Georger et al. found significantly improved tissue muscle oxygen saturation along with the increase in MAP by NE from 54 to 77 mmHg in patients with septic shock (36).
Fourthly, early NE administration could prevent harmful fluid overload. It is well-established that positive fluid balance is independently associated with worse outcomes in septic shock (37,38). In this respect, early NE administration could result in a reduced volume of infused fluids as reported by clinical studies (39,40) and thus in lowered risks of fluid overload.
Finally, early NE administration could improve the patients’ outcomes. Two retrospective studies found that the time to initiate NE was an independent factor associated with mortality: the earlier, the better (30,39). A recent single-center RCT including 101 patients with septic shock admitted to the emergency department, compared the impact on survival of early NE initiation (along with fluid administration: early NE group) with late NE initiation (after the failure of 30 mL/kg crystalloids to achieve the MAP target). The NE infusion started after 25 [20–30] and 120 [120–180] min in the early NE and late NE groups, respectively (31). A significant difference in the in-hospital survival in favor of the early NE group was reported (31). However, numerous limitations to that study preclude drawing a definitive conclusion. Another single-center RCT (CENSER study) (41) compared two groups of patients with septic shock: in one group (n=155), NE was administered in the first 2 hours from the onset of resuscitation {93 [72–114] min} while in the other group (n=155), NE was initiated only if fluid resuscitation (at least 30 mL/kg) failed. In the delayed NE group, NE was initiated 192 [150–298] min after the onset of resuscitation (41). The primary endpoint was the shock control at 6 hours from the onset, which was defined as MAP ≥65 mmHg with either urine flow ≥0.5 mL/kg/hour for two consecutive hours or decreased serum lactate ≥10% from baseline (41). The main result was that 76% of patients in the early NE initiation group vs. 48% of patients in the delayed NE initiation group achieved the primary endpoint. The mortality rate (secondary endpoint) was not different but a lower rate of cardiogenic pulmonary edema and of new-onset arrhythmia was found in the early NE group without a difference in ischemic events (41). Taken together, these results suggest that early NE initiation was effective and safe. The results of a much larger ongoing RCT testing early vasopressors in septic shock (CLOVERS) (https://clinicaltrials.gov/ct2/show/NCT03434028) with the primary outcome of 90-day of all-cause mortality are expected to draw more definitive conclusions.
Although there is some evidence that early initiation of NE should be preferred to delayed initiation (i.e., after full completion of fluid resuscitation), there is still some debate about whether NE should be administered at the same time of the commencement of fluid infusion or a little later. A retrospective analysis of 2,849 patients with septic shock suggested starting NE at least 1 hour after starting fluid infusion (42), which was in disagreement with results recently reported from a single-center RCT (see above) (31).
A simple way to identify the patients who need NE urgently is to look at the DAP, as a low DAP is mainly due to a depressed vascular tone, especially in the case of tachycardia (43). Thus, measuring a low DAP in this context should prompt urgent initiation of NE, even in the absence of central venous access (44).
How much should we give NE?
Is there an optimal blood pressure target?
There is a physiological relationship between organ blood flow and MAP, which is generally regarded as the perfusion pressure of most vital organs (Figure 1). Changes in MAP will result in no change in organ blood flow within a physiological “autoregulation” range of MAP. Nevertheless, below a certain critical value of MAP, organ blood flow will decrease along with the decrease in MAP (45). Autoregulation mechanisms are supposed to be impaired in septic shock, making the vital organs more vulnerable in the case of hypotension (46).
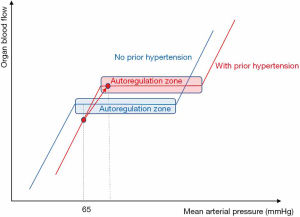
Based on previous data (28,47,48), there is a general agreement on the minimal MAP target (around 65 mmHg) to initially achieve during resuscitation of septic shock (2,3,27,49). By contrast, there is no consensus regarding the MAP value above which a further NE-induced increase in MAP would be harmful (50,51). It could be feared that a high dose of NE to achieve a higher MAP (e.g., 85 mmHg) would lead to excessive vasoconstriction and hence, impairment of microcirculation and ultimately in organ dysfunction. However, there is no robust evidence in favor of such harmful effects (52-57). Retrospective data suggest that a post-resuscitation MAP close or even higher than the pre-admission MAP results in a lower incidence of acute kidney injury (AKI) (52). In addition, several studies strongly suggest that increasing the NE dose to achieve MAP 85 mmHg was better than 65 mmHg in terms of microcirculation (54-57).
A large multicenter RCT (SEPSISPAM) that compared two ranges of MAP targets (65–70 vs. 80–85 mmHg) in patients with septic shock (n=776) did not show any difference in the mortality rate at 28 days (58). Occurrence of serious adverse events did not differ significantly between the two groups. However, the incidence of newly diagnosed atrial fibrillation was higher in the high-target group (7%) than in the low-target group (3%). Of note, a further analysis of the SEPSISPAM trial showed that resuscitation with MAP target between 80 and 85 mmHg was associated with higher arousal level as compared to a MAP target between 65 and 70 mmHg (59). Another RCT showed no difference in mortality and in the risk of cardiac arrhythmias when 60–65 mmHg was compared to 75–80 mmHg as MAP target ranges in unselected patients with septic shock (n=118) (60).
Nevertheless, a higher MAP target might be applied to some subgroups of patients. In the SEPSISPAM trial, benefits in terms of renal function (including the requirement of renal replacement therapy) were reported in the subgroup of patients with chronic hypertension when the higher MAP range was targeted (58). It is noteworthy that in this subgroup of patients, no difference in the incidence of atrial fibrillation was observed between the two MAP target arms (58). Benefits on renal function are consistent with the fact that in the case of chronic hypertension, the organ blood flow/pressure relationship may be rightward shifted so that a MAP value of 65–70 mmHg could not be on the “autoregulation” plateau (Figure 1). In this regard, a task force of the ESICM has suggested a higher than 65 mmHg in patients with prior chronic hypertension (49).
In addition, the organ perfusion pressure is represented by the difference between the upstream pressure and the downstream pressure. The MAP most often reflects the upstream pressure. In the large majority of cases, the downstream pressure is low compared to the MAP so that the MAP reflects the perfusion pressure. However, in the case of high venous pressures, (e.g., congestive heart failure or excessive fluid loading), MAP alone cannot reflect the actual organ perfusion pressure and the difference between MAP and central venous pressure (CVP) should be considered. In this regard, Ostermann et al. showed that the MAP-CVP difference but not MAP alone was associated with an increased risk of AKI (61). A cutoff of 60 mmHg for the MAP-CVP difference was found (61), suggesting that in cases of high CVP, a MAP target higher than 65 mmHg is necessary. Some investigators showed that the higher the CVP the higher the risk of new or persistent episodes of AKI in critically ill patients (62). By analogy, in patients with increased intra-abdominal pressure (IAP), the difference between MAP and IAP should be considered so that a higher MAP target could be necessary to ensure sufficient abdominal organ perfusion, awaiting any decision of abdominal decompression (63).
In summary, individualization of the MAP target is recommended (49). The initial MAP value of 65–70 mmHg in patients without chronic hypertension should be targeted. Targeting a higher MAP is reasonable in patients with chronic hypertension, and in cases of elevated CVP or IAP. In case of doubt or uncertainty, a “NE challenge” can work out the best perfusion pressure to recruit microvessels (64). Skin perfusion markers (65) such as the capillary refill time can be used to assess the effects of the vasopressor challenge as it was done in the ANDROMEDA-SHOCK trial (66,67).
Is there a maximum tolerable dose of NE?
The question of what would be the maximum tolerable dose of NE for achieving the MAP target is still not elucidated. NE at high doses, usually but not consensually defined as ≥1 µg/kg/min is sometimes used as rescue therapy in severe hypotensive patients (68-70). However, there is a “good” consensus among experts to start a second vasopressor in cases of refractory hypotension (3) to prevent the effects of excessive NE load (strategy of “decatecholaminization”). Indeed, high doses of NE may compromise the host immune system and promote bacterial growth (71) and may induce myocardial cell injury and oxidative stress (72). High mortality rates [90% (69) and 80% (68)] were reported in patients who received higher than 1 µg/kg/min NE in retrospective studies. Obviously, this cannot only be attributed to the drug toxicity but can also be explained by the severity of the sepsis-induced vascular damage. Nevertheless, another retrospective study showed a 40% of 28-day survival rate in septic shock patients who received more than 1 µg/kg/min NE for more than 1 hour and the incidence of serious digital or limb necrosis was about 12% in the survivors (70). In addition, a retrospective analysis of a large cohort of patients has suggested that the short-term application of very high doses of catecholamines (NE or epinephrine) does not influence outcomes (73). The results of the two latter studies (70,73) suggest that if the MAP has not yet been reached, the option of testing to increase NE at doses higher than 1 µg/kg/min may be acceptable, especially when vasopressin is not available as it is still the case in some countries. The question of adding low-doses corticosteroids (hydrocortisone) is still a matter of debate (74,75) as its influence on mortality is controversial. However, there is a good consensus among experts to suggest low-dose corticosteroids therapy in cases of refractory shock (3) as there is evidence that its use results in earlier shock reversal in patients with septic shock unresponsive to fluid and vasopressor therapy (76).
Finally, although it may seem paradoxical, early NE administration may be part of a strategy of decatecholaminization. In this regard, Bai et al. showed that compared to delayed NE administration (more than 2 hours after the onset of resuscitation), early NE administration was associated with a decrease in the total dose of NE over the first 24 hours and a shorter NE administration (39). Nevertheless, the strategy of adding vasopressin and maybe AT II in the future is seducing, as it would allow minimizing the side effects of each vasopressor (77). It can be expected that in the future, clinicians will individually select the appropriate combination of vasopressors based on relevant biomarkers indicating which endogenous “agent” and/or which receptor is the most deficient (77).
Conclusions
Today, NE is the first-line vasopressor in septic shock, and epinephrine and vasopressin remain the second-line therapy in cases of refractory shock (2,3). Early NE administration is recommended in order to achieve the initial MAP goal of 65 mmHg faster and to decrease the risk of fluid overload (3). The DAP could be used to identify patients who need NE urgently (43). The optimal MAP target should be individualized (49) as it depends on several factors such as history of chronic hypertension, values of CVP and IAP. In cases of refractory hypotension, increasing NE at high doses (≥1 µg/kg/min) might be an option although there is a current consensus in favor of adding other vasopressors such as vasopressin (2,3).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Glenn Hernández and Guo-wei Tu) for the series “Hemodynamic Monitoring in Critically Ill Patients” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.04.24). The series “Hemodynamic Monitoring in Critically Ill Patients” was commissioned by the editorial office without any funding or sponsorship. OH reports personal fees from Cheetah Medical, outside the submitted work. XM reports personal fees from Getinge/Pulsion and personal fees from Cheetah Medical, outside the submitted work. JLT reports personal fees from Getinge/Pulsion, outside the submitted work. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vincent JL, Jones G, David S, et al. Frequency and mortality of septic shock in Europe and North America: a systematic review and meta-analysis. Crit Care 2019;23:196. [Crossref] [PubMed]
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017;43:304-77. [Crossref] [PubMed]
- Scheeren TWL, Bakker J, De Backer D, et al. Current use of vasopressors in septic shock. Ann Intensive Care 2019;9:20. [Crossref] [PubMed]
- Hernández G, Teboul JL, Bakker J. Norepinephrine in septic shock. Intensive Care Med 2019;45:687-9. [Crossref] [PubMed]
- Russell JA, Walley KR, Singer J, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med 2008;358:877-87. [Crossref] [PubMed]
- Gordon AC, Mason AJ, Thirunavukkarasu N, et al. Effect of early vasopressin vs norepinephrine on kidney failure in patients with septic shock: The VANISH Randomized Clinical Trial. JAMA 2016;316:509-18. [Crossref] [PubMed]
- McIntyre WF, Um KJ, Alhazzani W, et al. Association of vasopressin plus catecholamine vasopressors vs catecholamines alone with atrial fibrillation in patients with distributive shock: A systematic review and meta-analysis. JAMA 2018;319:1889-900. [Crossref] [PubMed]
- Hajjar LA, Vincent JL, Barbosa Gomes Galas FR, et al. Vasopressin versus norepinephrine in patients with vasoplegic shock after cardiac surgery: the VANCS randomized controlled trial. Anesthesiology 2017;126:85-93. [Crossref] [PubMed]
- Nagendran M, Russell JA, Walley KR, et al. Vasopressin in septic shock: an individual patient data meta-analysis of randomised controlled trials. Intensive Care Med 2019;45:844-55. [Crossref] [PubMed]
- Hajjar LA, Zambolim C, Belletti A, et al. Vasopressin versus norepinephrine for the management of septic shock in cancer patients: the VANCS II randomized clinical trial. Crit Care Med 2019;47:1743-50. [Crossref] [PubMed]
- Sacha GL, Lam SW, Duggal A, et al. Predictors of response to fixed-dose vasopressin in adult patients with septic shock. Ann Intensive Care 2018;8:35. [Crossref] [PubMed]
- Liu ZM, Chen J, Kou Q, et al. Terlipressin versus norepinephrine as infusion in patients with septic shock: a multicentre, randomised, double-blinded trial. Intensive Care Med 2018;44:1816-25. [Crossref] [PubMed]
- Russell JA, Vincent JL, Kjolbye AL, et al. Selepressin, a novel selective vasopressin V1A agonist, is an effective substitute for norepinephrine in a phase IIa randomized, placebo-controlled trial in septic shock patients. Crit Care 2017;21:213. [Crossref] [PubMed]
- He X, Su F, Taccone FS, et al. A selective V(1A) receptor agonist, selepressin, is superior to arginine vasopressin and to norepinephrine in ovine septic shock. Crit Care Med 2016;44:23-31. [Crossref] [PubMed]
- Laterre PF, Berry SM, Blemings A, et al. Effect of selepressin vs placebo on ventilator- and vasopressor-free days in patients with septic shock: the SEPSIS-ACT. JAMA 2019;322:1476-85. [Crossref] [PubMed]
- Annane D, Vignon P, Renault A, et al. Norepinephrine plus dobutamine versus epinephrine alone for management of septic shock: a randomised trial. Lancet 2007;370:676-84. [Crossref] [PubMed]
- Belletti A, Nagy A, Sartorelli M, et al. Effect of continuous epinephrine infusion on survival in critically ill patients. A meta-analysis of randomized trials. Crit Care Med 2020;48:398-405. [Crossref] [PubMed]
- Tarvasmaki T, Lassus J, Varpula M, et al. Current real-life use of vasopressors and inotropes in cardiogenic shock - adrenaline use is associated with excess organ injury and mortality. Crit Care 2016;20:208. [Crossref] [PubMed]
- Khanna A, English SW, Wang XS, et al. Angiotensin II for the treatment of vasodilatory shock. N Engl J Med 2017;377:419-30. [Crossref] [PubMed]
- Ham KR, Boldt DW, McCurdy MT, et al. Sensitivity to angiotensin II dose in patients with vasodilatory shock: a prespecified analysis of the ATHOS-3 trial. Ann Intensive Care 2019;9:63. [Crossref] [PubMed]
- Busse LW, McCurdy MT, Ali O, et al. The effect of angiotensin II on blood pressure in patients with circulatory shock: a structured review of the literature. Crit Care 2017;21:324. [Crossref] [PubMed]
- Antonucci E, Agosta S, Sakr Y. Angiotensin II in vasodilatory shock: lights and shadows. Crit Care 2017;21:277. [Crossref] [PubMed]
- Sakr Y, Reinhart K, Vincent JL, et al. Does dopamine administration in shock influence outcome? Results of the Sepsis Occurrence in Acutely Ill Patients (SOAP) Study. Crit Care Med 2006;34:589-97. [Crossref] [PubMed]
- Levy B, Dusang B, Annane D, et al. Cardiovascular response to dopamine and early prediction of outcome in septic shock: a prospective multiple-center study. Crit Care Med 2005;33:2172-7. [Crossref] [PubMed]
- De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010;362:779-89. [Crossref] [PubMed]
- De Backer D, Aldecoa C, Njimi H, et al. Dopamine versus norepinephrine in the treatment of septic shock: a meta-analysis. Crit Care Med 2012;40:725-30. [Crossref] [PubMed]
- Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign Bundle: 2018 Update. Crit Care Med 2018;46:997-1000. [Crossref] [PubMed]
- Varpula M, Tallgren M, Saukkonen K, et al. Hemodynamic variables related to outcome in septic shock. Intensive Care Med 2005;31:1066-71. [Crossref] [PubMed]
- Vincent JL, Nielsen ND, Shapiro NI, et al. Mean arterial pressure and mortality in patients with distributive shock: a retrospective analysis of the MIMIC-III database. Ann Intensive Care 2018;8:107. [Crossref] [PubMed]
- Colon Hidalgo D, Patel J, Masic D, et al. Delayed vasopressor initiation is associated with increased mortality in patients with septic shock. J Crit Care 2020;55:145-8. [Crossref] [PubMed]
- Elbouhy MA, Soliman M, Gaber A, et al. Early use of norepinephrine improves survival in septic shock: earlier than early. Arch Med Res 2019;50:325-32. [Crossref] [PubMed]
- Hamzaoui O, Georger JF, Monnet X, et al. Early administration of norepinephrine increases cardiac preload and cardiac output in septic patients with life-threatening hypotension. Crit Care 2010;14:R142. [Crossref] [PubMed]
- Monnet X, Jabot J, Maizel J, et al. Norepinephrine increases cardiac preload and reduces preload dependency assessed by passive leg raising in septic shock patients. Crit Care Med 2011;39:689-94. [Crossref] [PubMed]
- Persichini R, Silva S, Teboul JL, et al. Effects of norepinephrine on mean systemic pressure and venous return in human septic shock. Crit Care Med 2012;40:3146-53. [Crossref] [PubMed]
- Hamzaoui O, Jozwiak M, Geffriaud T, et al. Norepinephrine exerts an inotropic effect during the early phase of human septic shock. Br J Anaesth 2018;120:517-24. [Crossref] [PubMed]
- Georger JF, Hamzaoui O, Chaari A, et al. Restoring arterial pressure with norepinephrine improves muscle tissue oxygenation assessed by near-infrared spectroscopy in severely hypotensive septic patients. Intensive Care Med 2010;36:1882-9. [Crossref] [PubMed]
- Boyd JH, Forbes J, Nakada T-a, et al. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med 2011;39:259-65. [Crossref] [PubMed]
- Vincent JL, Sakr Y, Sprung CL, et al. Sepsis occurrence in acutely ill patients investigators. sepsis in european intensive care units: results of the SOAP study. Crit Care Med 2006;34:344-53. [Crossref] [PubMed]
- Bai X, Yu W, Ji W, et al. Early versus delayed administration of norepinephrine in patients with septic shock. Crit Care 2014;18:532. [Crossref] [PubMed]
- Macdonald SPJ, Keijzers G, Taylor DM, et al. Restricted fluid resuscitation in suspected sepsis associated hypotension (REFRESH): a pilot randomised controlled trial. Intensive Care Med 2018;44:2070-8. [Crossref] [PubMed]
- Permpikul C, Tongyoo S, Viarasilpa T, et al. Early Use of Norepinephrine in Septic Shock Resuscitation (CENSER). A Randomized Trial. Am J Respir Crit Care Med 2019;199:1097-105. [Crossref] [PubMed]
- Waechter J, Kumar A, Lapinsky SE, et al. Interaction between fluids and vasoactive agents on mortality in septic shock: a multicenter, observational study. Crit Care Med 2014;42:2158-68. [Crossref] [PubMed]
- Hamzaoui O, Teboul JL. Importance of diastolic arterial pressure in septic shock: PRO. J Crit Care 2019;51:238-40. [Crossref] [PubMed]
- Delaney A, Finnis M, Bellomo R. Initiation of vasopressor infusions via peripheral versus central access in patients with early septic shock: A retrospective cohort study. Emerg Med Australas 2020;32:210-9. [Crossref] [PubMed]
- Johnson PC. Autoregulation of blood flow. Circ Res 1986;59:483-95. [Crossref] [PubMed]
- Pfister D, Siegemund M, Dell-Kuster S, et al. Cerebral perfusion in sepsis-associated delirium. Crit Care 2008;12:R63. [Crossref] [PubMed]
- LeDoux D, Astiz ME, Carpati CM, et al. Effects of perfusion pressure on tissue perfusion in septic shock. Crit Care Med 2000;28:2729-32. [Crossref] [PubMed]
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001;345:1368-77. [Crossref] [PubMed]
- Cecconi M, De Backer D, Antonelli M, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med 2014;40:1795-815. [Crossref] [PubMed]
- Khanna AK. Defending a mean arterial pressure in the intensive care unit: Are we there yet? Ann Intensive Care 2018;8:116. [Crossref] [PubMed]
- Lesur O, Delile E, Asfar P, et al. Hemodynamic support in the early phase of septic shock: a review of challenges and unanswered questions. Ann Intensive Care 2018;8:102. [Crossref] [PubMed]
- Moman RN, Ostby SA, Akhoundi A, et al. Impact of individualized target mean arterial pressure for septic shock resuscitation on the incidence of acute kidney injury: a retrospective cohort study. Ann Intensive Care 2018;8:124. [Crossref] [PubMed]
- Dubin A, Pozo MO, Casabella CA, et al. Increasing arterial blood pressure with norepinephrine does not improve microcirculatory blood flow: a prospective study. Crit Care 2009;13:R92. [Crossref] [PubMed]
- Jhanji S, Stirling S, Patel N, et al. The effect of increasing doses of norepinephrine on tissue oxygenation and microvascular flow in patients with septic shock. Crit Care Med 2009;37:1961-6. [Crossref] [PubMed]
- Thooft A, Favory R, Salgado DR, et al. Effects of changes in arterial pressure on organ perfusion during septic shock. Crit Care 2011;15:R222. [Crossref] [PubMed]
- Fiorese Coimbra KT, de Freitas FGR, Bafi AT, et al. Effect of increasing blood pressure with noradrenaline on the microcirculation of patients with septic shock and previous arterial hypertension. Crit Care Med 2019;47:1033-40. [Crossref] [PubMed]
- Kazune S, Caica A, Luksevics E, et al. Impact of increased mean arterial pressure on skin microcirculatory oxygenation in vasopressor-requiring septic patients: an interventional study. Ann Intensive Care 2019;9:97. [Crossref] [PubMed]
- Asfar P, Meziani F, Hamel JF, et al. High versus low blood-pressure target in patients with septic shock. N Engl J Med 2014;370:1583-93. [Crossref] [PubMed]
- Jouan Y, Seegers V, Meziani F, et al. Effects of mean arterial pressure on arousal in sedated ventilated patients with septic shock: a SEPSISPAM post hoc exploratory study. Ann Intensive Care 2019;9:54. [Crossref] [PubMed]
- Lamontagne F, Meade MO, Hebert PC, et al. Higher versus lower blood pressure targets for vasopressor therapy in shock: a multicentre pilot randomized controlled trial. Intensive Care Med 2016;42:542-50. [Crossref] [PubMed]
- Ostermann M, Hall A, Crichton S. Low mean perfusion pressure is a risk factor for progression of acute kidney injury in critically ill patients-A retrospective analysis. BMC Nephrol 2017;18:151. [Crossref] [PubMed]
- Legrand M, Dupuis C, Simon C, et al. Association between systemic hemodynamics and septic acute kidney injury in critically ill patients: a retrospective observational study. Crit Care 2013;17:R278. [Crossref] [PubMed]
- Malbrain ML, De Laet IE, De Waele JJ, et al. Intra-abdominal hypertension: definitions, monitoring, interpretation and management. Best Pract Res Clin Anaesthesiol 2013;27:249-70. [Crossref] [PubMed]
- Legrand M, De Backer D, Dépret F, et al. Recruiting the microcirculation in septic shock. Ann Intensive Care 2019;9:102. [Crossref] [PubMed]
- Hariri G, Joffre J, Leblanc G, et al. Narrative review: clinical assessment of peripheral tissue perfusion in septic shock. Ann Intensive Care 2019;9:37. [Crossref] [PubMed]
- Hernández G, Cavalcanti AB, Ospina-Tascón G, et al. Early goal-directed therapy using a physiological holistic view: the ANDROMEDA-SHOCK-a randomized controlled trial. Ann Intensive Care 2018;8:52. [Crossref] [PubMed]
- Hernández G, Ospina-Tascón GA, Damiani LP, et al. Effect of a resuscitation strategy targeting peripheral perfusion status vs serum lactate levels on 28-day mortality among patients with septic shock: the ANDROMEDA-SHOCK randomized clinical trial. JAMA 2019;321:654-64. [Crossref] [PubMed]
- Brown SM, Lanspa MJ, Jones JP, et al. Survival after shock requiring high-dose vasopressor therapy. Chest 2013;143:664-71. [Crossref] [PubMed]
- Martin C, Medam S, Antonini F, et al. Norepinephrine: not too much, too long. Shock 2015;44:305-9. [Crossref] [PubMed]
- Auchet T, Regnier MA, Girerd N, et al. Outcome of patients with septic shock and high-dose vasopressor therapy. Ann Intensive Care 2017;7:43. [Crossref] [PubMed]
- Stolk RF, van der Poll T, Angus DC, et al. Potentially inadvertent immunomodulation: norepinephrine use in sepsis. Am J Respir Crit Care Med 2016;194:550-8. [Crossref] [PubMed]
- Hartmann C, Radermacher P, Wepler M, et al. Non-hemodynamic effects of catecholamines. Shock 2017;48:390-400. [Crossref] [PubMed]
- Kastrup M, Braun J, Kaffarnik M, et al. Catecholamine dosing and survival in adult intensive care unit patients. World J Surg 2013;37:766-73. [Crossref] [PubMed]
- Venkatesh B, Finfer S, Cohen J, et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med 2018;378:797-808. [Crossref] [PubMed]
- Annane D, Renault A, Brun-Buisson C, et al. Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med 2018;378:809-18. [Crossref] [PubMed]
- Rygård SL, Butler E, Granholm A, et al. Low-dose corticosteroids for adult patients with septic shock: a systematic review with meta-analysis and trial sequential analysis. Intensive Care Med 2018;44:1003-16. [Crossref] [PubMed]
- Teboul JL, Duranteau J, Russell JA. Intensive care medicine in 2050: vasopressors in sepsis. Intensive Care Med 2018;44:1130-2. [Crossref] [PubMed]


