
Systemic anti-cancer treatment in malignant ovarian germ cell tumours (MOGCTs): current management and promising approaches
Background
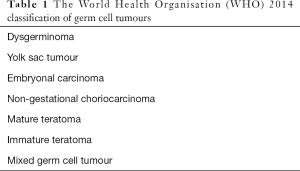
Non-epithelial ovarian cancers are histologically and clinically distinct rare tumours, including germ cell tumours and sex-cord stromal tumours, each of which is subdivided into several histological sub-types (1). Female malignant ovarian germ cell tumours (MOGCTs) accounts for only 2–5% of all ovarian malignancies (2). MOGCTs can occur in all age groups, with peak incidence in young girls aged 15 to 19 years old (3). MOGCTs are histologically heterogeneous neoplasms arising from the primordial germ cells of the embryonal gonad. Most common MOGCT histology subtypes include dysgerminomas, immature teratomas, yolk sac tumours, and mixed germ cell tumours. Other less frequent MOGCTs include embryonal carcinomas, choriocarcinomas, and struma ovarii, the latter defined as mature ovarian teratoma containing 50% or more thyroid tissue (4,5) (Table 1). MOGCTs classically manifest clinically with palpable mass and/or abdominal distension, lower abdominal pain, urinary symptoms, and feeling of fullness in the pelvis. According to the histologic subtype, MOGCTs can frequently cause serum tumour markers elevation. Such tumour markers include lactate dehydrogenase (LDH), alpha-fetoprotein (AFP), and human chorionic gonadotropin (HCG). In addition to clinical findings and conventional histological material, immunohistochemical markers and fluorescent in situ hybridisation (FISH) are useful for the establishment of the diagnosis. SALL4 and OCT4 are widely used, whereas SOX2 can be used to diagnose embryonal carcinoma and neuroectodermal tumours of teratomatous origin (5-7). MOGCTs share many characteristics with male testicular germ cell tumours, though MOGCTs can frequently spread to the peritoneum, showing the same pattern as the epithelial ovarian cancer. After appropriate multimodal management, the prognosis is quite good, with 5-year survival exceeding 85% (5-8). The majority of MOGCTs are diagnosed while confined to the ovary (stage I), and these early-stage tumours can be treated with surgery alone. Surgical staging represents the first step in the management of MOGCTs. Surgical procedures include exploratory laparotomy, peritoneal washing for cytology, omental biopsy, unilateral oophorectomy, and selective removal of enlarged lymph nodes. Among MOGCTs, dysgerminomas have a high risk of nodal spread. There is no consensus about the role of systematic lymphadenectomy. Nevertheless, the omission of staging peritoneal procedures seems to increase the recurrence rate without affecting survival rates. Nodal debulking surgery is only required in cases of residual disease following adjuvant chemotherapy. Bilateral salpingo-oophorectomy and hysterectomy can be considered in women who do not wish to preserve the ability to have children in the future (6,9). Fertility preservation is an important issue because MOGCTs typically affects young women of child-bearing potential. Fertility-sparing surgery is the mainstay of treatment in early-stage disease and can be also proposed in advanced stages after careful discussion with young patients who desire pregnancy (10). Cisplatin-based chemotherapy with surgery can provide good outcome even in the presence of advanced or incompletely resected disease (11). The role of second-look surgery is not entirely clear, but resection of residual teratoma represents the main indication (12). Centralisation of care can further improve the clinical outcomes. However, clinical trial data are severely missing due to the rarity of these malignancies, and progress has lagged behind other tumours. MOGCTs are largely equivalent to those originating from male germ cells with regards to many aspects, and majority of data on MOGCT chemotherapy are extrapolated from male germ cell tumours. Available literature mainly consists of retrospective data. Indeed, existing combination chemotherapies in use for MOGCTs are similar to standard regimens for male germ cell tumours (6,11). This review will focus on current chemotherapy options in the treatment of non-paediatric MOCGTs, highlighting also the scenario with regards to precision medicine and ongoing trials.
Full table
Current standard post-operative and pre-operative management with chemotherapy
Following surgery, cisplatin-based adjuvant chemotherapy is the standard of care in MOGCTs. However, early-stage dysgerminoma (stage IA and IB) and stage IA grade 1 (G1) immature teratoma are usually spared from adjuvant chemotherapy. The indication of adjuvant chemotherapy remains controversial in stage IA G2–G3 and stage IB–IC, whereas all patients with yolk sac tumour histology are offered adjuvant chemotherapy (7). The International Federation of Gynecology and Obstetrics (FIGO) classification is commonly adopted for MOGCT staging (13). In addition to advanced stage, other prognostic factors include age >45 years, incomplete surgical resection, and yolk sac tumour histology (7,14). Limited prospective trials have evaluated the use of BEP (bleomycin, etoposide and cisplatin) and other platinum-based combinations in the adjuvant setting, showing 5-year survival rates of 85% or higher, according to the stage at diagnosis (6,7,11). The PVB (cisplatin, vinblastine and bleomycin) regimen was widely used in the past, but it has been largely replaced by more tolerable combinations since the introduction of etoposide. BEP is the most popular regimen while EP (etoposide and cisplatin) can be considered in patients ineligible to bleomycin (7,11). Advanced age, poor kidney function, pulmonary comorbidity and smoking history are traditionally regarded as relative contraindications to bleomycin due to increased risk of bleomycin-induced pneumonitis (15,16). Some guidelines recommend obtaining baseline pulmonary function tests (PFTs) before Bleomycin commencement and repeat as clinically indicated thereafter. However, many institutions do not routinely perform baseline PFTs and mainly rely on patient’s selection in order to avoid drug-related pulmonary injury (17). In 1996, Bower et al. (18) published data of 59 subjects treated with POMB/ACE (cisplatinum, vincristine, methotrexate, bleomycin, actinomycin D, cyclophosphamide and etoposide). After a median follow-up of 7.7 years, the 3-year survival was 87.8%. Nevertheless, POMB/ACE did not demonstrate to be superior to BEP in male populations and is regarded as the regimen of choice for extensive metastatic and recurrent MOGCTs in selected institutions only (6,19). Williams et al. (20) enrolled around 40 patients with completely resected stage IB–III dysgerminoma to receive a regimen containing carboplatin plus etoposide for 3 cycles. The regimen was well tolerated, sparing those women from cisplatin-related peripheral neuropathy and hearing loss. After a median follow-up of around 7.8 years, none suffered from MOGCT relapse. Therefore, adjuvant carboplatin can be considered in cisplatin-ineligible women as an alternative regimen. Three cycles of BEP are currently recommended for early-stage radically resected MOGCTs while four cycles are usually adopted in more advanced stages (11). BEP chemotherapy was initially developed as a 5-day regimen requiring a brief inpatient stay in the hospital. A more convenient 3-day schedule was subsequently developed for outpatient administration. Evidence on BEP chemotherapy in MOGCT is mainly extrapolated from testicular cancer, but a large Taiwanese retrospective case series of patients treated with post-operative 3-day BEP chemotherapy reported good safety and efficacy profile in female MOGCTs (21). Advanced MOGCTs often exhibit an aggressive behaviour and require expedited workup and prompt delivery of chemotherapy. The role of preoperative chemotherapy in MOGCTs is not well defined and has not been investigated in randomised trials. It is usually offered to women with advanced stage and significant tumour burden not amenable to upfront fertility-sparing surgery (6,7,11). A weekly EP induction regimen can be adopted in the presence of poor performance status in order to obtain an initial response before proceeding with standard treatment (22). Standard preoperative chemotherapy regimens do not differ from those in the post-operative setting. In large case series published, the number of chemotherapy cycles ranged from one to three, according to treatment response and single institutional practice (11,23,24). Treatment of MOGCTs with brain metastasis at presentation can be challenging, but it usually involves the use of platinum-based chemotherapy. Upfront and/or additional radiotherapy and/or neurosurgery can be offered in particular clinical scenarios, according to individual decisions. Whenever possible, stereotactic radiotherapy is usually preferred over whole brain radiotherapy in order to achieve improved disease control with minimal effect on brain tissue, resulting in better cognitive outcomes. The use of multimodality treatment is more frequently adopted in MOGCTs relapsed with brain metastases, as chemo-resistance can be commonly observed in this setting (7,25). Following radical treatment, women are routinely followed-up with standardized surveillance protocol for either 5 or 10 years, according to the risk of relapse and institutional practice. Clinical examination, chest x-rays, computed tomography, magnetic resonance imaging, and tumour markers are the investigations regularly adopted by surveillance protocols in order to detect potentially curable relapse from MOGCTs (6,7). Additionally, MOGCT on follow-up should be assessed for late effects from chemotherapy such as increased risk of secondary malignancies and cardiovascular complications, sensory peripheral neuropathy, hearing loss, chronic fatigue, impaired fertility, interstitial pulmonary fibrosis, chronic kidney disease, and nontraumatic osteonecrosis (26,27). Relevant psychological and psychosexual sequelae from anti-cancer treatment can also require specialist intervention. Involvement of dedicated teenage and young adult specialist team is strongly recommended, as subjects in the transition between adolescence and adulthood are often unable to cope with the distress provoked by the novelty of illness, side effects from treatments, and hospital admissions (6,27).
Management of recurrent/refractory disease
Over the past decades, the use of validated surgery and chemotherapy protocols has led to high rates of remission in patients diagnosed with MOGCTs. However, around 15–20% with advanced disease at presentation will experience refractory disease. Whilst the management of recurrent or refractory disease is mainly derived from evidence extrapolated from the male population, women who relapse with MOGCT have poorer outcomes when compared with testicular germ cell tumours (6,7). The majority of recurrences from MOGCTs occur within the first two years of treatment and are mainly detected by rise in serum tumour markers and/or abnormal surveillance imaging. Due to the rarity of the disease and paucity of data, salvage treatment is quite complex and relies on prognostic factors, patient’s preference and single institutional experience (7,28). Patients with late relapses and/or growing teratoma syndrome can be managed with surgery alone. However, potentially radical treatment of early and late relapses very often involves the use of platinum-based chemotherapy. Management of recurrent MOGCTs should be restricted to high-volume institutions with experienced surgeons and, whenever possible, high-dose chemotherapy (HDCT) availability (6,7,11). Retrospective data show that long term survival was achieved in only 10% of recurrent MOGCTs treated with conventional-dose salvage chemotherapy. Yolk sac histology can be frequently seen at recurrence. Most commonly used conventional dose chemotherapy regimens for recurrent MOGCTs include TIP (paclitaxel, ifosfamide, cisplatin), TE/TP (paclitaxel and etoposide/paclitaxel and cisplatin), and Gem-TIP (gemcitabine added to TIP) (6,7). Treatment with palliative intent can also include GemOx (gemcitabine and oxaliplatin), VAC (vincristine/actinomycin D/cyclophosphamide), paclitaxel/gemcitabine, and other chemotherapeutic agents given as single agent or in combination (11,28,29). Retrospective data suggest that HDCT plus peripheral blood stem rescue may be superior to conventional-dose chemotherapy as front-line therapy for recurrent testicular germ cell tumour (30). The role of HDCT in MOGCTs still remains unclear, though some retrospective evidence suggests that HDCT can provide long term remission in around 30%-40% of patients (11,29). The ongoing phase 3 TIGER trial (31) will definitively assess the role of HDCT in relapse male germ cell tumours by comparing with the standard conventional-dose chemotherapy. Extrapolation of results from the TIGER trial will likely impact on MOGCT treatment algorithm.
Clinical trials and novel approaches
Optimising treatment selection and reducing the dose of chemotherapy in good prognostic groups and improving the outcomes in recurrent and refractory disease represent important objectives for the management of MOGCT in the foreseeable future. Guidelines for the management of adult MOGCT recommend BEP chemotherapy or other intensive chemotherapy regimens, resulting to potentially debilitating toxicity in long term survivors (24,27). Late toxicities are frequently underreported in clinical trials due to short follow-up, but some studies show that prevalence in germ cell tumour survivors can be relatively high. The long-term side effects of platinum-based chemotherapy for MOGCT are usually mild in nature but are largely irreversible and the severity is related to the total amount of chemotherapy received (24,32). Several single-nucleotide polymorphisms identified individuals more likely to develop cisplatin-related toxicities when compared with wild-type subjects. Variability in DNA repairing mechanisms, key transduction pathways and metabolism mediated via glutathione transferase are major areas of research in this field. Using a pharmacogenetic approach, a clinically meaningful genetic panel may be generated in the future to better predict both cisplatin-related efficacy and toxicity outcomes in various tumour types (33,34). Pediatric germ cell tumours are successfully treated replacing cisplatin with the more tolerable carboplatin and administering lower doses of bleomycin (24). Investigators from Shandong University are conducting a prospective trial aiming at adopting the standard chemotherapy regimen in use for ovarian carcinoma. In this phase 3 trial (35), over 100 MOGCT patients are being treated with either carboplatin plus paclitaxel or the standard BEP chemotherapy as adjuvant therapy. Progression-free survival (PFS) was selected as primary endpoint, whereas tolerability, overall survival (OS) and overall response rate in measurable disease will be evaluated as secondary endpoints. A multicentre French trial (36) is currently recruiting subjects affected by MOGCT and other rare non-epithelial ovarian tumours in complete remission after surgery and/or chemotherapy in order to investigate the sequelae deriving from the disease and its treatment. The trial consists of a 2-step case control study to assess chronic fatigue and quality of life, and chemotherapy-related late effects. Almost 500 study participants will complete several self-questionnaires exploring quality of life and living conditions. Cardiac, pulmonary, auditory and biological assessment will be also carried out. The study enrolment is restricted to subjects in remission more than 2 years after initial surgery or salvage treatment. The most promising approaches derive from testicular cancer trial protocols, some of them allowing enrolment of female MOGCT patients. The ANZUP 1302 (37) is an international phase 3 randomised trial of accelerated versus standard BEP chemotherapy for adult and paediatric male and female patients with metastatic germ cell tumour. The investigators aim at answering the question whether a 2-weekly BEP chemotherapy regimen would provide superior outcomes when compared with the standard 3-weekly schedule. Accelerating chemotherapy by administering the same dose of chemotherapy with shorter cycles can overcome the rapid regrowth of shrinking germ cell tumours during cytotoxic treatment, as suggested by the increased cure rate observed in other cancer types when accelerated schedules are administered (38). Key inclusion criteria of the ANZUP 1302 trial include age between 11 and 45 years, and intermediate or poor-risk definition as per International Germ Cell Cancer Collaborative Group (IGCCCG), which is commonly in use for testicular cancers (39). The experimental treatment for adults participants is the accelerated BEP schedule given as bleomycin 30,000 international units (IU) intravenous (IV) weekly on day 1 and 8, etoposide 100 mg/m2 on days 1–5 and cisplatin 20 mg/m2 on days 1–5 every 2 weeks for 4 cycles, followed by single agent bleomycin 30,000 IU IV once a week for a further 4 weeks to a total of 12 doses of bleomycin. This schedule was previously tested in a single arm phase 2 trial including 43 eligible testicular cancer patients, showing promising tolerability and efficacy data (40). The rate of febrile neutropenia was 12% while the 2-year PFS was 50% and 94% for poor- and intermediate prognosis patients, respectively (36). A large multicentre international phase 3 trial (41) is being conducted to evaluate the role of active surveillance and carboplatin-based chemotherapy in paediatric and adult germ cell tumours. Enrollment of MOGCT patients is allowed in this trial. According to the specific risk stratification criteria set by the protocol, subjects will be either observed with active surveillance or treated with BEP chemotherapy or the combination bleomycin, carboplatin and etoposide. The experimental arm consists of bleomycin IV on days 1, 8, and 15, etoposide IV on days 1–5, and carboplatin IV day 1. Both treatments repeat every 21 days for up to 3–4 cycles according to the risk stratification and in the absence of evidence of progressive disease or unacceptable toxicity. Patient will undergo a long follow-up to assess OS and PFS as primary outcome measures. Other endpoints include assessing the percentage of individuals with significant hearing loss and peripheral neuropathy. Huddart et al. (42) randomised 89 male patients with IGCCCG poor risk to receive either standard BEP chemotherapy or CBOP/BEP (carboplatin/bleomycin/vincristine/cisplatin/BEP) regimen in a phase 2 trial. The experimental arm was intended to be given for six cycles over 15 weeks. Primary end point was favourable response rate (FRR), defined as complete response or partial response with normal tumour markers. After a median follow-up of 58 months, the primary endpoint was met, as FRR was 74% [90% confidence interval (CI), 61–85%] for CBOP/BEP, and 61% for BEP (90% CI, 48–73%), respectively. However, the CBOP/BEP schedule resulted in increased toxicity, largely due to higher rate of haematological side effects. The efficacy data are quite promising but require confirmation in an international phase 3 trial. Table 2 summarises the main ongoing chemotherapy phase 3 trials that may change the standard of care of germ cell tumours over the coming years. Some efforts have been made to molecularly characterize MOGCTs, leading to the recognition of typical molecular characteristics for each of the histological subtypes, including ploidy indices, DNA copy number changes, and specific pattern of expression of mRNA, miRNA, and proteins. Unlike many somatic malignancies, MOGCTs and the male counterpart rarely develop genetic mutations in well-known oncogenes or tumour-suppressor genes, resulting in less suitability to precision medicine approach (43). Due to the low incidence and high cure rate, targeted therapies are more commonly tested in platinum-refractory germ cell tumours, but the results have been quite disappointing (44). Subset of germ cell tumours can acquire KRAS-activating mutations and other genetic alterations such as BRCA1/2, KIT, and MAPK, but the efficacy of targeted therapy and genomic features contributing to chemoresistance still remain to be elucidated (43). So far, no molecularly targeted treatment has shown clinically meaningful activity in unselected patient populations across several clinical trials, though tumour marker stabilization or short-term treatment responses have been described in some patients treated with targeted agents such as sunitinib, brentuximab vedotin or imatinib. Targeted trials based on molecular selection of patients have not been performed selectively in MOCGT (44,45). Preliminary data suggest that cyclin-dependent kinases 4/6 inhibitors may delay disease-related major clinical events in unresectable mature teratoma (46). KIT tyrosine kinase expression is crucial in the development of normal germ cells. Ovarian dysgerminomas and majority of testicular seminomas frequently present KIT mutations and overexpression, making imatinib and other tyrosine kinase inhibitors of the same class potentially useful drugs to counteract chemoresistance to cisplatin (47). However, phase 2 trials in both unselected and KIT-positive germ cell tumours failed to show significant anti-tumour activity of imatinib. The lack of significant clinical benefit in germ cell tumours is probably explained by the finding of mutations frequently occurring in the KIT enzymatic site, thus conferring reduced sensitivity to imatinib blockade. Indeed, mutations involving the kinase domain of KIT confer resistance to imatinib by affecting the binding of the drug while wild-type KIT and KIT with mutations at regulatory sites tend to confer sensitivity to imatinib (48,49). Immunotherapy offers remarkable opportunities of long survival by producing sustained remissions in metastatic cancers. The role of immune check point inhibitors in germ cell tumours still needs to be elucidated. PD-L1 overexpression in testicular germ cell tumours has been recently described, but the preliminary results shown by immune check point inhibitors in molecularly unselected germ cell tumours were not promising. Several immune-oncology combinations are being tested in clinical trials conducted on germ cell tumours (44,50). Recently, great enthusiasm has been generated by the documentation of impressive clinical responses of various solid and haematological tumour types in trials investigating genetically modified T cells expressing chimeric antigen receptors (CAR T-cell therapy). CAR T-cell therapy involves the removal of a patient’s own T cells and then transfecting them with the CAR gene, and subsequent T cell expansion and reinfusion them into the patient. The success of CAR T-cell therapy has been observed primarily in chemo-resistant haematological cancers, though this treatment modality is now starting to produce valuable outcomes in solid malignancies. Sustained clinical responses can be frequently observed in heavily pre-treated tumours, regardless of sensitivity to prior chemotherapy (51). CD30 is a member of the tumor necrosis factor receptor superfamily and is expressed on activated lymphocytes and a few other normal cells, including the germ cells. Preliminary in vivo data show that CD30-redirected CAR T-cell therapy might be useful as immunotherapy for embryonal carcinomas arising from the testis (52). Though extremely rare, embryonal carcinoma can originate from the ovaries and invariably express CD30 on their cell membranes. Targetable biomarkers for CAR T-cell therapy are expanding, but tumour rarity and heterogeneity are currently limiting the development of CAR T-cell therapy in MOGCT (43,53).

Full table
Conclusions
MOGCTs are rare entities with limited available literature. Multidisciplinary management and centralisation of care are crucial to achieve optimal outcomes. Fertility-sparing surgery and platinum-based regimens are the cornerstone of treatment, leading to high cure rates and acceptable quality of life. Prognosis of recurrent disease is dismal, calling for efforts to perform valuable multicentre clinical trials. Current chemotherapy protocols and algorithm for MOGCT largely rely on evidence extrapolated from testicular germ cell tumours. Studies aimed at better assessing the role of active surveillance and alternative chemotherapy in earlier stages are also warranted. The incorporation of targeted therapy and immunotherapy into the standard of care of MOGCT still requires more efforts.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Stergios Boussios and Nicholas Pavlidis) for the series “Ovarian Cancer: State of the Art and Perspectives of Clinical Research” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.04.15). The series “Ovarian Cancer: State of the Art and Perspectives of Clinical Research” was commissioned by the editorial office without any funding or sponsorship. SB served as the unpaid Guest Editor of the series. Dr. MM reports other from AstraZeneca, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Boussios S, Moschetta M, Zarkavelis G, et al. Ovarian sex-cord stromal tumours and small cell tumours: Pathological, genetic and management aspects. Crit Rev Oncol Hematol 2017;120:43-51. [Crossref] [PubMed]
- Boussios S, Attygalle A, Hazell S, et al. Malignant Ovarian Germ Cell Tumors in Postmenopausal Patients: The Royal Marsden Experience and Literature Review. Anticancer Res 2015;35:6713-22. [PubMed]
- Smith HO, Berwick M, Verschraegen CF, et al. Incidence and survival rates for female malignant germ cell tumors. Obstet Gynecol 2006;107:1075-85. [Crossref] [PubMed]
- Boussios S, Zarkavelis G, Seraj E, et al. Non-epithelial Ovarian Cancer: Elucidating Uncommon Gynaecological Malignancies. Anticancer Res 2016;36:5031-42. [Crossref] [PubMed]
- Prat J, Cao D, Carinelli S et al. Teratoma (Chapter 1: Tumours of the ovary). In: Kurman RJ, Carcangiu ML, Herrington CS, et al. editors. WHO Classification of Tumours of Female Reproductive Organs. 4th edition. IARC: Lyon, 2014:57-62.
- Tidy JA, Coleman RE, Harvey RA, et al. Management of female malignant ovarian germ cell tumours. RCOG Scientific Impact Paper No. 52. November 2016. Available online: https://www.rcog.org.uk/globalassets/documents/guidelines/scientific-impact-papers/sip_52.pdf
- Ray-Coquard I, Morice P, Lorusso D, et al. Non-epithelial ovarian cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv1-18. [Crossref]
- Siverino RO, Uccello A, Giunta ML, et al. Non-Seminomatous Germ Cell Tumor Metastasis to the Jaw: An Imaging Case Report. Iran J Radiol 2016;13:e27812. [Crossref] [PubMed]
- Liu Q, Ding X, Yang J, et al. The significance of comprehensive staging surgery in malignant ovarian germ cell tumors. Gynecol Oncol 2013;131:551-4. [Crossref] [PubMed]
- Di Tucci C, Casorelli A, Morrocchi E, et al. Fertility management for malignant ovarian germ cell tumors patients. Crit Rev Oncol Hematol 2017;120:34-42. [Crossref] [PubMed]
- Simone CG, Markham MJ, Dizon DS. Chemotherapy in ovarian germ cell tumors: A systematic review. Gynecol Oncol 2016;141:602-7. [Crossref] [PubMed]
- Zagamé L, Pautier P, Duvillard P, et al. Growing teratoma syndrome after ovarian germ cell tumors. Obstet Gynecol 2006;108:509-14. [Crossref] [PubMed]
- Prat J. FIGO Committee on Gynecologic Oncology. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet 2014;124:1-5. [Crossref] [PubMed]
- Mangili G, Sigismondi C, Gadducci A, et al. Outcome and risk factors for recurrence in malignant ovarian germ cell tumors: a MITO-9 retrospective study. Int J Gynecol Cancer 2011;21:1414-21. [Crossref] [PubMed]
- O'Sullivan JM, Huddart RA, Norman AR, et al. Predicting the risk of bleomycin lung toxicity in patients with germ-cell tumours. Ann Oncol 2003;14:91-6. [Crossref] [PubMed]
- Sleijfer S. Bleomycin-induced pneumonitis. Chest 2001;120:617-24. [Crossref] [PubMed]
- Watson RA, De La Peña H, Tsakok MT, et al. Development of a best-practice clinical guideline for the use of bleomycin in the treatment of germ cell tumours in the UK. Br J Cancer 2018;119:1044-51. [Crossref] [PubMed]
- Bower M, Fife K, Holden L, et al. Chemotherapy for ovarian germ cell tumours. Eur J Cancer 1996;32A:593-7. [Crossref] [PubMed]
- Husband DJ, Green JA. POMB/ACE chemotherapy in non-seminomatous germ cell tumours: outcome and importance of dose intensity. Eur J Cancer 1992;28:86-91. [Crossref] [PubMed]
- Williams S, Blessing JA, Liao SY, et al. Adjuvant therapy of ovarian germ cell tumors with cisplatin, etoposide, and bleomycin: a trial of the Gynecologic Oncology Group. J Clin Oncol 1994;12:701-6. [Crossref] [PubMed]
- Chen CA, Lin H, Weng CS, et al. Outcome of 3-day bleomycin, etoposide and cisplatin chemotherapeutic regimen for patients with malignant ovarian germ cell tumours: a Taiwanese Gynecologic Oncology Group study. Eur J Cancer 2014;50:3161-7. [Crossref] [PubMed]
- Alifrangis C, Agarwal R, Short D, et al. EMA/CO for high-risk gestational trophoblastic neoplasia: good outcomes with induction low-dose etoposide-cisplatin and genetic analysis. J Clin Oncol 2013;31:280-6. [Crossref] [PubMed]
- Talukdar S, Kumar S, Bhatla N, et al. Neo-adjuvant chemotherapy in the treatment of advanced malignant germ cell tumors of ovary. Gynecol Oncol 2014;132:28-32. [Crossref] [PubMed]
- Newton C, Murali K, Ahmad A, et al. A multicentre retrospective cohort study of ovarian germ cell tumours: Evidence for chemotherapy de-escalation and alignment of paediatric and adult practice. Eur J Cancer 2019;113:19-27. [Crossref] [PubMed]
- Feldman DR, Lorch A, Kramar A, et al. Brain Metastases in Patients With Germ Cell Tumors: Prognostic Factors and Treatment Options--An Analysis From the Global Germ Cell Cancer Group. J Clin Oncol 2016;34:345-51. [Crossref] [PubMed]
- Oechsle K, Hartmann M, Mehnert A, et al. Symptom burden in long-term germ cell tumor survivors. Support Care Cancer 2016;24:2243-50. [Crossref] [PubMed]
- Champion V, Williams SD, Miller A, et al. Quality of life in long-term survivors of ovarian germ cell tumors: a Gynecologic Oncology Group study. Gynecol Oncol 2007;105:687-94. [Crossref] [PubMed]
- Rashdan S, Einhorn LH. Salvage Therapy for Patients With Germ Cell Tumor. J Oncol Pract 2016;12:437-43. [Crossref] [PubMed]
- Reddy Ammakkanavar N, Matei D, Abonour R, et al. High-dose chemotherapy for recurrent ovarian germ cell tumors. J Clin Oncol 2015;33:226-7. [Crossref] [PubMed]
- Berger LA, Bokemeyer C, Lorch A, et al. First salvage treatment in patients with advanced germ cell cancer after cisplatin-based chemotherapy: analysis of a registry of the German Testicular Cancer Study Group (GTCSG). J Cancer Res Clin Oncol 2014;140:1211-20. [Crossref] [PubMed]
- Ongoing Clinical Trials in Testicular Cancer. The TIGER Trial. Oncol Res Treat 2016;39:553-6. [Crossref] [PubMed]
- Bissett D, Kunkeler L, Zwanenburg L, et al. Long-term sequelae of treatment for testicular germ cell tumours. Br J Cancer 1990;62:655-9. [Crossref] [PubMed]
- Khrunin AV, Moisseev A, Gorbunova V, et al. Genetic polymorphisms and the efficacy and toxicity of cisplatin-based chemotherapy in ovarian cancer patients. Pharmacogenomics J 2010;10:54-61. [Crossref] [PubMed]
- Roco A, Cayún J, Contreras S, et al. Can pharmacogenetics explain efficacy and safety of cisplatin pharmacotherapy? Front Genet 2014;5:391. [Crossref] [PubMed]
- TC or BEP in Treating Patients With Malignant Ovarian Germ Cell Tumors (MOGCT-01). ClinicalTrials.gov Identifier: NCT02429687.
- Living After a Rare Cancer of the Ovary: Chronic Fatigue, Quality of Life and Late Effects of Chemotherapy (VIVROVAIRE TR). ClinicalTrials.gov Identifier: NCT03418844.
- Lawrence NJ, Chan H, Toner G, et al. Protocol for the P3BEP trial (ANZUP 1302): an international randomised phase 3 trial of accelerated versus standard BEP chemotherapy for adult and paediatric male and female patients with intermediate and poor-risk metastatic germ cell tumours. BMC Cancer 2018;18:854. [Crossref] [PubMed]
- Möbus V. Adjuvant Dose-Dense Chemotherapy in Breast Cancer: Standard of Care in High-Risk Patients. Breast Care (Basel) 2016;11:8-12. [Crossref] [PubMed]
- International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol 1997;15:594-603. [Crossref] [PubMed]
- Grimison PS, Stockler MR, Chatfield M, et al. Accelerated BEP for metastatic germ cell tumours: a multicenter phase II trial by the Australian and New Zealand Urogenital and Prostate Cancer Trials Group (ANZUP). Ann Oncol 2014;25:143-8. [Crossref] [PubMed]
- Active Surveillance, Bleomycin, Carboplatin, Etoposide, or Cisplatin in Treating Pediatric and Adult Patients With Germ Cell Tumors. ClinicalTrials.gov Identifier: NCT03067181.
- Huddart RA, Gabe R, Cafferty FH, et al. A randomised phase 2 trial of intensive induction chemotherapy (CBOP/BEP) and standard BEP in poor-prognosis germ cell tumours (MRC TE23, CRUK 05/014, ISRCTN 53643604). Eur Urol 2015;67:534-43. [Crossref] [PubMed]
- Kraggerud SM, Hoei-Hansen CE, Alagaratnam S, et al. Molecular characteristics of malignant ovarian germ cell tumors and comparison with testicular counterparts: implications for pathogenesis. Endocr Rev 2013;34:339-76. [Crossref] [PubMed]
- De Giorgi U, Casadei C, Bergamini A, et al. Therapeutic Challenges for Cisplatin-Resistant Ovarian Germ Cell Tumors. Cancers (Basel) 2019;11:1584. [Crossref] [PubMed]
- Fenner MH, Beutel G, Grünwald V. Targeted therapies for patients with germ cell tumors. Expert Opin Investig Drugs 2008;17:511-22. [Crossref] [PubMed]
- Narayan V, Hwang WT, Lal P, et al. Cyclin-Dependent Kinase 4/6 Inhibition for the Treatment of Unresectable Mature Teratoma: Long-Term Follow-Up of a Phase II Study. Clin Genitourin Cancer 2016;14:504-10. [Crossref] [PubMed]
- Hoei-Hansen CE, Kraggerud SM, Abeler VM, et al. Ovarian dysgerminomas are characterised by frequent KIT mutations and abundant expression of pluripotency markers. Mol Cancer 2007;6:12. [Crossref] [PubMed]
- Einhorn LH, Brames MJ, Heinrich MC, et al. Phase II study of imatinib mesylate in chemotherapy refractory germ cell tumors expressing KIT. Am J Clin Oncol 2006;29:12-3. [Crossref] [PubMed]
- Oing C, Kollmannsberger C, Oechsle K, et al. Investigational targeted therapies for the treatment of testicular germ cell tumors. Expert Opin Investig Drugs 2016;25:1033-43. [Crossref] [PubMed]
- Semaan A, Haddad FG, Eid R, et al. Immunotherapy: last bullet in platinum refractory germ cell testicular cancer. Future Oncol 2019;15:533-41. [Crossref] [PubMed]
- June CH, O'Connor RS, Kawalekar OU, et al. CAR T cell immunotherapy for human cancer. Science 2018;359:1361-5. [Crossref] [PubMed]
- Hong LK, Chen Y, Smith CC, et al. CD30-Redirected Chimeric Antigen Receptor T Cells Target CD30+ and CD30- Embryonal Carcinoma via Antigen-Dependent and Fas/FasL Interactions. Cancer Immunol Res 2018;6:1274-87. [Crossref] [PubMed]
- Cossu-Rocca P, Jones TD, Roth LM, et al. Cytokeratin and CD30 expression in dysgerminoma. Hum Pathol 2006;37:1015-21. [Crossref] [PubMed]


