
Effectiveness and safety of benralizumab for severe asthma in clinical practice (J-BEST): a prospective study
Introduction
Bronchial asthma is one of the most common respiratory diseases, which affects individuals in all age-groups (1). The last twenty years have witnessed a considerable decrease in the number of emergency outpatient visits and hospitalizations due to acute exacerbations of bronchial asthma; in addition, there has been a steady decrease in deaths due to bronchial asthma since the mid 1990s (2). This phenomenon is mainly due to improved asthma control achieved by maintenance treatment with inhaled corticosteroids (ICS), either alone or in combination with a second controller, generally a long-acting beta2 agonist (LABA), depending on asthma severity (3). However, bronchial asthma is a heterogenous disease with considerable inter-individual variability with respect to the clinical course (4-6).
ICS therapy is the mainstay of treatment of bronchial asthma, which is supplemented with LABAs, long-acting muscarinic antagonists (LAMA), and leukotriene receptor antagonist (LTRA), as appropriate. Nonetheless, some patients do not adequately respond to these treatments (7). Anti immunoglobulin E (IgE) antibody, anti interleukin 5 (IL-5) antibody, anti IL-5 receptor alpha antibodies, and IL-4/13 receptor antibodies have been approved for maintenance treatment of severe asthma patients with eosinophilic phenotype who are not adequately controlled by standard of care (8). These new drugs may be effective in cases where conventional treatment fails to prevent asthma exacerbations.
Benralizumab is a humanized, fucosylated, monoclonal antibody that targets the IL-5 α receptor (9). In contrast to anti-IL-5 monoclonal antibodies including mepolizumab and reslizumab, benralizumab induces direct, rapid, and nearly complete depletion of blood eosinophils through enhanced antibody-dependent cell-mediated cytotoxicity (ADCC), an apoptotic process of eosinophil elimination involving natural killer cells (10). In two Phase III trials (SIROCCO and CALIMA), benralizumab administered in combination with high-dose ICS/LABA significantly reduced acute exacerbations of asthma and improved lung function and disease control in patients with severe uncontrolled asthma and blood eosinophil counts ≥300/µL as compared to placebo (11,12). In another Phase III trial (ZONDA), benralizumab significantly reduced the use of maintenance prednisone in oral corticosteroid (OCS)-dependent patients while maintaining asthma control (13). Statistical analyses of these phase III studies identified several baseline clinical factors associated with enhanced efficacy of benralizumab, regardless of blood eosinophil counts; these included OCS use, history of nasal polyposis, lung function (as assessed by prebronchodilator forced vital capacity), exacerbation frequency, and age at asthma diagnosis (13).
However, there are no prospective studies in clinical practice. Therefore, we performed a prospective study to assess the effects of benralizumab in patients with severe asthma.
Methods
J-BEST was a prospective, open-label, single-arm, single-center study in patients with severe asthma in clinical practice. This study was approved by the ethics committee at the Japanese Red Cross Medical Center (No.869) and registered at the University Hospital Medical Information Network (UMIN, 00003195). Written informed consent was obtained from all patients prior to their enrolment. Patients with uncontrolled severe asthma who were aged ≥20 years were eligible for inclusion. These patients had persistent symptoms despite regular treatment with high-dose inhaled ICS/LABA, and had also received LAMA and LTRA with or without OCS therapy and biological agents other than benralizumab at the step 5 for Global Initiative for Asthma (GINA) or at the step 4 for Japanese guidelines for bronchial asthma. This study was conducted from May 2018 to Mar 2019. Overall, 26 patients were enrolled.
Patients were prescribed a single subcutaneous injection of benralizumab (30 mg) administered at intervals of 4 weeks for the first three injections, and every 8 weeks thereafter. Haematological, clinical, functional, and pharmacotherapeutic parameters were recorded at baseline and at weeks 4 and 12 after initiation of benralizumab therapy. Hematological parameters included total eosinophil count, total basophil count, and serum IgE level. Other parameters recorded were asthma quality of life questionnaire (AQLQ) score, asthma control test (ACT) score, prebronchodilator FEV1 (only at baseline and at week 12), peak expiratory flow (PEF, only at baseline and at week 12), fractional exhaled nitric oxide (FeNO), oral prednisone dose, and adverse events (AEs). The primary endpoint was mean change in AQLQ and ACT scores from baseline at week 12. Additional endpoint was mean change in FEV1 from baseline at week 12. The safety endpoint was the frequency of AEs, which included any adverse medical event (including abnormal laboratory changes) that occurred after treatment, with or without a causal relationship with the treatment. AEs were recorded on a worksheet by patients and documented by physicians.
A sample size of 25 was calculated using an expected value of 0.75 and a threshold of 0.50 with a two-tailed statistical significance of α=0.05 and a power of at least 0.80. Allowing for missing data (due to patient dropout), we planned to recruit 26 patients to the present study. Descriptive statistics are presented as mean (95% confidence interval, CI) or as frequency (percentage). The Fischer exact test for categorical data and Mann-Whitney U test for numeric data were used to evaluate the significance of difference in the native and switched groups. Kruskal-Wallis test for paired data was used to evaluate the significance of difference in baseline, week 4 and week 12 of the time course of the treatment. All reported P values were two-sided, and P values <0.05 were considered indicative of statistical significance. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) (14).
Results
Baseline demographic and clinical characteristics of patients are summarized in Table 1. The mean age of patients was 66.8 years; 15 patients were women. Ten out of the 26 patients had previously received biological drugs (omalizumab: 2 patients; mepolizumab: 8 patients). The mean prior omalizumab and mepolizumab treatment duration were 12.5 months and 9.0 months, respectively.
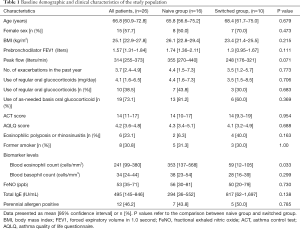
Full table
The time course of benralizumab treatment is described in Table 2. The AQLQ scores of patients showed a significant improvement over the study period [baseline: 4.2 (mean, 95% CI) (3.6–4.8); week 4: 5.4 (5.0–5.8) (P<0.001 vs. baseline); week 12: 5.6 (5.1–6.1) (P<0.0001 vs. baseline)]. The ACT scores also showed a significant improvement over the study period {baseline: 14 [11–17]; week 4: 19 [17–21] (P<0.001 vs. baseline); week 12: 20 [19–22] (P<0.001 vs. baseline)}. This improvement was maintained over the study period in 20 patients (77%) at week 12. FEV1 increased from 1.57 L (1.31–1.84) at baseline to 1.75 L (1.46–2.03) at week 12 (P=0.003). Peak flow increased from 314 L/min [255–373] at baseline to 369 L/min [307–431]at week 12 (P<0.001). Pulmonary function (FEV1) of 19 out of 26 patients (73.1%) improved over the study period at week 12. The dose of regular OCS decreased from 4.1 (1.6–6.5) mg at baseline to 1.2 (0–2.5) mg at week 12 (P=0.008); the number of patients on need-based OCS therapy decreased from 19 (73.1%) at baseline to 2 (7.7%) patients at week 12.
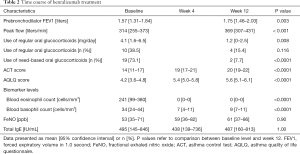
Full table
The total eosinophil count in blood showed a significant decrease over the study period [baseline: 241 [99–380] cells/mm3; week 4: 0 [0–0] (P=0.005 vs. baseline); week 12: 0 [0–0] (P=0.005 vs. baseline)]. Similarly, blood basophil count also showed a significant decrease over the study period: {baseline: 34 [24–44] cells/mm3; week 4: 7 [4–11] (P<0.001 vs. baseline); week 12: 9 [7–11] (P<0.001 vs. baseline)}. However there were no significant changes in the level of FeNO {baseline: 53 ppb [35–71]; week 4: 59 ppb [36–82]; week 12: 61 ppb [37–86], P=0.9}. Similarly, there was no significant change in total IgE level [baseline: 495 [145–846] IU/mL; week 4: 438 [139–736] (P=0.45 vs. baseline); week 12: 487 [160–813] (P=1.0 vs. baseline)].
In this study, 8 patients changed from mepolizumab to benralizumab. In these 8 patients, the total eosinophil count in blood showed a significant decrease over the study period {baseline: 32 [14–51] cells/mm3; week 4: 0 [0–0] (P=0.012 vs. baseline); week 12: 0 [0–0] (P=0.012 vs. baseline)}. Moreover, blood basophil count also showed a significant decrease at week 12: {baseline: 30 [18–42] cells/mm3; week 4: 11 [2–21] (P=0.091 vs. baseline); week 12: 10 [3–16] (P=0.046 vs. baseline)}.
Only one patient experienced slight headache that did not require any pharmacologic treatment.
Discussion
To the best of our knowledge, this is the first prospective study of the effect of benralizumab in patients with severe asthma in clinical practice. In this study, benralizumab was found to be highly effective against severe asthma at weeks 4 and 12 after administration.
Patients with severe asthma account for an estimated 5–10% of all asthma patients. The estimated cost of treatment of these patients accounts for as much as 50% of all costs of asthma treatment (15). Patients with severe asthma have been stratified based on phenotype and endotype; this has facilitated a better understanding of the pathophysiology of airway inflammation and enabled development of novel therapies for severe asthma (16). The Th2 cytokines (IL-4, IL-5, IL-9, and IL-13) play an important role in the pathogenesis and acute exacerbations of bronchial asthma (17). In recent years, anti-IgE antibodies were the first to be approved for treatment of severe asthma; subsequently, anti-IL-5 antibodies, anti-IL-5 receptor α antibodies, and IL-4/13 receptor antibodies were also approved for clinical use. Studies have demonstrated the effectiveness of biological products (16,18). These developments have heralded the era of precision medicine for severe asthma. Eosinophilic asthma, which is characterized by eosinophilic airway inflammation, accounts for approximately half of all patients with severe asthma (19). Asthmatic patients with high eosinophil counts have a strong predisposition to allergy, impaired respiratory function, and a high frequency of exacerbations (20).
In our study, benralizumab was effective as early as 4 and 12 weeks after administration. A significant improvement in subjective scores (ACT and AQLQ scores) was observed 4 weeks after administration of benralizumab; the effect was sustained even 12 weeks after administration of benralizumab. Prompt improvement in symptoms is a key imperative in patients with severe asthma; benralizumab administration was found to be very useful for patients with severe asthma in this study. Furthermore, FEV1 and peak flow after 12 weeks of benralizumab was significantly improved compared with pre-treatment levels. In addition, a significant decrease in the dose of OCS was observed 12 weeks after administration of benralizumab. It is well known that continuous administration of OCS may cause various complications, and it is very important to reduce the use of OCS for asthma in clinical practice (21).
In this study, the mean total eosinophil count in the blood at 4 weeks after benralizumab therapy was 0 cells/mm3. We had earlier reported a significant decrease in eosinophils in lung and bronchial tissues in surgically resected specimens after administration of benralizumab (22). We believe that the improvement in FEV1 and peak flow was attributable to the decrease in the number of tissue eosinophils consequent to the nil eosinophil count in the blood. In this study, the basophil count was also significantly decreased at week 4 and 12 after administration of benralizumab. Basophils are known to exhibit surface expression of anti-IL-5 receptor. In a study, treatment with mepolizumab (an anti-IL-5 antibody) did not led to a decrease in basophils; the decrease in blood basophils in the present study may reflect the difference between anti-IL-5 antibodies and anti-IL-5 receptor antibodies (7,23). In our study, 8 patients changed from mepolizumab to benralizumab. In these 8 patients, the both total eosinophil count and basophil count in blood showed a significant decrease in this study. This might be the reason why patients who switched from mepolizumab to benralizumab were also effective.
In our study, benralizumab did not reduce the levels of FeNO or total IgE level. These results are consistent with the results of previous clinical trials of benralizumab (11,12). The therapeutic effects of benralizumab might not depend on the levels of FeNO or total IgE level.
This study had several limitations. Although this study was a prospective study, it was conducted at a single health facility. Prospective multicenter studies may provide more definitive evidence. Second, there was the lack of a control group of severe asthma patients who were on standard of care in this study. In clinical practice, it was very difficult to set a control group without biological products because of patient’s care. Third, our study included both patients who were switched from other biological products to benralizumab and those who received a biological product for the first time. The study was conducted without preparing a special environment as much as possible for examination in clinical practice. In addition, we could not assess the patients who are more likely to benefit from switching to benralizumab. However this is considered to be an important point of view in clinical practice, the number of cases calculated in this study was not enough to compare in each group. Further investigations are needed to clarify this aspect.
Conclusions
Benralizumab conferred clinically significant benefits in patients with severe asthma with no associated serious AEs in the short term in clinical practice. More studies are required to provide more robust evidence of the benefits of benralizumab in particular as compared with other biologics approved for severe asthma in clinical practice. Future studies should identify treatable traits, as a step towards precision medicine for severe asthma.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.04.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the ethics committee at the Japanese Red Cross Medical Center (No. 869) and registered at the University Hospital Medical Information Network (UMIN, 00003195).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dusser D, Montani D, Chanez P, et al. Mild asthma: an expert review on epidemiology, clinical characteristics and treatment recommendations. Allergy 2007;62:591-604. [Crossref] [PubMed]
- Ichinose M, Sugiura H, Nagase H, et al. Japanese guidelines for adult asthma 2017. Allergol Int 2017;66:163-89. [Crossref] [PubMed]
- Reddel HK, FitzGerald JM, Bateman ED, et al. GINA 2019: a fundamental change in asthma management: Treatment of asthma with short-acting bronchodilators alone is no longer recommended for adults and adolescents. Eur Respir J 2019. [Crossref] [PubMed]
- Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014;43:343-73. [Crossref] [PubMed]
- Montuschi P, Barnes PJ. New perspectives in pharmacological treatment of mild persistent asthma. Drug Discov Today 2011;16:1084-91. [Crossref] [PubMed]
- Montuschi P.. Leukotrienes, antileukotrienes and asthma. Mini Rev Med Chem 2008;8:647-56. [Crossref] [PubMed]
- Israel E, Reddel HK. Severe and Difficult-to-Treat Asthma in Adults. N Engl J Med 2017;377:965-76. [Crossref] [PubMed]
- Baldacci S, Simoni M, Maio S, et al. Prescriptive adherence to GINA guidelines and asthma control: An Italian cross sectional study in general practice. Respir Med 2019;146:10-7. [Crossref] [PubMed]
- Kolbeck R, Kozhich A, Koike M, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol 2010;125:1344-53.e2. [Crossref] [PubMed]
- Pham TH, Damera G, Newbold P, et al. Reductions in eosinophil biomarkers by benralizumab in patients with asthma. Respir Med 2016;111:21-9. [Crossref] [PubMed]
- Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016;388:2115-27. [Crossref] [PubMed]
- FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016;388:2128-41. [Crossref] [PubMed]
- Nair P, Wenzel S, Rabe KF, et al. Oral Glucocorticoid-Sparing Effect of Benralizumab in Severe Asthma. N Engl J Med 2017;376:2448-58. [Crossref] [PubMed]
- Kanda Y.. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013;48:452-8. [Crossref] [PubMed]
- Nunes C, Pereira AM, Morais-Almeida M. Asthma costs and social impact. Asthma Res Pract 2017;3:1. [Crossref] [PubMed]
- Pepper AN, Renz H, Casale TB, et al. Biologic Therapy and Novel Molecular Targets of Severe Asthma. J Allergy Clin Immunol Pract 2017;5:909-16. [Crossref] [PubMed]
- Moore WC, Meyers DA, Wenzel SE, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med 2010;181:315-23. [Crossref] [PubMed]
- Santini G, Mores N, Malerba M, et al. Dupilumab for the treatment of asthma. Expert Opin Investig Drugs 2017;26:357-66. [Crossref] [PubMed]
- Nagase H.. Severe asthma in Japan. Allergol Int 2019;68:167-71. [Crossref] [PubMed]
- Berry A, Busse WW. Biomarkers in asthmatic patients: Has their time come to direct treatment? J Allergy Clin Immunol 2016;137:1317-24. [Crossref] [PubMed]
- Ekström M, Nwaru BI, Hasvold P, et al. Oral corticosteroid use, morbidity and mortality in asthma: A nationwide prospective cohort study in Sweden. Allergy 2019;74:2181-90. [Crossref] [PubMed]
- Izumo T, Terada Y, Tone M, et al. Rapid effects of benralizumab on severe asthma during surgery for residual tumor after advanced lung squamous cell carcinoma treatment with pembrolizumab. Respir Med Case Rep 2019;26:292-5. [Crossref] [PubMed]
- Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 2014;371:1198-207. [Crossref] [PubMed]


