Metformin in cancer prevention and therapy
The prevalence of diabetes is dramatically increasing worldwide reaching epidemic proportion. Landmark of diabetes, chronic hyperglycemia leads to the development and progression of life-treating complications, predominantly cardiovascular. The results of several studies indicate that people with diabetes (mainly type 2, T2DM) are also at substantially higher risk of cancer of the pancreas, liver, endometrium, breast, colon, rectum and urinary bladder compared to individuals without this chronic disease (1). However, the incidence of other types of cancer (e.g., lung, kidney, non-Hodgkin lymphomas) does not seem to be strongly associated with diabetes or the evidence is inconclusive (2). Interestingly enough, it has been suggested that diabetes is associated with a lower risk for prostate cancer (2,3). According to the American Diabetes Association and the American Cancer Society consensus report the relative risks imparted by diabetes are greatest (about two fold or higher) for cancers of the liver, pancreas, and endometrium, and lesser (about 1.2-1.5 fold) for cancers of the colon and rectum, breast, and bladder (2). Clinical observations indicate that the prevalence of diabetes in newly diagnosed cancer patients ranges from 8% to 18%, suggesting bidirectional association between these two disease (4,5). The association of diabetes and cancer was first reported as an incidental finding in 1932 (6). Nowadays, this coexistence is well recognized, however in spite of the intensive studies its mechanism still remains unclear. There is a general agreement that T2DM and cancer share several common potential risk factors (e.g., aging, sex, obesity, physical inactivity, diet, alcohol, and smoking). In T2DM, insulin resistance and hyperinsulinemia (either endogenous due to insulin resistance or induced by administration of exogenous insulin formulations) are considered to be independent risk factors for cancer development (1,2). In addition, hyperglycemia-related oxidative stress, accumulation of advances glycation end products as well as low-grade inflammation may also enhance the risk of malignant transformation (7,8). Recent publications have also suggested the link between hypoglycemic medications and cancer (8-11). The results of numerous preclinical, epidemiological and clinical studies suggested that metformin use is associated with inhibition of cancer cell growth and proliferation and reduction in all-cancer incidents in comparison with users of other hypoglycemic drugs. In the present work we discuss the proposed mechanism(s) of anticancer effect of metformin as well as preclinical and clinical data suggesting this beneficial effect.
Molecular action of metformin in cancer cell
The current proposed anticancer molecular action of metformin is mainly associated with the inhibition of the mammalian target of rapamycin complex 1 (mTORC1). The mTOR pathway plays a pivotal role in metabolism, growth and proliferation of cancer cell (12). Metformin is thought to inhibit mTORC1 pathway (Figure 1).
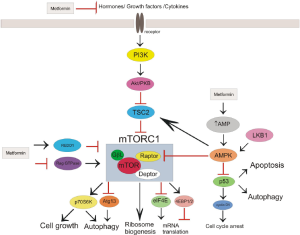
It is believed that systemic effect of metformin manifested by the reduction of circulating level of insulin and insulin-like growth factor 1 (IGF-1) might be associated with anticancer action (13). Insulin/IGF-1 is involved not only in regulation of glucose uptake but also in carcinogenesis through upregulation of insulin/IGF receptor signaling pathway (14). The excessive food consumption (insulin) leads to increased liver production of IGF-1 that binds to IGF-1 receptor and insulin receptor. Then, through insulin receptor substrate (IRS) the signal is transmitted to phosphoinositide 3-kinase (PI3K), and Akt/protein kinase B (PKB) that indirectly activates (not phosphorylates) mTORC1. Additionally, insulin receptor through growth factor receptor-bound protein 2 (GRB2) propagates signal to Ras/Raf/ERK pathway that drives cell growth. Evidences indicate that these pathways play important role in changes of cellular metabolism that are typical feature of tumor cells (15). Increased levels of circulating insulin/IGF1 and upregulation of insulin/IGF receptor signaling pathways were demonstrated to be involved in the formation of many types of cancer. Metformin was found to reduce insulin level, inhibit insulin/IGF signaling pathways, and modify cellular metabolism in normal and cancer cells (16).
Evidences suggest that the inhibition of mTOR pathway by metformin proceeds dependent and independent on AMP-activated protein kinase (AMPK) activation. AMPK phosphorylates tuberous sclerosis complex protein 2 (TSC2) that inhibits mTORC1 leading to decrease in protein synthesis and cell growth (17). Among the first studies that showed the participation of AMPK activation in antitumor action of metformin were researches performed on breast cancer cells (18,19). Dowling et al. showed that compound C, an inhibitor of AMPK, reversed inhibition of initiation of translation evoked by metformin (18). More recently, Mohammed et al. showed reduction of carcinoma spread in pancreas of transgenic mice fed with metformin (20). Additionally, pancreatic tissue of mice fed with metformin revealed a significant inhibition of mTOR, and an increase of phosphorylated AMPK and TSC2 (20). However, Gwinn el al. demonstrated that inhibition of mTOR could be independent on TSC2, since AMPK directly phosphorylates the rotor compartment of mTOR (21).
Several studies identified that liver kinase B1 (LKB1), a major upstream kinase of AMPK, may be involved in anticancer action of metfromin associated with inhibition of mTOR. In vitro and in vivo studies revealed that deletion of LKB1 function accelerated proliferation of tumor cell and sensitized them to activators of AMPK such as biguanide (22-24). Due to the fact that p53 expression and phosphorylation is regulated by AMPK and p53 is involved in cell metabolism and control of cell cycle its participation in metformin action is discussed. Growing evidences from in vivo and in vitro studies of various cancers revealed that metformin blocked cell cycle in G0/G1 phase with a significant decrease expression of G1 cyclins (including cyclin D1) without changes in p53 status (25-27). However, others researches indicated that inhibitory effect on cancer cell growth of metformin was associated with p53 activity (28-31). Taking together the results of preclinical studies are inconclusive whether antitumor action of metformin is associated with p53. Some investigators hypothesize that the dose of metformin may determine the effect of metformin. Yi et al. demonstrated on hepatoma cells that low concentration of metformin induced p53-dependent senescence, whereas higher doses induced apoptotic cell death (32).
Inhibition of mTOR by metformin independent on AMPK activation was demonstrated by Memmott et al. in mice lung cancer cells (16). Metformin evoked inhibition of mTOR pathway with accompanied decreasing activation of IGF-1/insulin receptor, Akt, extracellular signal-regulated kinase (ERK) without AMPK activation (16). Kalender et al. demonstrated in Drosophilla cells that inhibition of mTOR signaling induced by metformin occurred in the absence of AMPK. They reveal the existence of an alternative TSC1/2-mTOR AMPK-independent pathway mediated by RAG GTPase (33). Metformin was found to inhibit breast carcinoma cell growth through decreasing level of epidermal growth factor receptor 2 (HER2). This effect was mediated by inhibition of the mTOR effector, p70S6K1 (34). p70S6K is responsible for the phosphorylation of S6 ribosomal protein and thereby protein synthesis at the ribosome (35). Antiproliferative action of metformin related to enhancement of DNA-damage-inducible transcript 4 protein (DDIT4, REDD1) expression, a negative regulator of mTOR, was reported in prostate cancer cells by Ben Sahra et al. (36). This effect of metformin was also independent on AMPK activation (36).
The results of preclinical studies undoubtedly confirm the efficacy of metformin to inhibit cancer cell growth in vitro and to reduce tumor spread in animal models of various cancers. However, it should be stressed that molecular action of metformin is still investigated and seems to be affected by the type of tumor cell line.
Metformin and the risk of cancer
Metformin is the most commonly prescribed drug for T2DM. Its use in diabetes was shown to prevent macrovascular complications to the better extent than other oral hypoglycemic drugs as well as insulin (37,38). Additionally, the results of numerous epidemiologic studies repeatedly indicated that T2DM patients receiving metformin, compared to those taking other antidiabetic medications, had a decreased risk of the occurrence of various types of cancers (39). This observation was also confirmed by numerous meta-analyses that confirmed that metformin reduces cancer incidence by 30-50%.
Bowker et al. used databases from Saskatchewan Health (Canada) to examine the association between different therapeutic schedules of diabetes and cancer mortality in T2DM patients (10). It was observed that in T2DM patients using sulfonylureas (SU) or insulin the risk of cancer-related mortality was significantly increased compared to metformin users. A similar difference in cancer incidence in metformin users compared with SU was also reported by Evans et al. (40). The researchers used databases developed in Tayside (Scotland) to assess the influence of metformin therapy on the risk of cancer in patients with T2DM (40). They observed that metformin reduced the risk of cancer in patients with T2DM, both before and after adjusting for BMI. Additionally, they suggested the existence of the inverse relation between the dose of metformin and the risk of cancer.
Currie et al. performed a retrospective cohort study in 62,809 people older than 40 years, treated in U.K. by general practioners (41). Patients were on oral antidiabetic drugs and/or insulin. For the analysis the cohort was subdivided into four groups: metformin monotherapy, sulfonylurea monotherapy, combination therapy with metformin and sulfonylurea, or insulin. Insulin users were further subdivided into glargine, long-acting human insulin, biphasic analogue or human biphasic insulin. The observed risk of cancer in patients treated with basal human insulin alone vs. glargine alone was 1.24. Insulin therapy was associated with an increased risk for colorectal (HR =1.69) and pancreatic cancers (HR =4.63). However, when compared with metformin, this relation was not seen for breast and prostate cancers.
Franciosi et al. selected randomized studies comparing metformin and other hypoglycaemic agents as well as observational studies assessing the relation between exposure to metformin and cancer (42). Altogether, 12 randomized controlled trials and 41 observational studies met the inclusion criteria. They noted that in observational studies there was a significant association of exposure to metformin with the risk of cancer death, all malignancies, liver, colorectal, pancreas, stomach, and esophagus. Interestingly, such a relationship was not seen in randomized trials, what stresses the need for randomized trials to evaluate the efficacy of metformin as an anticancer agent.
Another meta-analysis of seventeen observational studies investigated the risk of all cancers and site-specific cancers in people with T2DM (43). Soranna et al. compared metformin with SU users. The meta-analysis showed that therapy with metformin use was associated with decreased risk for all cancer. Furthermore, except for colorectal cancer, metformin was not associated with any significant effect on the incidence of other cancers, for example, prostate and breast cancers.
In a large population-based study, a lower risk of cancer cancers was observed in patients treated with metformin in comparison with those received SU (44). The duration of diabetes was similar in both groups, but unfortunately the cause of death was not identified. That is why the researchers could not compare the association of the cancer-related mortality between metformin and SU users.
Chlebowski et al. assessed the association between diabetes, metformin use, and breast cancer among 68,019 postmenopausal women participating in Women’s Health Initiative clinical trials (45). Compared with women without diabetes, in diabetic woman the incidence of breast cancer was related to diabetes therapy. Diabetic women not treated with metformin had a slightly higher incidence of breast cancer. The association was observed for cancers positive for both estrogen receptor and progesterone receptor as well as those negative for HER2.
Home et al. (46) collected data for malignancies in Diabetes Outcome Progression Trial (ADOPT) and Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycemia in Diabetes (RECORD) studies. The results did not reveal significant differences in cancer incidence between metformin and rosiglitazone, however the incidence of cancer was slightly higher in SU group. One should remember that the number of malignancies was small in both trials.
Zhang et al. pooled the currently available data to examine the association between metformin therapy and colorectal cancer among patients with T2DM (47). More than 108,161 patients with T2DM were included into analysis, and once again they noted that metformin treatment was associated with a significantly lowest risk of colorectal cancer.
Noto et al. calculated pooled risk ratios (RRs) for overall cancer mortality and cancer incidence in 21,195 diabetic patients (48). Similarly to the results of other trials they noted that the use of metformin in diabetic patients was associated with significantly lower risks of both cancer incidence and mortality.
The positive correlation between metformin use and incidence of various type cancers was not universally noted by all investigators. Mamtani et al. analysed data from 87,600 patients with T2DM (49). They assessed the incidence of bladder cancer in new users of metformin and SU and did not see any association between metformin use and this type cancer.
It is still uncertain, whether the observed increased risk of cancer mortality in diabetic patients are related to a protective effect of metformin or negative effects of other therapies including SU and insulin (40). Again, if the difference in cancer-related mortality is related to the antidiabetic drugs, it may be associated with either the slower development of the cancer or better response to anticancer therapy. Additionally, one should also remember, that there are important differences in the characteristics of patients treated with metformin compared with other antidiabetic agents. These differences may be responsible for the observed differences in cancer incidence. In the United Kingdom metformin users had a higher BMI, a younger age, a lower systolic blood pressure, a lower prevalence of cardiovascular disease, and a higher proportion of aspirin and NSAID use as compared with second-generation SU users at the beginning of therapy (50,51).
Clinical evidence with metformin in cancer prevention and treatment
Biguanides were used in oncology more than 40 years ago as “metabolic rehabilitation” in breast, colorectal, or gastric cancers patients (52). The therapy with biguanides used with caloric restriction resulted in diminished tumor development and lower incidence of metastases (53). However, until now we do not have conclusive data on the role of metformin neither in cancer prevention nor the therapy both in diabetic and non-diabetic populations.
Several studies assessed the influence of metformin on metabolic status in cancer patients with and without diabetes. It was observed in nondiabetic woman that in the early stage breast cancer metformin reduced fasting insulin by 22% and improved several metabolic parameters (54). In a randomized study in woman with breast cancer, Campagnoli et al. observed that doses of metformin used routinely in diabetes decreased testosterone and insulin levels as well as several indices of insulin resistance (55). In another study in non-diabetic women with breast cancer the therapy with metformin resulted not only in reduced number of Ki67-positive cancer cells but also in changes in gene expression of molecules involved in the mTOR and AMPK pathways (56). In a randomized study, Hosono et al. showed that compared to control group metformin in small doses (250 mg/day) reduced colorectal aberrant crypt foci (regarded as surrogate marker for colorectal cancer) by 40% in non-diabetic patients (57).
Jiralerspong et al. observed 2,529 females with breast cancer. They noted increased incidence of complete response rates in metformin group, both in patients with and without diabetes (58). However, despite the increased incidence of complete response rates, metformin did not significantly improve survival. Margel et al. assessed the relation between duration of metformin therapy after prostate cancer diagnosis and mortality in patients with diabetes (59). The data were obtained from several databases in Ontario (Canada). In the cohort consisting of 3,837 patients, they noted that the longer duration of metformin treatment after diagnosis of prostate cancer was associated with a significant decrease not only in the risk of cancer-specific but also in all-cause mortality.
Metformin was also used as adjuvant therapy in cancer patients, and most of the cancer clinical trials of metformin use the same doses typically used to treat diabetes.
Summary
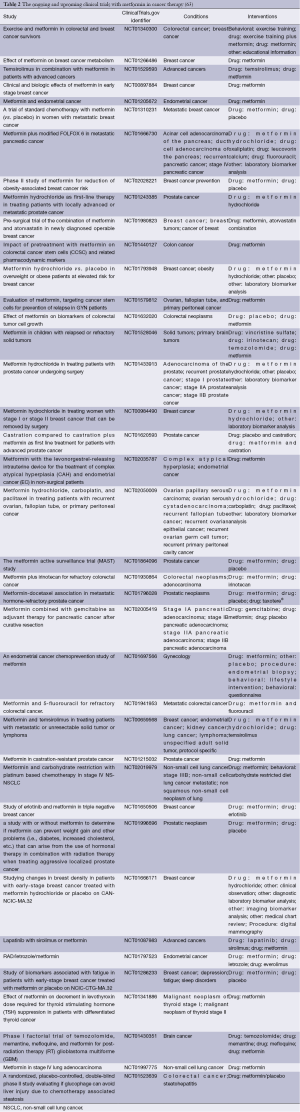
Preclinical evidence suggests that metformin appears to inhibit the proliferation and growth of certain types of cancer. Results of numerous clinical studies, although inconclusive, indicate that metformin use, and possibly cumulative duration of therapy and cumulative dose, is associated not only with decreased incidence of cancer in diabetic population, but also with the better outcome in cancer patients. Considering the possible variations in response to metformin in cancer patients it seems crucial to identify target populations for its use. However, factors contributing to better outcome in metformin users, such as genetic polymorphisms, are still to be elucidated (60). The definite data on the efficacy of metformin as neoadjuvant therapy in cancer patients is lacking. There are numerous trials underway in prostate cancer patients receiving androgen deprivation therapy as well as in patients with small benign thyroid nodules and insulin resistance (61,62). Altogether, there are currently more than 100 ongoing or upcoming clinical studies assessing the role of metformin in the therapy cancer (Tables 1 and 2). The vast majority of current trials assess the usefulness of metformin in cancer treatment, while several trials evaluate metformin in cancer prevention. Their results will permit to assess the place of metformin in cancer prevention and therapy, and define the target populations in the nearest future.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Smith U, Gale EM. Cancer and diabetes: are we ready for prime time? Diabetologia 2010;53:1541-4. [PubMed]
- Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care 2010;33:1674-85. [PubMed]
- Bonovas S, Filloussi K, Tsantes A. Diabetes mellitus and risk of prostate cancer: a meta-analysis. Diabetologia 2004;47:1071-8. [PubMed]
- Barone BB, Yeh HC, Snyder CF, et al. Postoperative mortality in cancer patients with preexisting diabetes: systematic review and meta-analysis. Diabetes Care 2010;33:931-9. [PubMed]
- Richardson LC, Pollack LA. Therapy insight: influence of type 2 diabetes on the development and outcomes of cancer. Nat Clin Pract Oncol 2005;2:48-53. [PubMed]
- Smith U, Gale EM. Does diabetes therapy influence the risk of cancer? Diabetologia 2009;52:1699-708. [PubMed]
- Azar M, Lyons TJ. Diabetes, insulin treatment and cancer: what is the evidence? F1000 Med Rep 2010;2. pii: 4.
- Drzewoski J, Drozdowska A, Sliwinska A. Do we have enough data to confirm the link between antidiabetic drug use and cancer development? Pol Arch Med Wewn 2011;121:81-7. [PubMed]
- Schiel R, Müller UA, Braun A, et al. Risk of malignancies in patients with insulin-treated diabetes mellitus: results of a population-based trial with 10-year follow-up (JEVIN). Eur J Med Res 2005;10:339-44. [PubMed]
- Bowker SL, Majumdar SR, Veugelers P, et al. Increased cancer-related mortality for patients with type 2 diabetes who use SU or insulin. Diabetes Care 2006;29:254-8. [PubMed]
- Hemkens LG, Grouven U, Bender R, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia 2009;52:1732-44. [PubMed]
- Chiang GG, Abraham RT. Targeting the mTOR signaling network in cancer. Trends Mol Med 2007;13:433-42. [PubMed]
- Memmott RM, Mercado JR, Maier CR, et al. Metformin prevents tobacco carcinogen-induced lung tumorigenesis. Cancer Prev Res (Phila) 2010;3:1066-76. [PubMed]
- Drzewoski J, Drozdowska A, Sliwińska A. Do we have enough data to confirm the link between antidiabetic drug use and cancer development? Pol Arch Med Wewn 2011;121:81-7. [PubMed]
- LeRoith D, Roberts CT Jr. Insulin-like growth factors and cancer. Ann Intern Med 1995;122:54-9. [PubMed]
- Memmott RM, Mercado JR, Maier CR, et al. Metformin prevents tobacco carcinogen-induced lung tumorigenesis. Cancer Prev Res (Phila) 2010;3:1066-76. [PubMed]
- Yoshida S, Hong S, Suzuki T, et al. Redox regulates mammalian target of rapamycin complex 1 (mTORC1) activity by modulating the TSC1/TSC2-Rheb GTPase pathway. J Biol Chem 2011;286:32651-60. [PubMed]
- Dowling RJ, Zakikhani M, Fantus IG, et al. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res 2007;67:10804-12. [PubMed]
- Zakikhani M, Dowling R, Fantus IG, et al. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res 2006;66:10269-73. [PubMed]
- Mohammed A, Janakiram NB, Brewer M, et al. Antidiabetic Drug Metformin Prevents Progression of Pancreatic Cancer by Targeting in Part Cancer Stem Cells and mTOR Signaling. Transl Oncol 2013;6:649-59. [PubMed]
- Gwinn DM, Shackelford DB, Egan DF, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 2008;30:214-26. [PubMed]
- Shaw RJ, Kosmatka M, Bardeesy N, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A 2004;101:3329-35. [PubMed]
- Algire C, Amrein L, Bazile M, et al. Diet and tumor LKB1 expression interact to determine sensitivity to anti-neoplastic effects of metformin in vivo. Oncogene 2011;30:1174-82. [PubMed]
- Shackelford DB, Abt E, Gerken L, et al. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell 2013;23:143-58. [PubMed]
- Hadad SM, Hardie DG, Appleyard V, et al. Effects of metformin on breast cancer cell proliferation, the AMPK pathway and the cell cycle. Clin Transl Oncol 2013; [PubMed]
- Ben Sahra I, Laurent K, Giuliano S, et al. Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res 2010;70:2465-75. [PubMed]
- Zhuang Y, Miskimins WK. Cell cycle arrest in Metformin treated breast cancer cells involves activation of AMPK, downregulation of cyclin D1, and requires p27Kip1 or p21Cip1. J Mol Signal 2008;3:18. [PubMed]
- Malki A, Youssef A. Antidiabetic drug metformin induces apoptosis in human MCF breast cancer via targeting ERK signaling. Oncol Res 2011;19:275-85. [PubMed]
- Janjetovic K, Harhaji-Trajkovic L, Misirkic-Marjanovic M, et al. In vitro and in vivo anti-melanoma action of metformin. Eur J Pharmacol 2011;668:373-82. [PubMed]
- Nelson LE, Valentine RJ, Cacicedo JM, et al. A novel inverse relationship between metformin-triggered AMPK-SIRT1 signaling and p53 protein abundance in high glucose-exposed HepG2 cells. Am J Physiol Cell Physiol 2012;303:C4-13. [PubMed]
- Cerezo M, Tichet M, Abbe P, et al. Metformin blocks melanoma invasion and metastasis development in AMPK/p53-dependent manner. Mol Cancer Ther 2013;12:1605-15. [PubMed]
- Yi G, He Z, Zhou X, et al. Low concentration of metformin induces a p53-dependent senescence in hepatoma cells via activation of the AMPK pathway. Int J Oncol 2013;43:1503-10. [PubMed]
- Kalender A, Selvaraj A, Kim SY, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab 2010;11:390-401. [PubMed]
- Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. The antidiabetic drug metformin suppresses HER2 (erbB-2) oncoprotein overexpression via inhibition of the mTOR effector p70S6K1 in human breast carcinoma cells. Cell Cycle 2009;8:88-96. [PubMed]
- Chung J, Kuo CJ, Crabtree GR, et al. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell 1992;69:1227-36. [PubMed]
- Ben Sahra I, Regazzetti C, Robert G, et al. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res 2011;71:4366-72. [PubMed]
- United Kingdom Diabetes study group, United Kingdom Prospective Diabetes Study (UKPDS). 13: Relative efficacy of randomly allocated diet, sulphonylurea, insulin, or metformin in patients with newly diagnosed non-insulin dependent diabetes followed for three years. BMJ 1995;310:83-8. [PubMed]
- Kourelis TV, Siegel RD. Metformin and cancer: new applications for an old drug. Med Oncol 2012;29:1314-27. [PubMed]
- Quinn BJ, Kitagawa H, Memmott RM, et al. Repositioning metformin for cancer prevention and treatment. Trends Endocrinol Metab 2013;24:469-80. [PubMed]
- Evans JM, Donnelly LA, Emslie-Smith AM, et al. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005;330:1304-5. [PubMed]
- Currie CJ, Poole CD, Jenkins-Jones S, et al. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care 2012;35:299-304. [PubMed]
- Franciosi M, Lucisano G, Lapice E, et al. Metformin Therapy and Risk of Cancer in Patients with T2DM: Systematic Review. PLoS One 2013;8:e71583. [PubMed]
- Soranna D, Scotti L, Zambon A, et al. Cancer risk associated with use of metformin and sulfonylurea in type 2 diabetes: a meta-analysis. Oncologist 2012;17:813-22. [PubMed]
- Ruiter R, Visser LE, van Herk-Sukel MP, et al. Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: results from a large population-based follow-up study. Diabetes Care 2012;35:119-24. [PubMed]
- Chlebowski RT, McTiernan A, Wactawski-Wende J, et al. Diabetes, Metformin, and Breast Cancer in Postmenopausal Women. J Clin Oncol 2012;30:2844-52. [PubMed]
- Home PD, Kahn SE, Jones NP, et al. Experience of malignancies with oral glucose-lowering drugs in the randomised controlled ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) clinical trials. Diabetologia 2010;53:1838-45. [PubMed]
- Zhang ZJ, Zheng Z, Kan H, et al. Reduced Risk of Colorectal Cancer With Metformin Therapy in Patients With T2DM. A meta-analysis. Diabetes Care 2011;34:2323-8. [PubMed]
- Noto H, Goto A, Tsujimoto T, et al. Cancer Risk in Diabetic Patients Treated with Metformin: A Systematic Review and Meta-analysis. PLoS One 2012;7:e33411. [PubMed]
- Mamtani R, Pfanzelter N, Heynes K, et al. Incidence of bladder cancer in patients with type 2 diabetes treated with metformin or SU. Diabetes Care 2014;37:1910-7. [PubMed]
- Tzoulaki I, Molokhia M, Curcin V, et al. Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database. BMJ 2009;339:b4731. [PubMed]
- Ioannou GN, Boyko EJ. Metformin and Colorectal Cancer Risk in Diabetic Patients. Diabetes Care 2011;34:2336-7. [PubMed]
- Quinn BJ, Kitagawa H, Memmott RM, et al. Repositioning metformin for cancer prevention and treatment. Trends Endocrinol Metab 2013;24:469-80. [PubMed]
- Dilman VM, Berstein LM, Yevtushenko TP, et al. Preliminary evidence on metabolic rehabilitation of cancer patients. Arch Geschwulstforsch 1988;58:175-83. [PubMed]
- Goodwin PJ, Pritchard KI, Ennis M, et al. Insulin-lowering effects of metformin in women with early breast cancer. Clin Breast Cancer 2008;8:501-5. [PubMed]
- Campagnoli C, Pasanisi P, Abbà C, et al. Effect of different doses of metformin on serum testosterone and insulin in non-diabetic women with breast cancer: a randomized study. Clin Breast Cancer 2012;12:175-82. [PubMed]
- Hadad S, Iwamoto T, Jordan L, et al. Evidence for biological effects of metformin in operable breast cancer: a pre-operative, window-of-opportunity, randomized trial. Breast Cancer Res Treat 2011;128:783-94. [PubMed]
- Hosono K, Endo H, Takahashi H, et al. Metformin suppresses colorectal aberrant crypt foci in a short-term clinical trial. Cancer Prev Res (Phila) 2010;3:1077-83. [PubMed]
- Jiralerspong S, Palla SL, Giordano SH, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol 2009;27:3297-302. [PubMed]
- Margel D, Urbach DR, Lipscombe LL, et al. Metformin use and all-cause and prostate cancer-specific mortality among men with diabetes. J Clin Oncol 2013;31:3069-75. [PubMed]
- Quinn BJ, Kitagawa H, Memmott RM, et al. Repositioning metformin for cancer prevention and treatment. Trends Endocrinol Metab 2013;24:469-80. [PubMed]
- Nobes JP, Langley SE, Klopper T, et al. A prospective, randomized pilot study evaluating the effects of metformin and lifestyle intervention on patients with prostate cancer receiving androgen deprivation therapy. BJU Int 2012;109:1495-502. [PubMed]
- Rezzónico J, Rezzónico M, Pusiol E, et al. Metformin treatment for small benign thyroid nodules in patients with insulin resistance. Metab Syndr Relat Disord 2011;9:69-75. [PubMed]
- A registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. Available online: www.ClinicalTrials.gov