
Serum levels of olfactomedin 4: a biomarker for asthma control state in asthmatics
Introduction
Asthma, a multifactorial chronic airway inflammatory disease that affects 300 million people worldwide (1), is characterized by chronic airway inflammation leading to bronchial hyperresponsiveness. Inflammatory cells, such as eosinophils and neutrophils, are important pro-inflammatory cells in the pathogenesis of asthma and, based on the inflammatory cell count in induced sputum, asthma phenotypes (2). Different clinical characteristics, such as treatment response, are also known to be associated with these inflammatory phenotypes (3). Inflammation in asthma patients can be monitored using traditional biomarkers, including total neutrophil count, sputum eosinophil count ratio, serum periostin level, and fractional exhaled nitric oxide (FeNO) concentration (4,5). However, sputum neutrophil count and FeNO concentrations of detection is limited by various factors, and studies have also reported that sputum neutrophil count is poorly related to blood neutrophil count (6). In addition, many primary health centers do not have the facilities to detect sputum cell counts. Currently, there are no serum biomarkers that effectively reflect neutrophil inflammation in asthma, although potential biomarkers for identification are beginning to emerge.
Olfactomedin 4 (OLFM4, also known as HGC-1 and GW112) is a member of the olfactory inhibitory protein family that has a relatively diverse helical domain at the amino terminus and a well-preserved olfactory domain at the carboxyl terminus (7). OLFM4 is constitutively expressed by neutrophils and in epithelial cells in the gastrointestinal tract (8). Furthermore, OLFM4 is the target gene of the Notch signaling pathway, suggesting it has a possible role in notch-mediated cell differentiation, proliferation, and immune response to inflammation (9). OLFM4 may offer a potential target for these therapies. Notably, a recent microarray analysis study showed high RNA levels of OLFM4 in the blood of asthma patients (10). Based on the results of previous studies, we hypothesized that OLFM4 may play a role in inflammatory immune response in asthma; serum OLFM4 protein expression level may be elevated in asthma patients, which may in turn hold potential clinical value for application in asthmatic diseases.
To test our hypothesis, we conducted an observational study to measure OLFM4 levels in asthmatic patients (controlled asthmatic and uncontrolled asthmatic) and healthy controls. At the same time, Spearman’s correlation analysis and stepwise regression analysis were used to analyze and compare OLFM4 and clinical parameters.
Methods
Study subjects
Seventy-nine subjects (comprising uncontrolled asthmatics, n=35; controlled asthmatics, n=14; and healthy controls, n=30) were enrolled at the Department of Respiratory Medicine, The Second Clinical Medical College of Jinan University between 1st August, 2019 and 10th October, 2019. The diagnosis of asthma was made in line with the criteria of the Global Initiative for Asthma (GINA) 2015 (11). Asthma Control Test (ACT) score was used to determine if the subjects’ asthma was well controlled (a score between 20 and 24) or uncontrolled (a score of below 20). Samples from the healthy control group were obtained from the physical examination center. The study received ethical approval from the Ethics Committee of the College of Science, The Second Clinical Medical College of Jinan University. The participants were requested to complete a questionnaire to provide demographic information. Anthropometric measurements, including height (cm), weight (kg), age, gender, smoking, and allergies, were taken. Body mass index (BMI) was calculated as weight (kg) divided by height (cm) in square meters.
Study measurements
Pulmonary function tests were performed using a SYSTEM 21® device (Minato Medical Science Co., Osaka, Japan) according to the criteria of the American Thoracic Society (ATS)/European Respiratory Society and the Japanese Respiratory Society. Pulmonary function was measured and included percentage of predicted volume (FEV1% pred). FeNO levels were measured using a NIOX MINO® device (Aerocrine AB, Solna, Sweden) according to the manufacturer’s instructions and the ATS guidelines. Peripheral blood cell and sputum cell counts were performed on each patient. Fasting blood samples were drawn and centrifuged, and the serum was placed in plain polystyrene tubes on the same day. The serum samples were sent to the laboratory for storage at −80 °C. Serum hs-CRP and OLFM4 were measured using the enzyme-linked immunosorbent assay (ELISA) kit (Cloud-Clone Corp, CCC, USA).
Statistical analysis
The Shapiro-Wilk test was used to evaluate normality of data. The data are expressed in terms of mean (± standard deviation) and median (quartile range) of parametric and nonparametric data, respectively. For parametric data, Student’s t-test and one-way ANOVA were used to compare two or more groups. For non-parametric data, the Mann-Whitney and Kruskal-Wallis tests were used to compare two or more groups. The classification data were compared by chi-square test or Fisher's exact test. Parametric and non-parametric data were compared using Dunnett’s test and Steel’s test. Stepwise regression analysis and Spearman’s correlation coefficient were used to measure correlation. We applied a Bonferroni correction to determine significance when multiple comparisons were being made. The P value was also corrected with linkage disequilibrium (LD)-adjusted Bonferroni correction. Statistical analysis was performed using SPSS version 19.0.0 (IBM, New York, USA) and Sigma Prot version 11.0 (Systat Software Inc., Illinois, USA).
Results
Clinical characteristics of asthma
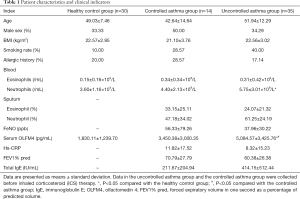
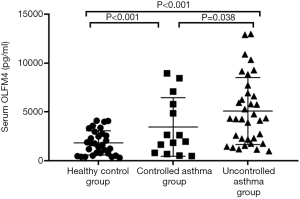
A total of 79 subjects (14 controlled asthmatics, 35 uncontrolled asthmatic, and 30 healthy controls) were enrolled in this study. The clinical characteristics of each group are shown in Table 1. The uncontrolled asthma group had higher serum OLFM4 than the controlled asthma group. The serum OLFM4 level and blood neutrophil count of the uncontrolled asthma group were higher than those of the healthy control group (P<0.001) (Figure 1). There were no significant differences in serum OLFM4 level or blood neutrophil count between the controlled asthma and healthy control groups. There were also no significant differences in age, gender, smoking rate, allergy rate, sputum eosinophil, FeNO, hs-CRP, FEV1% pred, or total IgE between these three groups.
Full table
Association between serum OLFM4 levels and the clinical parameters of asthmatic patents
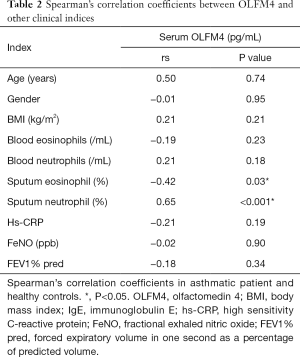
A correlation between OLFM4 and sputum neutrophil was still found in the asthma patients after LD-adjusted Bonferroni correction. However, there was no significant correlation between OLFM4 and sputum eosinophil after LD-adjusted Bonferroni correction (P<0.005). No significant correlations were found between OLFM4 and age, gender, BMI, blood eosinophils, blood neutrophil, FeNO, or FEV1% pred (Table 2).
Full table
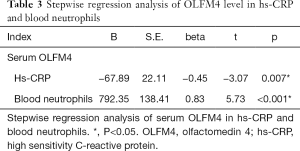
We performed stepwise regression analysis of OLFM4 and the clinical parameters. The P value was corrected with LD-adjusted Bonferroni correction. The results indicated that hs-CRP and blood neutrophil count were positively correlated with OLFM4 after LD-adjusted Bonferroni correction (Table 3).
Full table
Discussion
The accurate assessment of disease severity and control status in asthma patients is based on the prospect of obtaining biomarkers using non-invasive and inexpensive methods. These markers can be used to identify clinical and therapeutic response phenotypes, and to evaluate disease changes and diagnosis. These biomarkers may come from blood, bronchoalveolar lavage, bronchial biopsy, induced sputum, or exhaled condensate. However, a consensus is yet to be reached for the identification and application of ideal and specific biomarkers in asthma. The reasons for this include changes in marker concentrations, methods of sample collection/evaluation, the need for specific tools and skills, and the invasive nature of sampling techniques. Serum marker detection holds the advantage of being a simple sampling method with less trauma for the patient. Therefore, the development of novel serum/sputum biomarkers with increased sensitivity and specificity may facilitate the diagnosis of asthma in the future.
This is the first study to put forward serum OLFM4 level as a serum biomarker for assessing asthma. Serum OLFM4 level was significantly higher in the uncontrolled asthmatic subjects compared to the controlled asthmatic and healthy subjects. At the same time, blood neutrophil count in the uncontrolled asthma group was higher than that in the healthy control group. Serum OLFM4 levels and blood neutrophil count in the controlled asthmatic subjects were similar when compared to those of the healthy subjects. In addition, after LD-adjusted Bonferroni correction, serum OLFM4 level showed a negative correlation with sputum eosinophil, although no statistical significance was observed. The level of serum OLFM4 was positively correlated with neutrophil in the sputum of asthmatic patients, and this correlation was statistically significant. After LD-adjusted Bonferroni correction, stepwise regression analysis indicated that hs-CRP and blood neutrophil count were positively correlated with OLFM4.
Previous studies have reported that granulocytes such as eosinophils and neutrophils are key cells in the inflammation of the airways in asthma and are associated with persistently elevated inflammatory markers (12). Elevated eosinophil count in the blood and sputum is a biologic inflammatory marker that is associated with the increased risk of future asthma exacerbation and extra financial cost in a real-world setting (13). Neutrophils are generally thought to be a consistent feature in asthma exacerbation (14). Inflammatory neutrophils are also able to alleviate airway inflammation through producing the cytotoxic proteases and reactive oxygen species, which are associated with decline in lung function and dysfunction of small airways (15). Meanwhile, OLFM4, which was first cloned from human proto-granulocytes, has many functions, such as promoting cell proliferation, regulating cell adhesion and metastasis, inhibiting cell apoptosis, and immune defense (9).
In this study, serum OLFM4 levels were significantly higher in the uncontrolled asthmatic subjects compared to the controlled asthmatic and healthy subjects. We speculate that these results may be attributed to an increased number of neutrophils and subsequently increased OLFM4 expression in asthma patients. Brand et al. proved that OLFM4 transcription is associated with severity of disease in children with viral lower respiratory tract infections (16). Our results also showed that serum OLFM4 levels were higher in the controlled asthmatic subjects than in the healthy subjects. These findings, together with our findings, suggest that serum OLFM4 may be involved in the pathogenesis of asthma, and that changes in serum OLFM4 levels may serve as an indicator of asthma control status.
In addition, a positive correlation was found between sputum neutrophil and serum OLFM4 levels. However, there was no significant correlation between sputum eosinophil and serum OLFM4 after LD-adjusted Bonferroni correction. This suggests that there may be high levels of OLFM4 in sputum in patients with uncontrolled asthma, and OLFM4 may act as a marker of neutrophilic inflammation in the airways. Gersemann et al. achieved similar results; they found that in healthy controls, OLFM4 staining was predominantly located in the lower crypts, and that glycoprotein was not a protective factor for surface epithelium and mucus. However, in inflammatory bowel disease, OLFM4 immunostaining extended to the surface of the intestinal epithelium, and its presence was found in mucus. They suggested that OLFM4 may act as a substitute for mucin during inflammation and bacterial attack (17). Our stepwise regression analysis revealed a significant correlation between serum OLFM4 and blood neutrophils, indicating that the level of serum OLFM4 is increased in neutrophil-dominated inflammation. In our next study, we will further explore the molecular mechanism of OLFM4 in asthma.
Previous studies have shown a significant relationship to exist between elevated hs-CRP levels and respiratory symptoms such as wheezing, dyspnea after exertion, and nighttime cough (18). Another previous study also demonstrated the correlation between increased serum hs-CRP level and decreased respiratory function. Hs-CRP was demonstrated to be a potentially sensitive marker of asthma severity and disease control (19). In the present study, a positive correlation was found between serum hs-CRP values and serum OLFM4. In addition, serum OLFM4 levels were found to be significantly higher in the uncontrolled asthmatic subjects than in the controlled asthmatic subjects. These results suggest that OLFM4 levels increase as a result of uncontrolled bronchial asthma. However, the present results did not demonstrate a significant correlation between serum OLFM4 levels and FEV1% pred and other lung function indicators.
However, this study had some limitations which must be addressed. The primary limitation was that the sample size was small and the study was not longitudinal by design. OLFM4 levels could not be repeatedly measured during the study period, and so the effect of clinical intervention on OLFM4 expression changes could not be determined. We also need to take into account the influence of other factors, such as specificity, complications, and drugs. Therefore, further research is needed to verify the practical application value of this new biomarker in clinical practice.
Conclusions
In summary, serum OLFM4 values may be used to assess airway inflammation in asthma patients. In future, OLFM4 may serve as an indicator in uncontrolled asthma patients due to its ease of clinical use. OLFM4 may also serve as an indicator to classify subtypes of asthma. Further longitudinal studies are needed to investigate the role of OLFM4 in assessing asthma severity and control status.
Acknowledgments
The Second Clinical Medical College of Jinan University.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.213). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethical clearance was granted by The Second Clinical Medical College of Jinan University who also approved the research (Grant no. LL-KY-2019227). All of the participants were informed about the risk and potential outcomes of the procedure. Informed consent was obtained from each patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol 2015;16:45-56. [Crossref] [PubMed]
- Huang W, Song Y, Wang L. Wenshen decoction suppresses inflammation in IL-33-induced asthma murine model via inhibiting ILC2 activation. Ann Transl Med 2019;7:570. [Crossref] [PubMed]
- Baines KJ, Simpson JL, Wood LG, et al. Sputum gene expression signature of 6 biomarkers discriminates asthma inflammatory phenotypes. J Allergy Clin Immunol 2014;133:997-1007. [Crossref] [PubMed]
- Ricciardolo FL, Sorbello V, Ciprandi G. FeNO as biomarker for asthma phenotyping and management. Allergy Asthma Proc 2015;36:e1-8. [Crossref] [PubMed]
- Matsumoto H. Serum Periostin: A Novel Biomarker for Asthma Management. Allergol Int 2014;63:153-60. [Crossref] [PubMed]
- Perlikos F, Hillas G, Loukides S. Phenotyping and Endotyping Asthma Based on Biomarkers. Curr Top Med Chem 2016;16:1582-6. [Crossref] [PubMed]
- Oue N, Sentani K, Noguchi T, et al. Serum olfactomedin 4 (GW112, hGC-1) in combination with Reg IV is a highly sensitive biomarker for gastric cancer patients. Int J Cancer 2009;125:2383-92. [Crossref] [PubMed]
- Clemmensen SN, Bohr CT, Rørvig S, et al. Olfactomedin 4 defines a subset of human neutrophils. J Leukoc Biol 2012;91:495-500. [Crossref] [PubMed]
- Liu W, Rodgers GP. Olfactomedin 4 expression and functions in innate immunity, inflammation, and cancer. Cancer Metastasis Rev 2016;35:201-12. [Crossref] [PubMed]
- Bigler J, Boedigheimer M, Schofield JPR, et al. A Severe Asthma Disease Signature from Gene Expression Profiling of Peripheral Blood from U-BIOPRED Cohorts. Am J Respir Crit Care Med 2017;195:1311-20. [Crossref] [PubMed]
- Reddel HK, Bateman ED, Becker A, et al. A summary of the new GINA strategy: a roadmap to asthma control. Eur Respir J 2015;46:622-39. [Crossref] [PubMed]
- Vidal G A. Use of inflammatory markers for monitoring paediatric asthma. Rev Chil Pediatr 2015;86:206-13. [PubMed]
- Zeiger RS, Schatz M, Li Q, et al. The association of blood eosinophil counts to future asthma exacerbations in children with persistent asthma. J Allergy Clin Immunol Pract 2015;3:283-7.e4. [Crossref] [PubMed]
- Busse WW. A role for neutrophils in asthma exacerbations. Nat Med 2017;23:658-9. [Crossref] [PubMed]
- Sharma A, Simonson TJ, Jondle CN, et al. Mincle regulates autophagy to control neutrophil extracellular trap formation. J Infect Dis 2017;215:1040. [Crossref] [PubMed]
- Brand HK, Ahout IM, de Ridder D, et al. Olfactomedin 4 Serves as a Marker for Disease Severity in Pediatric Respiratory Syncytial Virus (RSV) Infection. PLoS One 2015;10:e0131927. [Crossref] [PubMed]
- Gersemann M, Becker S, Liu W, et al. OLFM4 – A Bacterial Induced Epithelial Peptide in IBD Downstream of Notch. Gastroenterology 2011;140:S-498-9. [Crossref]
- Monadi M, Firouzjahi A, Hosseini A, et al. Serum C-reactive protein in asthma and its ability in predicting asthma control, A case-control study. Caspian J Intern Med 2016;7:37-42. [PubMed]
- Sileem AE, Embarak S, Meleha MS. Serum eosinophilic cationic protein and high sensitive C-reactive protein as alternative parameters for differentiation of severity stages and monitoring control in bronchial asthma patients. Egypt J Chest Dis Tuberc 2014;63:765-70. [Crossref]


