
Comprehensive management of breast augmentation with polyacrylamide hydrogel injection based on 15 years of experience: a report on 325 cases
Introduction
Polyacrylamide hydrogel (PAAG) is a polymer synthesized from 2.5% acrylamide and 97.5% water (1). It was once considered a non-biodegradable hydrogel that was non-toxic, non-sensitizing, and non-teratogenic (2,3). After its introduction from Ukraine in 1997, it was widely used in China as a soft tissue filler for the repair of soft tissue contours and breast augmentation (3). However, after long-term clinical application, many complications, such as pain, induration, displacement, deformation, milk deposition, and psychological fear, were reported (4). There were even some reports that PAAG may cause breast cancer (5,6). Patients who received PAAG breast injections have been negatively impacted, both physically and mentally. Therefore, on April 30, 2006, PAAG was banned from the clinical application by the Chinese Food and Drug Administration (CFDA).
During the 10 years that PAAG was approved for use, 200,000 women in China were estimated to have undergone breast augmentation by PAAG injection (7). Nowadays, a large number of symptomatic and asymptomatic patients whose breasts were augmented with the gel injection have continued to seek medical advice. Although aspiration surgery was once used for gel removal, it turned out to be inefficient (8). Worse still, the blind operation can easily lead to hydrogel diffusion and injury to the breast. The secondary complications may emerge endlessly, and surgical revision would be extremely complicated. On the other hand, no consensus has been reached for the reconstruction of breast malformation after PAAG removal. Currently, there is a lack of standardized clinical management regarding the removal of the gel and breast reconstruction. Patients seeking help with PAAG removal will continue to emerge for a long time.
This work concluded our algorithm for the management of breast augmentation with PAAG injection based on the 325 patients who had PAAG removed at our hospital from 2003 to 2018. It is the largest cohort of cases reported to date.
Methods
Clinical data
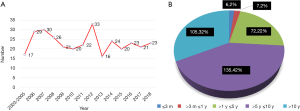
Between January 2003 and July 2018, 325 female patients (650 breasts) who had undergone PAAG injection for breast augmentation in other hospitals and clinics presented to our hospital. The number of patients per year and their history relating to the PAAG injection are recorded in Figure 1. There was no obvious downward trend. The inclusion criteria of the patients consisted of the following: (I) history of PAAG breast injection; (II) appeal to remove the gel; (III) availability of clinical data of original surgery; and (IV) accepting the potential complications of the operation and breast deformity after gel removal.
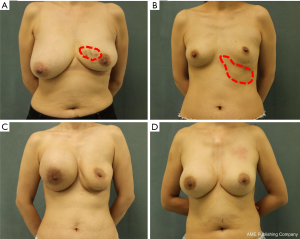
All patient characteristics are summarized in Table 1. Pain-related complications included stabbing pain, distending pain, pressing pain, and referred pain, and the areas of pain were the breast, axilla, chest, and back. Induration included single, multiple, and diffuse indurations. Deformation included atrophy, ptosis, and asymmetry. Displacement included the axilla, forearms, the thoracic-abdominal wall, and even the perineum. Systemic symptoms included headaches, palpitations, hypodynamia, and upper limb numbness, among others. The asymptomatic cases related to harm caused by the gel. Examples of complications are illustrated in Figure 2.

Full table
Surgical technique
Most of the patients were scanned by magnetic resonance imaging (MRI) preoperatively to disclose the general distribution of the gel and its infiltration into the muscle and gland. A semi-annular incision to the lower edge of the areola was made under general anesthesia. Dissection was carried out through the subcutaneous tissue to expose the mammary gland, and then the mammary gland was cut through until the capsule of the PAAG was exposed. The contained PAAG was then removed by aspiration. In case there was multiple subcutaneous sinuses, a careful and thorough intraoperative exploration was necessary to ensure that the PAAG was removed as completely as possible. After this, a large amount of physiological saline was irrigated using a catheter, and the breast was massaged while irrigation took place to make the residual injection particles easier to suck out. If the capsule was thick or the tissue degeneration was obvious, it was necessary to remove the capsule completely, including some muscles and glandular tissue, and send it for pathological examination. Finally, the gland and muscle tissue was probed carefully with the finger. Drainage was maintained until the total drainage was less than 20 mL per day. An elastic garment was worn by the patients for about 2 weeks at least. If the PAAG was significantly displaced elsewhere, additional incisions would need to be considered.
Postoperative follow-up
The breast ultrasound was recommended to determine whether there was PAAG residue 6 months after the operation. The augmentation module of BREAST-Q was used to evaluate patient satisfaction with the operation and their postoperative quality of life (9). The BREAST-Q score calculated through the Q-score program. P<0.05 was regarded as significant. SPSS 25.0 software was used to analyze the data. Fisher’s exact test was used to detect any significant differences between preoperative and postoperative satisfaction (10). The scores of preoperative and postoperative results were divided into three groups: immediate breast reconstruction (IBR), delayed breast reconstruction (DBR), and no breast reconstruction (NBR). Data were also collected for the postoperative course, including complications and aesthetic evaluation.
Results
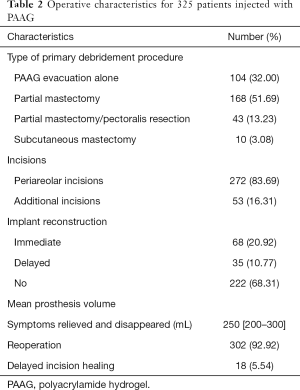
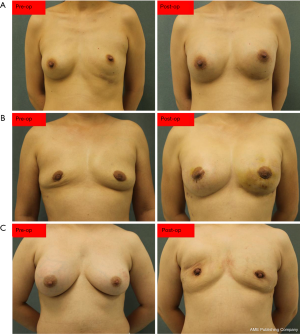
Besides the PAAG evacuation and capsulectomy, the surgery had to include partial mastectomy or partial pectoralis muscle resection or subcutaneous mastectomy in most of the patients due to the extensive infiltration of the PAAG. However, complete removal of the PAAG was impossible. Due to the strong desire of some patients, breast reconstruction with prosthesis implantation was performed. All the results are detailed in Table 2. The average hospital stay was 9 days (range, 3–23 days). The period of follow-up was 12 months. Two-hundred and eight patients were available for follow-up. The results of the follow-up evaluation are summarized in Tables 3 and 4. All of the categories presented with statistically significant differences (P<0.05), which demonstrated the effectiveness of our surgical management. However, for the patients without breast reconstruction, although PAAG-related complications and psychological fears were reduced or even disappeared altogether, their quality of life was significantly lower than that of patients with IBR or DBR. Due to completely different management, no comparison was implemented among the three groups. The surgical results are set out in Figure 3.
Full table

Full table

Full table
Discussion
Complications of PAAG injection
PAAG was banned by the CFDA in 2006 because of several associated complications, including contour abnormalities, abnormal skin sensation, pain, induration, malignant breast tumors, aseptic inflammation, leakage, and hematoma (4). Apart from malignant breast tumors, these complications were found in our patients, and the top three complications were pain, induration, and deformation (Table 1). Although there was no patient with breast cancer caused by PAAG found in our department, it has been reported in several recent studies (5,6). Recent studies suggested that PAAG may decompose acrylamide monomers under multiple factors such as body fluids, various enzymes, and mechanical stimuli after injection into the body, with carcinogenic and toxic effects on the nervous and reproductive systems (1,11-13).
PAAG was often injected into the retro mammary pocket and then formed a thin capsule that could break due to gravity, pressure, or trauma. It may migrate along with the loose connective tissue and extend to the adjacent area including the inframammary fold, axilla, infraclavicular region, abdomen, and even the perineum. These may lead to changes in breast shape. Induration was usually caused by the incorrect injection, improper postoperative massage, and uneven distribution of the PAAG. Pathology results of induration showed extensive fibrous tissue proliferation generated by the stimulation of the PAAG. Pain may be related to tissue degeneration, local tissue adhesion, infection, and aseptic inflammation. Poor intraoperative disinfection and bacterial contamination of the filler may result in acute infection.
Most of these complications could be relieved by removing the PAAG and severely degenerated tissue. However, some complications affect surgery choice. Infection is a contraindication for implants. Several complications make thorough PAAG removal challenging and create unsuitable conditions for immediate implant reconstruction, which include extensive PAAG displacement (involving the back, lower abdomen, upper arm, and sternum), extensive infiltration, dispersive induration, and confusing injection level. Limited PAAG displacement may deform the inframammary fold (IMF), which could be reconstructed by suture and flap techniques in most cases (14-16). If there is not adequate tissue to reconstruct IMF, DBR might be a better choice.
Surgical method
Currently, there are three common methods for PAAG removal: blunt aspiration, direct visualization surgery, and endoscopic surgery. We recommend the second option. As far as we were concerned, the efficiency of the blunt aspiration was limited by the following aspects: first, the channels generated by the back-and-forth movement would disseminate the PAAG, making its distribution more chaotic and damage to tissue more serious. Second, scar formation and fibrous tissue hyperplasia after aspiration may increase the difficulty of possible secondary surgery. Moreover, even under the guidance of ultrasound, the suction method could not completely remove the infiltrating capsule and fascia. Finally, it was impossible for the aspiration method to allow for evaluation of the extent of damage to the pectoralis major or the gland during surgery, causing dilemmas for the subsequent breast reconstruction. When we performed surgery on 26 patients (8%) with a history of aspiration, we could clearly see the PAAG residue in the breast. In addition to local tissue fibrosis, the breast structural confusion was more serious than in those who had no history of aspiration therapy. As for the endoscopic surgery, although the incision and postoperative scar were better concealed, its insufficient exposure of the pocket resulted in a more complicated surgical operation with higher technical requirements and higher costs. More importantly, the surgical effect was not widely confirmed. Perhaps for patients who especially want to avoid a breast scar, it is an alternative.
There were two types of incision options for surgery under direct vision: the semi-periareola incision and the IMF approach. However, we advocated that the periareola incision endowed a better surgical vision, and it was the easiest way to reach the surgical area. The advantages of our surgical methods were concluded as follows: (I) operation under direct vision was more efficient and safer to remove the hydrogel in the cyst completely; (II) the infiltrated capsule and the surrounding extensive necrotic and degenerative tissues could be removed simultaneously; (III) the hydrophilicity of the hydrogel was utilized during the operation so that the PAAG nodules, which were difficult to reach absorbed water and swelled. The enlarged nodules were then easy to find for surgical resection, and (IV) the residual PAAG content was reduced to the minimum by irrigating the pocket with saline repeatedly. Nevertheless, the limitation of periareola incision was that the surgical incision was relatively small for patients with the small areola, and the operation was difficult in patients with the distant displacement of PAAG to the abdominal wall.
The IMF approach had the following shortcomings: (I) it was difficult or even impossible to dissect the infiltrated capsule and fascia through the IMF incision; (II) the surgical area was not well exposed, and it was difficult to clearly see the various quadrants of the breast compared to the areola incision, especially in patients with PAAG displacement to the subclavian and axilla region; and (III) postoperative scars were more obvious. Of course, this approach has its own advantages in certain situations and was particularly suitable for the few patients in which the injection was shifted toward the abdominal wall. It may also cause relatively less damage to breast tissue compared with periareola incision.
There is an additional point to note: important structures such as muscles and nerves should be preserved as much as possible, although this may lead to residual PAAG. If the PAAG had invaded those structures severely, an experienced surgeon was required to weigh the pros and cons.
Postoperative reconstruction
Breast deformity after the removal of the PAAG caused a serious negative impact on patients’ quality of life. However, for security reasons, we had to control the indications for reconstructive surgery strictly. We believe that at least two points should be met: (I) a strong desire in the patient for breast reconstruction; and (II) adequate healthy soft tissue for coverage of the prosthesis.
As for the timing of breast reconstruction, we divided the patients into two groups of IBR and DBR. Patients with no signs of acute inflammation and no obvious PAAG residue in the implant pocket were candidates to undergo IBR. However, patients with acute inflammation, as well as those with unclear PAAG residue, could only be offered DBR based on the results of the review six months after the debridement operation. We especially did not recommend breast reconstruction surgery for patients with breast skin ulceration or for patients with severe gland and pectoralis major damage after the PAAG removal.
The placement of the prosthesis after PAAG removal has been another topic of discussion. Although most studies recommended sub-pectoral or dual plane breast reconstruction, we preferred to place the implant into the residual cavity, which meant a pre-pectoral plane for several considerations. First, the reconstruction based on the residual cavity was anatomically less invasive, technically less challenging, less time consuming, and be associated with less pain, as it obviated the need to elevate the pectoralis major muscle. In addition, nearly half of the PAAG-injected patients had complained of pain, and they showed significant resistance to the postoperative pain of reconstruction surgery during the preoperative conversation. Cattelani et al. evaluated pre-pectoral breast reconstruction and confirmed less postoperative pain, faster recovery from postoperative upper extremity functional morbidity as well as economic advantages compared to sub-muscular technology (17). Second, pre-pectoral reconstruction eliminated animation deformity and resulted in more natural breast shape, particularly during the adduction of the humerus. As many studies have indicated, even subtle animation deformity can be particularly bothersome for regular performance of upper body exercise (18-20). Third, the incidence of possible hematoma and seroma was lower. A suitable prosthesis utilized and filled the residual cavity. However, the sub-pectoral procedure meant that another pocket needed to be dissected, which may lead to a higher probability of dead space formation. Fourth, the majority of patients had enough well-perfused tissue to cover the prosthesis, although part of their glands was removed due to PAAG infiltration. After all, debridement surgery in patients with PAAG injection breast augmentation does not require complete removal of the mammary gland as radical surgery in breast cancer patients does. Moreover, more and more studies had verified that pre-pectoral implant-based breast reconstruction was a viable reconstructive option (21-23). The remaining breast tissue was considered capable of prosthetic coverage and superior to the acellular dermal matrices (ADM). Our postoperative follow-up results supported this argument. However, it was important to be aware of the need to retain as much of the healthy autologous glandular tissue as possible during the debridement surgery, which required an experienced surgeon to weigh the pros and cons. Fifth, the incidence rate of visible rippling over the permanent implants was lower compared to traditional pre-pectoral breast reconstruction. Due to gravity, the PAAG displacement was more common in the lower breast glands. Therefore, debridement surgery did not require extensive removal of the upper breast gland, which meant there was thicker tissue to cover the prosthesis. Finally, there were some issues that could not be ignored in the sub-pectoral reconstruction of the breast. Partial and complete loss of normal muscle fiber architecture had been documented when evaluating biopsies with electron microscopy after sub-pectoral tissue expansion and breast reconstruction (24). There were also reports of significant reductions in function and strength among patients with sub-pectoral implants (25,26). In the rare cases in which adequate tissue coverage was not available, we recommended a second stage sub-pectoral reconstruction. Our preliminary results suggested that this approach could lead to predictable outcomes and aesthetically stable reconstructions. To date, there has been no instance reported of animation deformity or capsular contracture.
However, this method is not suitable for patients without adequate healthy soft tissue coverage. Additionally, the residual cavity after gel removal was generally irregular, and the size of the residual cavity was often inconsistent with the size of the prosthesis, which may easily lead to the displacement of the prosthesis and the morphological change of the breast. Meticulous attention should be focused on eliminating dead space as much as possible by repositioning excess subcutaneous tissue to fill the spaces and collapse the pocket, which could prevent seroma formation.
Furthermore, if the residual cavity was too small, it could be peeled off to the ideal size along the base of the residual cavity. On the contrary, proper suture and reinforcement of the lower pole and external wall of the residual cavity were needed to reduce the residual cavity and fix the prosthesis position because the residual cavity was mainly weak at the lower and outer sides due to the gravity and pressure direction. Many Chinese scholars also used ADM to repair and strengthen residual cavities or cover the prosthesis, but the clinical efficacy of ADM in PAAG-injected patients still lacked further verification. Fourthly, when creating the reconstructive pocket, care should be taken to maintain a uniform thickness of residual glands, which is necessary to rebuild the natural feel of the breast. If not, it could be remedied by autologous fat transplantation, which happened on only one patient in our series. However, due to the poor local blood supply and severe scar hyperplasia, we recommended that autologous fat graft could be performed in small amounts several times to prevent fat liquefaction and necrosis. At present, there are many reports that adipose-derived stem cells in autologous fat can improve local scars, and even differentiate and proliferate to produce new fat particles (27-29). Therefore, the autologous fat graft may be an alternative to repair breast deformities in selected patients.
Conclusions
With the guidance of preoperative MRI images, surgery including PAAG evacuation, pathologic tissue excision, and pocket irrigation via the periareolar incision was a reliable method to ensure that the maximal removal of PAAG. Immediate or secondary breast reconstruction by subglandular placement of silicone prostheses showed a reliable mid-term effect.
Acknowledgments
Funding: This work was financially supported by the National Key R&D Program of China (2019YFA0110500) and the National Nature Science Foundation of China (No. 81701922 and No. 81873941).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.68). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of the Tongji Medical College, Huazhong University of Science and Technology. Patients provided written consent for the use of their images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Amended final report on the safety assessment of polyacrylamide and acrylamide residues in cosmetics. Int J Toxicol 2005;24 Suppl 2:21-50. [Crossref] [PubMed]
- Okubo M, Hyakusoku H, Kanno K, et al. Complications after injection mammaplasty. Aesthetic Plast Surg 1992;16:181-7. [Crossref] [PubMed]
- Christensen LH, Breiting VB, Aasted A, et al. Long-term effects of polyacrylamide hydrogel on human breast tissue. Plast Reconstr Surg 2003;111:1883-90. [Crossref] [PubMed]
- Cheng NX, Wang YL, Wang JH, et al. Complications of breast augmentation with injected hydrophilic polyacrylamide gel. Aesthetic Plast Surg 2002;26:375-82. [Crossref] [PubMed]
- Cheng NX, Liu LG, Hui L, et al. Breast cancer following augmentation mammaplasty with polyacrylamide hydrogel (PAAG) injection. Aesthetic Plast Surg 2009;33:563-9. [Crossref] [PubMed]
- Xiao ZB, Liu Y. The relationship between breast cancer and breast augmentation with injected polyacrylamide gel: two case reports. J Plast Reconstr Aesthet Surg 2008;61:981-2. [Crossref] [PubMed]
- Chen L, Sha L, Huang SP, et al. Treatment for displacement of PAAG mixture after injection augmentation mammoplasty. Int J Clin Exp Med 2015;8:3360-70. [PubMed]
- Qiao Q, Wang X, Sun J, et al. Management for postoperative complications of breast augmentation by injected polyacrylamide hydrogel. Aesthetic Plast Surg 2005;29:156-61; discussion 162. [Crossref] [PubMed]
- Pusic AL, Klassen AF, Scott AM, et al. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg 2009;124:345-53. [Crossref] [PubMed]
- Jung SH. Stratified Fisher's exact test and its sample size calculation. Biometrical journal Biometrische Zeitschrift 2014;56:129-40. [Crossref] [PubMed]
- Jin R, Luo X, Wang X, et al. Complications and Treatment Strategy After Breast Augmentation by Polyacrylamide Hydrogel Injection: Summary of 10-Year Clinical Experience. Aesthetic Plast Surg 2018;42:402-9. [Crossref] [PubMed]
- Virk-Baker MK, Nagy TR, Barnes S, et al. Dietary acrylamide and human cancer: a systematic review of literature. Nutrition Cancer 2014;66:774-90. [Crossref] [PubMed]
- Xi TF, Fan CX, Feng XM, et al. Cytotoxicity and altered c-myc gene expression by medical polyacrylamide hydrogel. J Biomed Mater Res A 2006;78:283-90. [Crossref] [PubMed]
- Nava M, Quattrone P, Riggio E. Focus on the breast fascial system: a new approach for inframammary fold reconstruction. Plast Reconstr Surg 1998;102:1034-45. [Crossref] [PubMed]
- Handel N, Jensen JA. An improved technique for creation of the inframammary fold in silicone implant breast reconstruction. Plast Reconstr Surg 1992;89:558-62. [Crossref] [PubMed]
- Terao Y, Taniguchi K, Tomita S. A new method for inframammary fold recreation using a barbed suture. Aesthetic Plast Surg 2015;39:379-85. [Crossref] [PubMed]
- Cattelani L, Polotto S, Arcuri MF, et al. One-Step Prepectoral Breast Reconstruction With Dermal Matrix-Covered Implant Compared to Submuscular Implantation: Functional and Cost Evaluation. Clinical Breast Cancer 2018;18:e703-11. [Crossref] [PubMed]
- Nigro LC, Blanchet NP. Animation Deformity in Postmastectomy Implant-Based Reconstruction. Plast Reconstr Surg Glob Open 2017;5:e1407. [PubMed]
- Spear SL, Schwartz J, Dayan JH, et al. Outcome assessment of breast distortion following submuscular breast augmentation. Aesthetic Plast Surg 2009;33:44-8. [Crossref] [PubMed]
- Vidya R, Iqbal FM. A Guide to Prepectoral Breast Reconstruction: A New Dimension to Implant-based Breast Reconstruction. Clinical Breast Cancer 2017;17:266-71. [Crossref] [PubMed]
- Bernini M, Calabrese C, Cecconi L, et al. Subcutaneous Direct-to-Implant Breast Reconstruction: Surgical, Functional, and Aesthetic Results after Long-Term Follow-Up. Plast Reconstr Surg Glob Open 2016;3:e574. [PubMed]
- Jones G, Antony AK. Single stage, direct to implant pre-pectoral breast reconstruction. Gland Surg 2019;8:53-60. [Crossref] [PubMed]
- Gabriel A, Maxwell GP. Prepectoral Breast Reconstruction in Challenging Patients. Plast Reconstr Surg 2017;140:14s-21s. [Crossref] [PubMed]
- Gur E, Hanna W, Andrighetti L, et al. Light and electron microscopic evaluation of the pectoralis major muscle following tissue expansion for breast reconstruction. Plast Reconstr Surg 1998;102:1046-51. [Crossref] [PubMed]
- Banbury J, Yetman R, Lucas A, et al. Prospective analysis of the outcome of subpectoral breast augmentation: sensory changes, muscle function, and body image. Plast Reconstr Surg 2004;113:701-7; discussion 708-11. [Crossref] [PubMed]
- Sarbak JM, Baker JL Jr. Effects of breast augmentation on pectoralis major muscle function in the athletic woman. Aesthet Surg J 2004;24:224-8. [Crossref] [PubMed]
- Kapur SK, Katz AJ. Review of the adipose derived stem cell secretome. Biochimie 2013;95:2222-8. [Crossref] [PubMed]
- Krastev T, van Turnhout A, Vriens E, et al. Long-term Follow-up of Autologous Fat Transfer vs Conventional Breast Reconstruction and Association With Cancer Relapse in Patients With Breast Cancer. JAMA Surg 2019;154:56-63. [Crossref] [PubMed]
- Caruana G, Bertozzi N, Boschi E, et al. Role of adipose-derived stem cells in chronic cutaneous wound healing. Annali Italiani Di Chirurgia 2015;86:1-4. [PubMed]


