
Incidence of patients with bone metastases at diagnosis of solid tumors in adults: a large population-based study
Introduction
Bones are one of the most common sites of metastases for many types of solid cancers (1-4). Bone metastases have an increased risk of serious skeletal-related events (SREs), such as pathological fractures, pain, hypercalcemia, and spinal cord compressions, which can seriously impair patients’ quality of life (5-9). Bone metastases also lead to a significant increase in mortality and morbidity (10-12).
In the United States, around 350,000 people die each year from bone metastasis (13). Several patients with bone metastasis and SREs are affected by breast or prostate cancer, while lower rates are observed in patients with lung, kidney, thyroid, or other cancers (4,14). The incidence rate of bone metastases in the United States is still unknown, and estimates have varied from 21,000–400,000 per annum. Though bone metastases can impact the mortality and quality of life of patients with cancer, more extensive population-based studies researching the incidence and prognosis of patients with bone metastases are lacking. Previous studies have shown that the prevalence of bone metastases is more than 70% in patients with metastatic breast and prostate cancer, and approximately 30% in metastatic renal cell carcinoma (1,12,15-18). However, there are no studies which provide information on the incidence of bone metastasis in other common cancers or systemic malignancies. Also, earlier studies cannot reflect the recent incidence and survival trends of patients with bone metastases (19).
Our study was conducted to estimate the incidence and prognosis of patients with bone metastases using the Surveillance, Epidemiology, and End Results (SEER) database that includes information on cancer incidence, treatment, and survival for approximately 30% of the American population (20). Specifically, we estimated the incidence proportion of patients’ bone metastases among solid tumors, considering tumor histology at the time of initial diagnosis.
Methods
Data source and cohort population
For our study, the SEER database was used. Inclusion criteria were adult patients (age ≥18 years) with a diagnosis of an invasive solid tumor originating outside of the bone and joints between January 1, 2010, and December 31, 2016. Patients were excluded if information relating to the presence or absence of bone metastases was unavailable. Other exclusion criteria were patients with diagnosis of carcinoma in situ and patients with a diagnosis of a rare tumor such as thymus cancer, heart cancer, mediastinum cancer, pleura cancer, spleen cancer, reticuloendothelial cancer, skin cancer, connective and soft tissue cancer, adrenal gland cancer, parathyroid gland cancer, other endocrine gland cancer, mesothelioma, Kaposi sarcoma, and lymphoma. For the survival analysis, patients were excluded if they were diagnosed at the time of the autopsy or at the issuing of the death certificate, or if they had unknown survival time or survival status.
Statistical analysis
Total numbers and incidence proportions of patients who were diagnosed with bone metastases were computed and then stratified by cancer type. The patients with lung cancer were classified by tumor histology using the International Classification of Disease for Oncology, 3rd Edition (ICD-O-3). Metastatic stage was conducted following the 7th edition of the American Joint Committee on Cancer staging manual, and then we defined patients with metastatic cancer as a subset with metastatic disease. We defined patients with bone metastases as a subset with bone metastases. The incidence proportion was defined as the number of patients diagnosed with bone metastases and a specific primary cancer divided by the total number of individuals diagnosed with that primary cancer; we also defined a second incidence proportion in which the denominator was restricted to patients with metastatic disease. The metastatic status of the brain, lung, and liver was also available, and we used it to characterize the extent of systemic disease, and subsequently calculated the incidence and median survival of patients with bone metastases classified by the extent of systemic disease. For survival estimates, we used the Kaplan-Meier method, taking into account a P value ≤0.05 as significant. The statistical analysis was generated and visualized with SPSS software (version 18; IBM Corp., USA).
Results
First, we identified 9,316,084 patients aged ≥18 years who were diagnosed with an invasive solid malignancy originating outside of the bone and joints between January 1, 2010 and December 31, 2016. The SEER database includes information on cancer incidence, treatment, and survival for approximately 30% of the American population. Patients were excluded in the cohort if the carcinoma is was in situ. Patients with an unknown bone metastases stage were excluded, leaving 2,470,634 patients for analysis. We then selected most of the sites where cancer often occurred, leaving 2,207,796 patients for the final incidence analysis (Figure 1).
Between 2010–2016, a total of 2,207,796 patients had a diagnosis of cancer from common solid organs, and 426,594 patients had metastatic disease. We found 113,317 patients with bone metastases, which accounted for 5.13% of all patients, and 26.56% of those patients had metastatic disease.
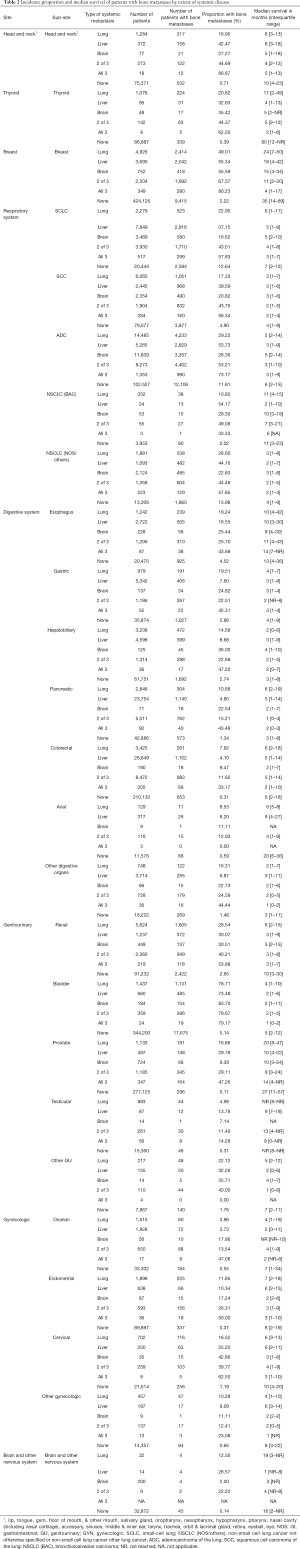
Next, we found that the rate of bone metastases varied widely by primary cancer type (Table 1; Figure 2). As shown in Table 1, the bone metastasis rate is the highest in lung cancer. More specifically, the rate of bone metastases is 23.19% for small-cell lung cancer (SCLC), 22.50% in non-small cell lung cancer not otherwise specified and others [NSCLC (NOS/others)], 20.28% for adenocarcinoma of the lung (ADC), 8.44% in squamous cell carcinoma of the lung (SCC), and 4.11% in bronchioloalveolar carcinoma [NSCLC (BAC)]. In analyzing the gastrointestinal tumors, the rate of bone metastases is 7.99% in the esophagus, 4.47% in the gastric system, 4.42% in the hepatobiliary system, 3.80% in the pancreas, 3.26% in other digestive organs, 1.24% in colorectum, and 1.00% in the anus. Among patients with renal cancer, prostate and breast cancer,16.08%, 5.69%, and 3.73% of patients were respectively found to have bone metastases.
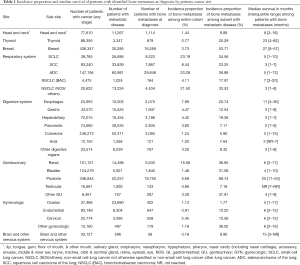
Full table
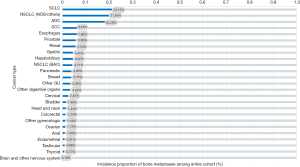
Moreover, Table 1 and Figure 3 show the incidence proportion of patients with bone metastases among the metastatic subset (patients with stage IV disease at diagnosis). The incidence of bone metastases among the metastatic subset is 88.74% in prostate cancer, 53.71% in breast cancer, and 38.65% in renal cancer. In descending order, patients with other cancers of the genitourinary system (except renal, bladder, prostate, testicular) (37.91%), ADC (36.86%), other gynecologic cancers (except ovarian, endometrial, and endometrial cancer) (36.02%), SCLC (34.56%), NSCLC (NOS/others) (33.55%), and bladder cancer (31.08%), showed an incidence proportion of bone metastases of >30%.
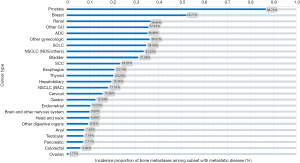
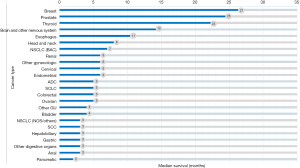
Table 1 and Figure 4 show the median survival time of patients with bone metastases in different systemic malignancies. The median survival time among patients with breast cancer and bone metastases, prostate cancer, and bone metastases and thyroid cancer, and bone metastases are 27, 25, and 23 months, respectively. The survival time of the 3 cancers mentioned above is higher than the others. The median survival time of other tumors with bone metastases is less than 10 months. In general, survival is worse in patients with digestive system cancer and bone metastases compared with other types of primary cancer. The median survival time in patients with hepatobiliary, gastric, and anal tumors is 3 months. Among patients with pancreatic tumor and bone metastases, the median survival time is 2 months.
Incidence proportion and median survival time of patients with bone metastases, as organized based on the presence or absence of brain, liver, and lung metastases, are shown in Table 2. In summary, the incidence of bone metastasis was higher, and survival time was shorter among patients with more extensive metastases at diagnosis. The incidence of bone-only metastasis was 13.98% in NSCLC (NOS/others), 12.64% in SCLC, and 11.81% in ADC. In descending order, patients with bladder cancer (5.14%), SCC (4.90%), esophageal cancer (4.52%), gastric cancer (2.86%), hepatobiliary cancer (2.74%), renal cancer (2.65%), breast cancer (2.22%), and NSCLC (BAC) (2.02%) showed an incidence proportion of bone metastases of >2%. The median survival time among patients with bone-only metastases in thyroid cancer, breast cancer, prostate cancer, and anal cancer was 60, 35, 27, and 20 months, respectively.
Full table
For patients with head and neck cancer, the incidence of comorbidity with liver metastases and bone metastasis was 42.47%. Among patients with was cancer, the incidence of comorbidity with liver metastasis and bone metastasis is higher in NSCLC (BAC) (54.17%), ADC (53.73%), and NSCLC (NOS/others) (44.10%) than in SCC (39.59%) and SCLC (37.15%). Furthermore, we found that the incidence of comorbidity of brain metastases and bone metastasis was higher than other sites among patients with digestive system cancer and gynecologic cancer.
Table S1 shows the incidence proportions of patients diagnosed with bone metastases, classified according to primary cancer, age, race, and gender. Median survival estimates, and those of age, race, gender, and cancer type, are displayed in Table S2.
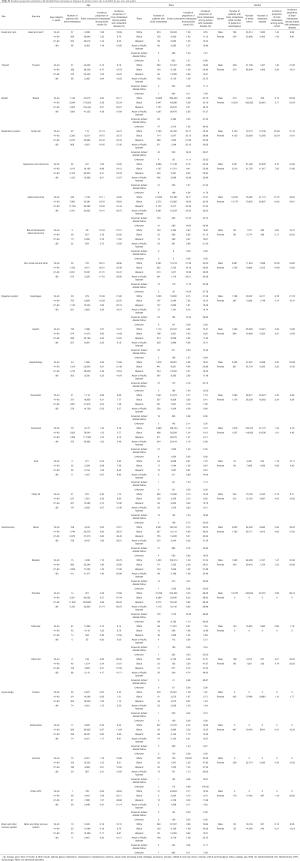
Full table
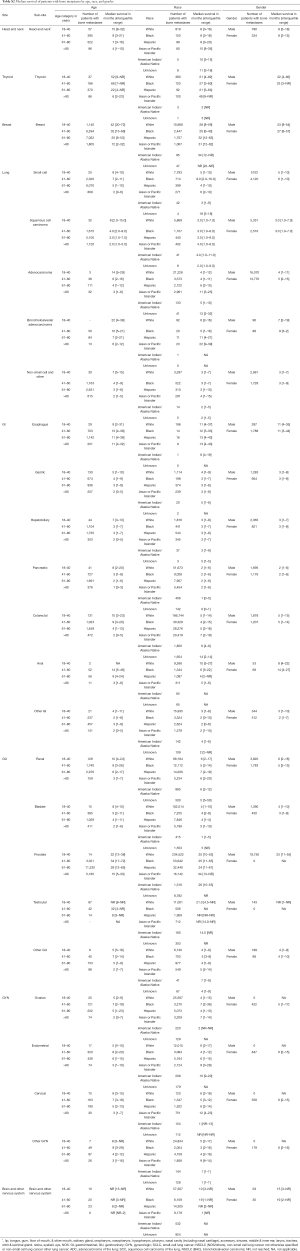
Full table
Discussion
In our study, we showed the number and incidence proportion of patients with bone metastases and the prognosis of identified bone metastases among patients with cancer of the digestive system with the lowest median survival time. To our knowledge, this is the first epidemiologic study of bone metastases using the entire SEER database. Roodman et al. pointed out that the exact prevalence of bone metastasis remains unknown, and patients with bone metastases are usually incurable (21). Therefore, it is probable that our study may have widespread applications and could be useful in the formation of screening paradigms for bone metastases, clinical treatment and trial design, and counseling of different subsets of patients with cancer.
Incidence of bone metastasis
In 1997, Coleman et al. reported that the incidence of bone metastasis was 30–40% for patients with lung cancer, which is higher than our results (22). In 2013, Sathiakumar et al. reported that the incidence of bone metastasis among lung cancer patients was 19.8%, based on data from 1999 to 2006 (23). Al Husaini et al. pointed out that the incidence of skeletal metastasis in advanced-stage lung cancer was 30–40% (24).
In our study, we found that the incidence of bone metastases was 16.89% in patients with newly diagnosed lung cancer and 33.10% in patients with metastatic lung cancer. A comparison of our findings with those of other studies confirms that the rate of bone metastasis among lung cancer is gradually decreasing, which has contributed to the popularization of screening and the development of effective treatment strategies. Additionally, we found the incidence of bone metastases among patients with SCLC to be higher than that of patients with non-small cell lung cancer (NSCLC). Yerushalmi et al. found that the incidence of bone metastases among patients with breast cancer had decreased steadily over 3 time periods (25) (1989–1991: 7.5%, 1992–1997: 5.3%, 1998–2001: 3.5%). Jensen et al. noted that the incidence rate of bone metastases among patients with breast cancer was 3.6% in a population of 35,912 patients (19). In this study, the incidence was slightly lower than that reported by earlier studies. Pietropaoli et al. indicated that only approximately 1% of patients with stage IV carcinoma of the head and neck had concomitant bone metastases (26), which is similar to our results.
Previous studies have reported that the incidence rate of bone metastases in patients with hepatocellular carcinoma ranges from 3% to 20% (27,28). These findings are consistent with our results. However, the studies just mentioned above only discussed the incidence rate of bone metastases in single cancers. There is no study which systematically analyzes the incidence of bone metastases in different cancers types. Our study shows that lung cancer is most likely to present with bone metastasis, which may support recent screening guidelines. Previous studies have shown that the incidence rate of bone metastases in metastatic prostate cancer is over 80%, while bone metastases occur in 65–80% of patients with metastatic breast cancer (29-34). Our study also indicates that the incidence proportion of bone metastases is high in patients with breast or prostate cancer. Previous studies have shown that bone metastases occur in approximately 30% of patients with invasive bladder cancer and renal cancer (35-38). In our study, the incidence rate of bone metastases was 16.08% and 1.48% in renal cancer and bladder cancer, respectively. Furthermore, bone metastases accounted for 38.65% and 31.08% of metastatic renal and bladder cancers, respectively. Though the rate of bone metastases is not high in bladder cancer, bone cancer accounts for a relatively large portion of the metastatic sites among patients with metastatic bladder cancer. Therefore, we must pay attention to the screening of bone metastases in this setting.
Survival
Our results show that cancer presented at diagnosis with bone metastases with the longest median survival time is breast cancer (27 months), followed by prostate cancer (25 months), and thyroid cancer (23 months). Previous studies had shown that the median survival time is 30 and 28 months among breast cancer patients with bone metastases and prostate cancer patients with bone metastases (39,40). These results are similar to ours. Bhatia reported that the prognosis of hepatocellular carcinoma with bone metastasis is extremely poor, with a median survival of only 1–2 months (41). We also found that the median survival time is the shortest in cancers of the digestive system. Silvestris et al. indicated that the median survival was 6 months in gastric cancer patients after bone metastasis diagnosis (42). Our results showed the median survival is 3 months among gastric cancer patients with bone metastases, which was a little shorter than the earlier study.
Clinical implications
Bone metastases are associated with an increased risk of mortality for patients with cancer and may lead to a poor quality of life (17,43,44). The early detection of bone metastases may minimize morbidity and mortality and lead to a better quality of life (45-47), while also being a fundamental step in anticancer treatment (48-50). The National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology also recommended routine screening bone metastases in patients with SCLC, prostatic cancer, and high-metastasis-risk renal cancer (51-54). Our results support the current guidelines as these cancers are all at high risk of the development of bone metastases, although the routine use of bone screening is not recommended in NSCLC. Our data showed that the incidence of bone metastases at diagnosis in NSLCLC is relatively high. Therefore, in patients with a diagnosis of stage IV NSCLC, special focus should be dedicated to the screening of the bones.
Furthermore, screening of bone metastases is not routinely performed for patients with esophagus cancer (55). However, our data revealed a 7.99% and 20.74% incidence proportion of bone metastases in patients with esophagus cancer and metastatic esophagus cancer, respectively. Therefore, routine screening of bone metastases is necessary for patients with these cancers.
As screening was not routinely performed in these patients, bone metastases are always discovered only as a result of SREs, which may be a more advanced disease that shortens survival (56) and often requires surgical intervention or a more complex treatment plan. However, surgery for pathological fracture and loss of motor function and mobility might also increase mortality (5). Our data show a relatively high rate of bone metastasis in these populations—one which may be underestimated. Therefore, our findings may support the need to routinely screen for bone metastases at diagnosis for these patients.
As for patients with head and neck cancers, the incidence of comorbidity for liver metastasis and bone metastases is high. Patients with breast and bladder cancer have a high incidence of comorbidity with bone metastasis and brain, liver, or lung metastasis. Therefore, a diagnosis of bone metastases may be a strong signal that other sites of metastases may exist in patients. For lung cancer, we should pay attention to the comorbidity of bone metastases and liver metastases, while for digestive system cancer and gynecologic cancer, we may be more concerned about the comorbidity of bone metastases and brain metastases.
Previous studies have shown that patients with bone-only metastases have a better prognosis (57-59). For instance, previous investigators pointed out that the median survival time of patients with breast cancer and bone-only metastasis was about 20–50 months, which is much longer than multiple sites metastasis (60-63). This result is consistent with our findings. The incidence of bone-only metastasis is high in NSCLC (NOS/others), SCLC, ADC, bladder cancer, and esophageal cancer. So, for patients with these cancers, we must find a specific metastasis status. Because the treatment of bone-only metastasis is different from other sites or multiple sites metastasis (60), identifying bone-only metastasis may help clarify the clinical course, improve the prognosis for patients with bone-only metastasis, and estimate median survival time more accurately (64,65).
Our data also have value for the design of clinical trials. The data in our study may help investigators quantify the specific number of patients needed to be excluded from the trial enrollment, with bone metastasis as an exclusion criterion. Moreover, for studies or trials which are related to bone metastases, our study can provide generalizable estimates of incidence and prognosis for use in calculations and some trial design.
Limitations
The present study has some potential limitations. Firstly, we only identified bone metastases at initial cancer diagnosis, and, because SEER cannot provide information relating to disease recurrence, we could not screen patients with bone metastases after initial diagnosis. Secondly, we do not have information relating to the number size and exact location of the bone metastases. Thirdly, screening was not conducted across all malignancies, and therefore some data of metastases might have been missed. Finally, treatment information for the metastatic sites was not provided, so we could not study the treatment received by each patient.
Although this study has several limitations, it provides new information regarding the epidemiology of bone metastasis. Incidence of bone metastasis and the specific proportion of patients with bone metastases among different cancer types could help in the development of the formation of screening paradigms for bone metastases, clinical treatment and trial design, and counseling of different subsets of patients with cancer.
Conclusions
The results of this study provide population-based estimates of the incidence of bone metastasis and the specific incidence proportion of patients with bone metastasis diagnosis of solid tumors. We have shown that prostate cancer and breast cancer are most likely to occur with bone metastases. Additionally, the rate of bone metastasis was more than 20% in patients with lung, renal, bladder, thyroid, and esophageal cancers. We also found that the median survival time was more than 20 months in bone metastatic breast cancer, prostate cancer, and thyroid cancer. Conversely, the median survival time was the shortest in gastrointestinal, lung, and gynecologic cancer with bone metastases. These data may help clinicians in their justification of using of bone screening, which may also have an important role in clinical trial design and better prognosis. The findings can support the decision of screening of the bone and extracranial metastases for patients with high-risk primary malignancy.
Acknowledgments
Funding: This work was funded by the National Natural Science Foundation of China (No. 81501933), the Zhejiang Provincial Medical and Health Technology Foundation of China (No. 2018KY129), Wenzhou Leading Innovative Talent Project (No. RX2016004) and the Wenzhou Municipal Science and Technology Bureau (No. Y20170389). The funders had no role in the design, execution, or writing of the study.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.55). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ruatta F, Derosa L, Escudier B, et al. Prognosis of renal cell carcinoma with bone metastases: Experience from a large cancer centre. Eur J Cancer 2019;107:79-85. [Crossref] [PubMed]
- Qiu MZ, Shi SM, Chen ZH, et al. Frequency and clinicopathological features of metastasis to liver, lung, bone, and brain from gastric cancer: A SEER-based study. Cancer Med 2018;7:3662-72. [Crossref] [PubMed]
- Tahara RK, Brewer TM, Theriault RL, et al. Bone Metastasis of Breast Cancer. Adv Exp Med Biol 2019;1152:105-29. [Crossref] [PubMed]
- Suva LJ, Washam C, Nicholas RW, et al. Bone metastasis: mechanisms and therapeutic opportunities. Nat Rev Endocrinol 2011;7:208-18. [Crossref] [PubMed]
- Norgaard M, Jensen AO, Jacobsen JB, et al. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007). J Urol 2010;184:162-7. [Crossref] [PubMed]
- Hernandez RK, Wade SW, Reich A, et al. Incidence of bone metastases in patients with solid tumors: analysis of oncology electronic medical records in the United States. BMC Cancer 2018;18:44. [Crossref] [PubMed]
- Tanaka R, Yonemori K, Hirakawa A, et al. Risk Factors for Developing Skeletal-Related Events in Breast Cancer Patients With Bone Metastases Undergoing Treatment With Bone-Modifying Agents. Oncologist 2016;21:508-13. [Crossref] [PubMed]
- Santoni M, Conti A, Procopio G, et al. Bone metastases in patients with metastatic renal cell carcinoma: are they always associated with poor prognosis? J Exp Clin Cancer Res 2015;34:10. [Crossref] [PubMed]
- Santini D, Procopio G, Porta C, et al. Natural history of malignant bone disease in renal cancer: final results of an Italian bone metastasis survey. PLoS One 2013;8:e83026. [Crossref] [PubMed]
- Guo Q, Zhang C, Guo X, et al. Incidence of bone metastasis and factors contributing to its development and prognosis in newly diagnosed renal cell carcinoma: a population-based study. Cancer Manag Res 2018;10:2935-44. [Crossref] [PubMed]
- Nguyen QN, Chun SG, Chow E, et al. Single-Fraction Stereotactic vs Conventional Multifraction Radiotherapy for Pain Relief in Patients With Predominantly Nonspine Bone Metastases: A Randomized Phase 2 Trial. JAMA Oncol 2019;5:872-8. [Crossref] [PubMed]
- Escudier B, Powles T, Motzer RJ, et al. Cabozantinib, a New Standard of Care for Patients With Advanced Renal Cell Carcinoma and Bone Metastases? Subgroup Analysis of the METEOR Trial. J Clin Oncol 2018;36:765-72. [Crossref] [PubMed]
- Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer 2011;11:411-25. [Crossref] [PubMed]
- Ell B, Kang Y. SnapShot: Bone Metastasis. Cell 2012;151:690-690.e1. [Crossref] [PubMed]
- Maurizi A, Rucci N. The Osteoclast in Bone Metastasis: Player and Target. Cancers (Basel) 2018. [Crossref] [PubMed]
- Umer M, Mohib Y, Atif M, et al. Skeletal metastasis in renal cell carcinoma: A review. Ann Med Surg (Lond) 2018;27:9-16. [Crossref] [PubMed]
- Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 2001;27:165-76. [Crossref] [PubMed]
- Hage WD, Aboulafia AJ, Aboulafia DM. Incidence, location, and diagnostic evaluation of metastatic bone disease. Orthop Clin North Am 2000;31:515-28. vii. [Crossref] [PubMed]
- Jensen AO, Jacobsen JB, Norgaard M, et al. Incidence of bone metastases and skeletal-related events in breast cancer patients: a population-based cohort study in Denmark. BMC Cancer 2011;11:29. [Crossref] [PubMed]
- Surveillance, Epidemiology, and End Results (SEER) Program () Research Data (1973-2012), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2015, based on the November 2014 submission.www.seer.cancer.gov
- Roodman GD. Mechanisms of bone metastasis. N Engl J Med 2004;350:1655-64. [Crossref] [PubMed]
- Coleman RE. Skeletal complications of malignancy. Cancer 1997;80:1588-94. [Crossref] [PubMed]
- Sathiakumar N, Delzell E, Morrisey MA, et al. Mortality following bone metastasis and skeletal-related events among patients 65 years and above with lung cancer: A population-based analysis of U.S. Medicare beneficiaries, 1999-2006. Lung India 2013;30:20-6. [Crossref] [PubMed]
- Al Husaini H, Wheatley-Price P, Clemons M, et al. Prevention and management of bone metastases in lung cancer: a review. J Thorac Oncol 2009;4:251-9. [Crossref] [PubMed]
- Yerushalmi R, Woods R, Kennecke H, et al. Patterns of relapse in breast cancer: changes over time. Breast Cancer Res Treat 2010;120:753-9. [Crossref] [PubMed]
- Pietropaoli MP, Damron TA, Vermont AI. Bone metastases from squamous cell carcinoma of the head and neck. J Surg Oncol 2000;75:136-41. [Crossref] [PubMed]
- Attili VS, Babu KG, Lokanatha D, et al. Bone metastasis in hepatocellular carcinoma: need for reappraisal of treatment. J Cancer Res Ther 2008;4:93-4. [Crossref] [PubMed]
- Okazaki N, Yoshino M, Yoshida T, et al. Bone metastasis in hepatocellular carcinoma. Cancer 1985;55:1991-4. [Crossref] [PubMed]
- Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer 1987;55:61-6. [Crossref] [PubMed]
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351:1502-12. [Crossref] [PubMed]
- Pezaro C, Omlin A, Lorente D, et al. Visceral disease in castration-resistant prostate cancer. Eur Urol 2014;65:270-3. [Crossref] [PubMed]
- Halabi S, Kelly WK, Ma H, et al. Meta-Analysis Evaluating the Impact of Site of Metastasis on Overall Survival in Men With Castration-Resistant Prostate Cancer. J Clin Oncol 2016;34:1652-9. [Crossref] [PubMed]
- Body JJ, Quinn G, Talbot S, et al. Systematic review and meta-analysis on the proportion of patients with breast cancer who develop bone metastases. Crit Rev Oncol Hematol 2017;115:67-80. [Crossref] [PubMed]
- Yanae M, Fujimoto S, Tane K, et al. Increased risk of SSEs in bone-only metastatic breast cancer patients treated with zoledronic acid. J Bone Oncol 2017;8:18-22. [Crossref] [PubMed]
- Wallmeroth A, Wagner U, Moch H, et al. Patterns of metastasis in muscle-invasive bladder cancer (pT2-4): An autopsy study on 367 patients. Urol Int 1999;62:69-75. [Crossref] [PubMed]
- Woodward E, Jagdev S, McParland L, et al. Skeletal complications and survival in renal cancer patients with bone metastases. Bone 2011;48:160-6. [Crossref] [PubMed]
- Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 2008;372:449-56. [Crossref] [PubMed]
- Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115-24. [Crossref] [PubMed]
- Gong Y, Zhang J, Ji P, et al. Incidence proportions and prognosis of breast cancer patients with bone metastases at initial diagnosis. Cancer Med 2018;7:4156-69. [Crossref] [PubMed]
- Teoh JY, Tsu JH, Yuen SK, et al. Prognostic significance of time to prostate-specific antigen (PSA) nadir and its relationship to survival beyond time to PSA nadir for prostate cancer patients with bone metastases after primary androgen deprivation therapy. Ann Surg Oncol 2015;22:1385-91. [Crossref] [PubMed]
- Bhatia R, Ravulapati S, Befeler A, et al. Hepatocellular Carcinoma with Bone Metastases: Incidence, Prognostic Significance, and Management-Single-Center Experience. J Gastrointest Cancer 2017;48:321-5. [Crossref] [PubMed]
- Silvestris N, Pantano F, Ibrahim T, et al. Natural history of malignant bone disease in gastric cancer: final results of a multicenter bone metastasis survey. PLoS One 2013;8:e74402. [Crossref] [PubMed]
- Diel IJ. Effectiveness of bisphosphonates on bone pain and quality of life in breast cancer patients with metastatic bone disease: a review. Support Care Cancer 2007;15:1243. [Crossref] [PubMed]
- Martin M, Bell R, Bourgeois H, et al. Bone-related complications and quality of life in advanced breast cancer: results from a randomized phase III trial of denosumab versus zoledronic acid. Clin Cancer Res 2012;18:4841-9. [Crossref] [PubMed]
- Weinfurt KP, Castel LD, Li Y, et al. Health-related quality of life among patients with breast cancer receiving zoledronic acid or pamidronate disodium for metastatic bone lesions. Med Care 2004;42:164-75. [Crossref] [PubMed]
- Pockett RD, Castellano D, McEwan P, et al. The hospital burden of disease associated with bone metastases and skeletal-related events in patients with breast cancer, lung cancer, or prostate cancer in Spain. Eur J Cancer Care (Engl) 2010;19:755-60. [Crossref] [PubMed]
- Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2002;2:584-93. [Crossref] [PubMed]
- Gravel G, Tselikas L, Moulin B, et al. Early detection with MRI of incomplete treatment of spine metastases after percutaneous cryoablation. Eur Radiol 2019;29:5655-63. [Crossref] [PubMed]
- Onoue K, Nishio M, Yakami M, et al. CT temporal subtraction improves early detection of bone metastases compared to SPECT. Eur Radiol 2019;29:5673-81. [Crossref] [PubMed]
- Hirai T, Shinoda Y, Tateishi R, et al. Early detection of bone metastases of hepatocellular carcinoma reduces bone fracture and paralysis. Jpn J Clin Oncol 2019;49:529-36. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Kidney Cancer. Version 2.2018. Available online: https://www.nccn.org/
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Small Cell Lung Cancer. Version 2.2018. Available online: https://www.nccn.org/
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Squamous Cell Skin Cancer. Version 2.2018. Available online: https://www.nccn.org/
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Prostate Cancer. Version 3.2016. Available online: https://www.nccn.org/
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Esophageal and Esophagogastric Junction Cancers. Version 1.2017. Available online: https://www.nccn.org/
- Oefelein MG, Ricchiuti V, Conrad W, et al. Skeletal fractures negatively correlate with overall survival in men with prostate cancer. J Urol 2002;168:1005-7. [Crossref] [PubMed]
- Soran A, Ozmen V, Ozbas S, et al. Randomized Trial Comparing Resection of Primary Tumor with No Surgery in Stage IV Breast Cancer at Presentation: Protocol MF07-01. Ann Surg Oncol 2018;25:3141-9. [Crossref] [PubMed]
- Lee SJ, Park S, Ahn HK, et al. Implications of bone-only metastases in breast cancer: favorable preference with excellent outcomes of hormone receptor positive breast cancer. Cancer Res Treat 2011;43:89-95. [Crossref] [PubMed]
- Shen L, Dong J, Li S, et al. M1 stage subdivision and treatment outcome of patients with bone-only metastasis of nasopharyngeal carcinoma. Oncologist 2015;20:291-8. [Crossref] [PubMed]
- Niikura N, Liu J, Hayashi N, et al. Treatment outcome and prognostic factors for patients with bone-only metastases of breast cancer: a single-institution retrospective analysis. Oncologist 2011;16:155-64. [Crossref] [PubMed]
- Domchek SM, Younger J, Finkelstein DM, et al. Predictors of skeletal complications in patients with metastatic breast carcinoma. Cancer 2000;89:363-8. [Crossref] [PubMed]
- Coleman RE, Smith P, Rubens RD. Clinical course and prognostic factors following bone recurrence from breast cancer. Br J Cancer 1998;77:336-40. [Crossref] [PubMed]
- Plunkett TA, Smith P, Rubens RD. Risk of complications from bone metastases in breast cancer. implications for management. Eur J Cancer 2000;36:476-82. [Crossref] [PubMed]
- Sun XS, Liang YJ, Liu SL, et al. Subdivision of Nasopharyngeal Carcinoma Patients with Bone-Only Metastasis at Diagnosis for Prediction of Survival and Treatment Guidance. Cancer Res Treat 2019;51:1259-68. [Crossref] [PubMed]
- He S, Wang Y, Peng H, et al. Pretreatment Alkaline Phosphatase and Epstein-Barr Virus DNA Predict Poor Prognosis and Response to Salvage Radiotherapy in Patients with Nasopharyngeal Carcinoma and Metachronous Bone-Only Metastasis. J Cancer 2017;8:417-24. [Crossref] [PubMed]



