
MicroRNA-18a induces epithelial-mesenchymal transition like cancer stem cell phenotype via regulating RKIP pathway in pancreatic cancer
Introduction
Pancreatic cancer is a devastating invasive disease and is the fourth most common cause of deaths attributed to cancer in the USA (1).The high mortality of pancreatic cancer could, at least partly, be associated with metastasis. Epithelial to mesenchymal transition(EMT) plays a key role in metastasis of pancreatic cancer (2). Thus, understanding the molecular mechanism of EMT in pancreatic cancer is important in terms of improving pancreatic cancer prognosis. Previous research has found that Raf-1 kinase inhibitor protein (RKIP) is a suppressor of metastasis in human cancers (3). It is proposed that RKIP is a metastasis suppressor and a promising marker for predicting prognosis of pancreatic cancer. However, the function of RKIP in pancreatic cancer is still unclear (4).
It is well-known that MicroRNAs (miRNAs/miR), as small non-coding RNAs with 19–25 nucleotides, play an essential role as post-transcriptional regulators to gene expression (5). Multiple genes regulated by miRNAs play crucial roles in migration, EMT, invasion, and tumor metastasis (6).
This study aimed to explore the activity of RKIP in pancreatic cancer, so as to understand the molecular mechanism of metastasis of pancreatic cancer.
Methods
Sample collection of pancreatic cancer tissues
Cancer tissues from subjects with pancreatic cancer and adjacent normal tissues were collected tour hospital. All tissues were confirmed by pathologists.
Cell line PANC-1
Pancreatic ductal adenocarcinoma cell Line PANC-1was obtained from the Chinese Academy of Sciences (Shanghai, China) and cultured with Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum (Invitrogen, San Diego, CA, USA) at 37 °C with 5% CO2.
Transfection experiments
RKIP expressing vectors were purchased from (Invitrogen, San Diego, CA, USA). Negative control miRNA (anti-miR) andpre-microRNA-181a (pre-miR-181a) were purchased from Ambion. The transfection process was conducted according to the manufacturers’ instructions. The incubation period lasted for 6 hours, before the medium was removed, and the cells were cultured with another medium for a further 48 hours.
Western blot analysis
Incubation with primary antibodies anti-RKIP, anti-N-Cadherin, anti-E-Cadherin, anti-SNAIL1, anti-Vimentin, anti-TGFB1, anti-Fibronectin, anti-CD44, anti-Tspan8 and anti-β-actin took place overnight at 4 °C. All primary antibodies were purchased from company (1:500; Abcam, Cambridge, MA, USA). IRDye TM-800 conjugated anti-rabbit secondary antibodies (Li-COR, Biosciences, Lincoln, NE, USA) were applied at room temperature for 2 hours. OdysseyTM Infrared Imaging System (Gene Company, Lincoln, NE, USA) was used to determine the specific protein.
Sphere growth and invasion assay
Cells (2.5×103/mL) were seeded for seven days on 0.5% agar precoated 6-well plates in serum-free RPMI1640/1 mM Na-pyruvate. Invasion assay was based on the protocol of the previous study (7) .
Quantitative polymerase chain reaction (qPCR) for microRNAs and RKIP
qPCR was performed as previously described (8). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as a housekeeping gene for controlling RNA loading. The PCR primer sequences were: GAPDH: Forward-5'-CGGAGTCAACGG ATTTGGTCGTAT-3', Reverse-5'-AGCCTT CTCCATGGTGGTGAAGAC-3'. RKIP: Forward-5'-TATGCCGGTGGACCTCAG-3', Reverse-5'-CCTACTTCCCAGACAG-3'. Power SYBR Green PCR Master Mix (TAKARA Cat No. RR820W) was used in this study.
Immunofluorescence analyses
Immunofluorescence analyses were performed according to the manufacturer’s instructions. Briefly, anti-RKIP antibody was applied to stain transfected cells. The secondary antibodies were Alexa Fluor 488 goat anti-rabbit IgG antibody and Coverslips (Invitrogen). Use of a confocal laser-scanning microscope (Leica Microsystems, Bensheim, Germany) allowed us to make microscopic observations. The intensity of fluorescence was assessed in a small number of viewing areas with 300 cells for each coverslip, and ImageJ 1.37v software (http://rsb.info.nih.gov/ij/index.html) was used to conduct analysis.
Statistical analysis
Data analysis was conducted using SPSS20.0 software (SPSS Inc., II, Chicago, IL, USA), and the mean level of index between the two groups was determined by Student’s t-test. A P value <0.05 was deemed to be statistically significant.
Results
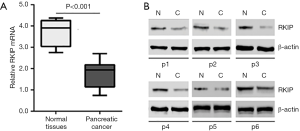
Pancreatic cancer sees lower RKIP expression
To assess the difference between the expression of RKIP mRNA in pancreatic cancer tissues in comparison with its expression in normal adjacent tissues, aqPCR was carried out for both sets of tissues. mRNA was separated from 56 pairs of cancerous tissues and adjacent normal tissues. Our results indicated that RKIP mRNA expression in the cancerous tissues were decreased in comparison with the adjacent normal tissues (Figure 1A). Western blotting was applied to identify protein isolated from the 6 pairs of cancer and adjacent normal tissues, and the results showed that the cancer tissues had lower RKIP expression (Figure 1B).
RKIP inhibits EMT in pancreatic cancer PANC-1 cells
To establish how RKIP operates in relation to pancreatic cancer, PANC-1 cells with RKIP-expressing plasmids were transfected. The results revealed that PANC-1cells transfected with RKIP-expressing plasmids showed slight changes in morphology in PANC-1 cells (Figure 2A) and higher expression of RKIP protein (Figure 2B).
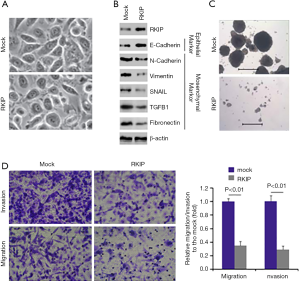
To further verify that MET induced the changes of cell morphology. Expression levels of epithelial and mesenchymal markers were determined between PANC-1 cells that had been transfected with RKIP-expressing plasmids and the empty-vector cells. We found the E-Cadherin (epithelial marker) to be higher and N-Cadherin, Vimentin, SNAIL, TGFB1, and Fibronectin (mesenchymal markers) to be lower in PANC-1 cells that had received RKIP-expressing plasmids (Figure 2B). Our study also identified an RKIP-induced decrease in sphere formation in PANC-1 cells (Figure 2C). We also found that RKIP overexpression could suppress migration and invasion (Figure 2D) in the cells.
miR-181a can degrade RKIP in PANC-1 cells
Our results established that an increase inmiR-181a was found in cells treated by pre-miR-181a (Figure 3A,B), as well as showing RKIP protein in the pre-miR-181a-transfected cells to be evidently suppressed (Figure 3C).
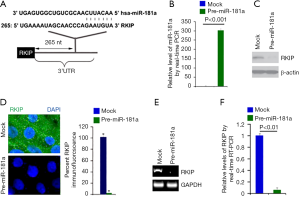
Furthermore, immunofluorescence analysis was carried out in PANC-1 cells transfected with pre-miR-181a and control miR. In correspondence with the findings of the western blot, the results of immunofluorescence found RKIP protein to be demonstrably suppressed in the cells that had received pre-miR-181a (Figure 3D).
RT-PCR and real-time PCR were then carried out to measure the expression of RKIP mRNA in PANC-1 cells transfected with pre-miR-181a or control miR. The RT-PCR results identified significant downregulation of RKIP mRNA (Figure 3E) in the cells transfected with pre-miR-181a. Real-time PCR also came up with the same results (Figure 3F).
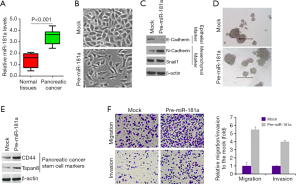
miR-181a can promote EMT
This study showed that expression of miR-181a was higher in cancerous tissues than in adjacent normal tissues (Figure 4A). Overexpression of miR-181a caused significant changes in PANC-1 cells morphology (EMT) (Figure 4B). The E-Cadherin (epithelial marker) was inhibited and N-Cadherin and SNAIL1 (mesenchymal markers) were induced by miR-181a in PANC-1 cells (Figure 4C). We also noted that miR-181a in PANC-1 cells increased sphere formation (Figure 4D). In addition, we found that miR-181a could suppress pancreatic cancer stem cell (CSC) markers (CD44 and Tspan8) in PCNA-1 cells (Figure 4E). To detect whether miR-181a could regulate migration and invasion, we performed invasion and migration assay. We found that miR-181a could indeed promote migration and invasion (Figure 4F) in the cells.
Discussion
Raf-1 can lead to the development of tumor-related processes. Deregulated or constitutively active Raf-1 protein, for example, can contribute to changes in cells (9). Amplification and mutation of upstream regulators of Raf-1, including receptor tyrosine kinases (10) and Ras (11), often results in signaling deregulation in tumors via the Raf/MEK/ERK cascade. The activation of Raf-1 has been identified in cells which express oncogenic B-Raf proteins (12). More recently, it has been proposed that RKIP is a metastasis suppressor and could be a promising marker for predicting a better prognosis in pancreatic cancer (4). In line with earlier reports, we found that in pancreatic cancer there is a downregulation in RKIP expression. RKIP can inhibit EMT in line with CSC phenotype in PANC-1 cells. MEK/ERK signaling is avital part of a wide-ranging group of cellular functions including cell proliferation, differentiation and survival, and it can have a negative impact on the regulation of mouse embryonic stem cell (mESC) self-renewal by antagonizing STAT3 activity (13). We reason that RKIP might inhibit EMT by regulating MEK/ERK. Chemotherapy is a key therapy in the strategic treatment of pancreatic, but it does not succeed at killing all tumor cells, at least in part due to drug resistance of an intrinsic or acquired nature. Emerging evidence indicates that CSCs and EMT-type cells play an important and complicated role in anticancer drug resistance. Thus, we believe that RKIP had a significant effect on drug resistance in the treatment of pancreatic cancer.
EMT cells can have features similar to CSCs, and CSCs exhibit mesenchymal phenotype under most circumstances. A correlation between aberrant miRNA expression, and the presence of CSCs and the acquisition of an EMT phenotype (14) has been identified. We found that miR-18a can promote EMT consistent with CSC phenotype by directly degrading RKIP in pancreatic cancer PANC-1 cells.
PTEN is crucial for maintaining stem cells and a decrease can result in CSCs clones emerging and proliferating (15). The expression of PTEN can be suppressed by miR-181a in pancreatic cancer (16). Thus, we reasoned that miR-181a promotes CSC-like features, at least partly, by regulating RKIP and PTEN in pancreatic cancer. Moreover, miR-181a can promote migration by inhibiting MAP2K4 in pancreatic cancer. All the results indicate that miR-181 operates like an oncogene by controlling different tumor suppressive genes in pancreatic cancer.
In conclusion, we established that miR-181a induces EMT phenotype by regulating RKIP in pancreatic cancer. MicroRNA-18a may be a novel target for treatment of pancreatic cancer in future.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.195). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yang C, Luo G, Cheng H, et al. Potential biomarkers to evaluate therapeutic response in advanced pancreatic cancer. Transl Cancer Res 2018;7:208-18. [Crossref]
- Sarkar FH, Li Y, Wang Z, et al. Pancreatic cancer stem cells and EMT in drug resistance and metastasis. Minerva Chir 2009;64:489-500. [PubMed]
- Chen Y, Ouyang GL, Yi H, et al. Identification of RKIP as an invasion suppressor protein in nasopharyngeal carcinoma by proteomic analysis. J Proteome Res 2008;7:5254-62. [Crossref] [PubMed]
- Song SP, Zhang SB, Li ZH, et al. Reduced expression of Raf kinase inhibitor protein correlates with poor prognosis in pancreatic cancer. Clin Transl Oncol 2012;14:848-52. [Crossref] [PubMed]
- Inui M, Martello G, Piccolo S. MicroRNA control of signal transduction. Nat Rev Mol Cell Biol 2010;11:252-63. [Crossref] [PubMed]
- Dong Y, Sun Y, Huang Y, et al. Depletion of MLKL inhibits invasion of radioresistant nasopharyngeal carcinoma cells by suppressing epithelial-mesenchymal transition. Ann Transl Med 2019;7:741. [Crossref] [PubMed]
- Lu Y, Chopp M, Zheng X, et al. MiR-145 reduces ADAM17 expression and inhibits in vitro migration and invasion of glioma cells. Oncol Rep 2013;29:67-72. [Crossref] [PubMed]
- Meng F, Henson R, Wehbe-Janek H, et al. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 2007;133:647-58. [Crossref] [PubMed]
- Heidecker G, Huleihel M, Cleveland JL, et al. Mutational activation of c-raf-1 and definition of the minimal transforming sequence. Mol Cell Biol 1990;10:2503-12. [Crossref] [PubMed]
- Marmor MD, Skaria KB, Yarden Y. Signal transduction and oncogenesis by ErbB/HER receptors. Int J Radiat Oncol Biol Phys 2004;58:903-13. [Crossref] [PubMed]
- Pruitt K, Der CJ. Ras and Rho regulation of the cell cycle and oncogenesis. Cancer Lett 2001;171:1-10. [Crossref] [PubMed]
- Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 2004;116:855-67. [Crossref] [PubMed]
- Li J, Wang G, Wang C, et al. MEK/ERK signaling contributes to the maintenance of human embryonic stem cell self-renewal. Differentiation 2007;75:299-307. [Crossref] [PubMed]
- Wang Z, Li Y, Ahmad A, et al. Targeting miRNAs involved in cancer stem cell and EMT regulation: An emerging concept in overcoming drug resistance. Drug Resist Updat 2010;13:109-18. [Crossref] [PubMed]
- Ciuffreda L, Falcone I, Incani UC, et al. PTEN expression and function in adult cancer stem cells and prospects for therapeutic targeting. Adv Biol Regul 2014;56:66-80. [Crossref] [PubMed]
- Liu J, Xu D, Wang Q, et al. LPS induced miR-181a promotes pancreatic cancer cell migration via targeting PTEN and MAP2K4. Dig Dis Sci 2014;59:1452-60. [Crossref] [PubMed]


